Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ueha, R.; Cotaoco, C.; Kondo, K.; Yamasoba, T. Management and Treatment for Dysphagia in Neurodegenerative Disorders. Encyclopedia. Available online: https://encyclopedia.pub/entry/53839 (accessed on 07 February 2026).
Ueha R, Cotaoco C, Kondo K, Yamasoba T. Management and Treatment for Dysphagia in Neurodegenerative Disorders. Encyclopedia. Available at: https://encyclopedia.pub/entry/53839. Accessed February 07, 2026.
Ueha, Rumi, Carmel Cotaoco, Kenji Kondo, Tatsuya Yamasoba. "Management and Treatment for Dysphagia in Neurodegenerative Disorders" Encyclopedia, https://encyclopedia.pub/entry/53839 (accessed February 07, 2026).
Ueha, R., Cotaoco, C., Kondo, K., & Yamasoba, T. (2024, January 15). Management and Treatment for Dysphagia in Neurodegenerative Disorders. In Encyclopedia. https://encyclopedia.pub/entry/53839
Ueha, Rumi, et al. "Management and Treatment for Dysphagia in Neurodegenerative Disorders." Encyclopedia. Web. 15 January, 2024.
Copy Citation
Patients with neurodegenerative disorders (NDDs) often experience functional dysphagia, which may involve dysfunction in a specific phase of swallowing or in the entire process. Distinguishing the etiology of dysphagia can be difficult, and it is important to always look out for signs pointing to NDD as the cause. Thorough diagnostic work-up is essential, and it includes a comprehensive history and physical examination, alongside swallowing function tests, such as fiberoptic endoscopic evaluation of swallowing, videofluoroscopic swallowing study, and high-resolution manometry. Management requires a multidisciplinary approach with a treatment plan tailored to each patient.
dysphagia
neurodegenerative disorders
Parkinson’s disease
multiple system atrophy
amyotrophic lateral sclerosis
Alzheimer’s disease
surgery
1. The Differentiation of Diseases Causing Dysphagia
Formulating differential diagnoses for swallowing disorders involves distinguishing among various conditions that can lead to impaired swallowing function. Dysphagia is categorized into organic swallowing disorders (static dysphagia) and functional swallowing disorders (dynamic dysphagia). Organic dysphagia results from structural abnormalities such as tumor lesions or inflammatory conditions extending from the oral cavity to the esophagus. It can also manifest as mechanical impediments to bolus passage due to irregularities in the passage or compression from the surrounding tissues. In contrast, functional dysphagia is characterized by normal anatomy but abnormal bolus transport function from the oral cavity to the esophagus. Other potential causes include eating disorders and psychiatric conditions [1][2]. Neurological disorders mainly present with functional dysphagia [3][4][5]. A comprehensive assessment of the patient’s symptoms and clinical findings is essential to accurately diagnose the underlying cause of dysphagia.
After differentiating whether dysphagia is organic or functional, a flowchart for evaluating the underlying cause of dysphagia, based on the presence of consciousness disorders, is illustrated in Figure 1. A flowchart for differentiating the causative disease of dysphagia according to whether the course of dysphagia is acute in onset or slowly progressive is shown in Figure 2. In patient assessments, it is essential to prioritize a comprehensive interview, encompassing the current and past medical history, family background, medication usage, and other pertinent details, before proceeding to a meticulous evaluation of the oral and pharyngeal regions. Thorough psychosomatic assessments are highly important.
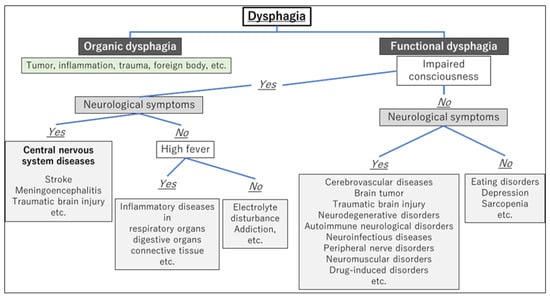
Figure 1. Flowchart for evaluating the underlying cause of dysphagia based on the presence of consciousness disorders.
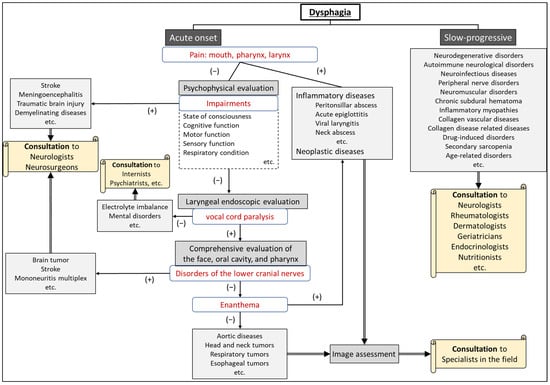
Figure 2. Flowchart for differentiating the etiology of dysphagia according to whether the onset is acute or slow-progressive.
2. Neurodegenerative Disorders Causing Dysphagia
2.1. Representative Neurodegenerative Disorders Causing Dysphagia
Major neurodegenerative disorders (NDDs) that cause dysphagia include Parkinson’s disease (PD), progressive supranuclear palsy (PSP), spinocerebellar ataxia (SCA), multiple system atrophy (MSA), amyotrophic lateral sclerosis (ALS), spinal muscular atrophy (SMA), corticobasal degeneration (CBD), Alzheimer’s disease (AD), and Huntington’s disease, and so on.
2.2. Parkinson’s Disease
PD is a neurodegenerative disorder characterized by symptoms such as resting tremor, muscle rigidity, and bradykinesia. More than 80% of patients with Parkinson’s disease develop dysphagia during the course of the disease; however, the degree of dysphagia does not necessarily correlate with the severity of PD [6]. In PD, all voluntary and involuntary motor processes in swallowing can be impaired: cognitive stage impairment due to depression and cognitive dysfunction [5], preparatory and oral stage impairment due to tremor and rigidity, pharyngeal stage impairment due to delayed swallowing reflex, decreased pharyngeal contractility, laryngeal elevation impairment [7][8], esophageal stage impairment due to upper esophageal sphincter (UES) dysfunction, and esophageal peristalsis impairment [9]. Furthermore, it should be noted that dysphagia can occur as a side effect of therapeutic medications in patients with PD [10]. Bilateral vocal fold movement disorders may also occur in patients with PD [11][12]. Tracheostomy, laser arytenoidectomy, or aspiration prevention surgery may be performed for progressive airway narrowing and severe dysphagia [11][12][13].
2.3. Multiple System Atrophy
MSA is a neurodegenerative disorder characterized by a combination of autonomic failure plus cerebellar syndrome and/or parkinsonism. MSA is categorized into two main subtypes: MSA-parkinsonian type (MSA-P), which is more like PD, and MSA-cerebellar type (MSA-C), which is associated with balance and coordination problems [14]. Dysphagia is a frequent and disabling symptom of MSA that occurs within five years [14][15]. Abnormalities in the oral and pharyngeal phases of swallowing, along with esophageal dysfunction and aspiration, occur in MSA and worsen as the disease progresses [14][16][17]. Bilateral vocal fold movement impairment (abduction disorder) occurs more frequently in MSA than in PD [18] and is characterized by an exacerbation of vocal cord dysfunction during sleep [19]. Depending on the progression of MSA, procedures such as tracheostomy or aspiration prevention surgery may be necessary [13][20].
2.4. Amyotrophic Lateral Sclerosis
ALS, also known as Lou Gehrig’s disease, is a progressive NDD. ALS affects the nerve cells in the brain and spinal cord. It specifically targets motor neurons, which are the nerve cells responsible for controlling muscle movements, including those used in talking, chewing, swallowing, and breathing [21]. Dysphagia is a significant and progressive symptom in ALS [22]. Dysphagia in ALS correlates significantly with bulbar onset and oral and pharyngeal swallowing impairment [22][23], while the esophageal phase is relatively preserved at the early stage of the disease [24]. Dysphagia contributes to malnutrition, dehydration, and aspiration pneumonia and accounts for a significant proportion of deaths in patients with ALS [22]. Early intervention and comprehensive management of dysphagia are crucial for addressing the risk of aspiration and its impact on the well-being of patients with ALS [25], and preventing aspiration is a critical aspect of managing ALS-related dysphagia [26]. Tracheostomy or aspiration prevention surgery may be performed because of respiratory deterioration or progression of dysphagia [13][26][27].
3. The Evaluation of Patients with Suspected Neurodegenerative Disorders
3.1. Patients with Neurodegenerative Disorders among New Outpatient Visits
In outpatient visits, some patients presenting with dysphagia or dysarthria as a chief complaint may have a background of NDDs. Patient data over approximately a decade (beginning in 2013) at the University of Tokyo Hospital showed that among 7910 patients attending the voice and swallowing clinic, 4973 (62.9%) had dysphagia, vocal fold movement disorder, or dysarthria. Of these, 1044 (13.2%) had already been diagnosed with NDDs (unpublished data). Among 3929 patients (49.7%) without a diagnosed underlying condition during the initial outpatient visits, 37 (1.0%) had suspected NDDs during otolaryngological examinations. Following referral to a neurologist, 19 patients (0.5%) were diagnosed with NDDs. This means that NDDs are hidden in the background of a small number of patients who present with dysphagia as their chief complaint.
3.2. Physical Signs and Oro-pharyngo-laryngeal Findings Suggesting Neurodegenerative Disorders
When the following findings are observed during medical interviews and physical assessments, it is recommended to proceed with the examination with consideration for NDDs:
-
Weight loss;
-
Gait disturbance/stumbling;
-
Muscle weakness;
-
Tremor;
-
Reduced facial expression;
-
Cognitive impairment;
-
Respiratory dysfunction.
When the following findings are observed upon examination of the oral cavity, pharynx, and larynx, the possibility of neurodegenerative disorders should be considered in conjunction with other physical examination findings:
-
Dysarthria;
-
Speech impairment (hoarseness, low volume);
-
Tongue atrophy and limited tongue movement;
-
Velopharyngeal insufficiency (during articulation and/or swallowing);
-
Vocal fold movement impairment;
-
Reduced pharyngeal contraction;
-
Decreased pharyngo-laryngeal sensation.
Dysarthria can be confirmed not only through pronunciation assessments, such as “/pa//ta//ka/,” “/ra//na/,” and “/ŋa//ŋa/,” but also through the repetition of tongue twisters when evaluating [28]. Tongue atrophy, tremors, and motor dysfunction are characteristic features observed in a variety of neuromuscular disorders [29]. Notably, tongue atrophy frequently manifests in conditions like ALS with bulbar involvement, muscular dystrophy, and severe myasthenia gravis. Tongue tremor is observed in conditions such as PD, essential tremor, and MSA-P [30][31]. When assessing tongue motor dysfunction, a finding of the mandible moving with the tongue is a compensatory maneuver for tongue motor impairment. Velopharyngeal insufficiency should be assessed during both swallowing and vocalization (/ka/, /ŋa/). Velopharyngeal insufficiency may be present during swallowing, even when open nasal speech is not present (velopharyngeal closure is possible during speech), and vice versa. ALS should be suspected when there is a discrepancy between velopharyngeal closure during swallowing and speech [32]. Velopharyngeal insufficiency can also occur in multiple sclerosis, Guillain-Barré syndrome, myasthenia gravis, muscular dystrophy, etc [33]. Vocal cord dysfunction (VCD) has been reported in various neurological disorders, including PD and MSA, and rarely in ALS and Guillain-Barre syndrome [34][35]. Reduced pharyngeal contraction and decreased pharyngolaryngeal sensation are commonly associated with a range of NDDs. In ALS, sensory disturbances are generally not evident until the disease has significantly advanced.
4. The Management of Dysphagia in Neurodegenerative Disorders
4.1. Meal-Time Management
Managing dysphagia in patients with NDDs involves providing support and guidance tailored to their current condition. Despite the frequent progressive nature of these disorders, there is the possibility of temporary improvement through pharmacological treatment, particularly in PD. Therefore, addressing swallowing difficulties in accordance with a patient’s specific conditions is advisable. Depending on the degree of the patient’s functional impairment, nutritional and swallowing guidance should include specific instructions regarding the following components: [36][37][38][39]
-
Modifications of bolus texture (liquid thickness, swallowing diet)
For individuals with dysphagia, adjusting the physical properties of the diet according to swallowing function is important to lessen the risk of choking and subsequent aspiration pneumonia [40]. Increasing the bolus thickness has been identified as a means of lowering the risk of airway penetration. However, these adjustments in the physical characteristics of the bolus are associated with reduced palatability and an increase in pharyngeal residue, potentially amplifying the risk of post-swallow aspiration [41].
-
Modifications of bolus size (the amount of food placed in the mouth at one time)
The modification of bolus size is a critical consideration in dysphagia management and should be tailored to the specific needs of the individual patient. Medical professionals may increase the bolus size to stimulate a swallow response or decrease it for patients who require multiple swallows per bolus. Larger bolus volumes have been associated with faster pharyngeal transit, while smaller volumes may be safer for swallowing in some populations [42][43].
-
Feeding posture
Effectively addressing feeding posture in the context of dysphagia, especially in individuals with neurological impairments, such as those found in ALS, involves specific head and body positioning techniques to optimize the safety and efficiency of the swallowing process. These strategies aim to reduce the risk of aspiration and ensure a satisfactory nutritional intake. [25][44][45]
-
Compensatory swallowing techniques, such as chin tuck, Supraglottic Swallow, and head rotation
In addition, guidance should be provided based on the patient’s medical and physical condition, including adjustments to meal settings, thoughtful supervision, posture modifications, and the utilization of self-help devices such as spoons of different sizes and lengths.
4.2. Swallowing Rehabilitation
The establishment of short- and long-term goals for patients undergoing swallowing rehabilitation varies depending on whether the condition is a progressive disease with no established treatment or a disease with available therapeutic approaches. However, the key points in the management of all swallowing disorders are conservative treatment, pharmacotherapy, and rehabilitation (swallowing voice, and speech). Swallowing training includes indirect exercises performed without the use of food, such as ice massages to facilitate the swallowing reflex [46], head-lift exercises (Shaker exercise) to strengthen laryngeal elevation [47][48][49], respiratory muscle training [50][51], and neuromuscular electrical stimulation [41][52]. In contrast, swallowing training can also include direct exercises that involve the use of food, such as effortful swallows and multiple swallows. Certain compensatory techniques used during eating are also a very common component of swallowing training, such as the chin-tuck maneuver and head rotation. Optimal intervention entails a multidisciplinary approach, with collaboration among physicians, speech-language pathologists, nurses, dietitians, and other healthcare professionals [1][41][53].
- ▪
-
Indirect exercises
-
Facilitating the swallowing reflex: ice massage [46]
-
Ice massage is a technique designed to trigger the swallowing reflex by lightly rubbing and applying pressure to the posterior tongue, tongue base, velum, and posterior pharyngeal wall with an ice stick for 10 s.
- Strengthening laryngeal elevation: resistance-based exercises and head-lift exercises (Shaker exercise) [47][48][49][54]
These exercises aim to strengthen the muscles involved in swallowing, particularly in patients with reduced superior and anterior movements of the hyolaryngeal complex. These include head-lift (Shaker) exercises, Mendelsohn Maneuver, effortful swallowing, and chin-tuck maneuver against resistance. Head-lift (Shaker) exercises involve lying flat on the back and lifting the head to look at the toes while keeping the shoulders down for 60 s and repeating this three times. The second part consists of a repetitive movement: lifting the head to look at the chin, lowering it to the bed, and repeating this 30 times for three sets.
This exercise is used to strengthen the muscles of respiration and is weakened by various conditions including ALS, PD, and chronic obstructive pulmonary disease. This can also help improve speaking, swallowing, and coughing, as these functions use the related muscles.
- Cough reflex exercise [54]
This exercise aims to strengthen the muscles involved in swallowing and enhance airway protection. This results in an improved closure of the larynx and enhanced coordination between swallowing and coughing to prevent aspiration.
Neuromuscular electrical stimulation is a noninvasive therapy that aims to improve the coordination, endurance, sensory feedback, and timing of the muscles involved in swallowing. This treatment is often used in combination with traditional treatments to improve swallowing.
- ▪
-
Direct exercises
This technique aims to recruit more motor units during swallowing, increase muscle demand, and create a muscle-training/strengthening effect. It involves continuously exerting force on the neck while maintaining the larynx in the maximally elevated position during swallowing.
- Multiple swallows [57]
Multiple swallows involves a series of repeated swallows to clear a single bolus from the oropharyngeal cavity. This aims to enhance the ability to modulate the timing, force, and coordination of the multiple muscles involved in swallowing.
- Alternate swallows [54]
This technique involves repeated swallows that alternate between solid foods and liquids as the bolus. This process is repeated several times. The purpose of this approach is to facilitate the movement of food and liquid through the swallowing process, helping to ensure safe and efficient swallowing.
- ▪
-
-
Chin-tuck maneuver [60]
-
This maneuver helps redirect food and liquid from the airway, reducing the risk of aspiration. It entails the individual tucking their chin toward their chest before or during swallowing. This position reduces the space between the tongue base and the back of the throat and increases pharyngeal pressure to facilitate bolus movement.
Head rotation during swallowing can be performed as a compensatory technique for individuals with dysphagia. Studies have shown that head rotation can improve swallowing in patients with unilateral oropharyngeal dysphagia. This maneuver helps redirect food and liquids away from the airway, reducing the risk of aspiration.
- Supraglottic Swallow [63]
The Supraglottic Swallow is a technique to prevent aspiration during swallowing. It involves the voluntary closing of the vocal folds by holding one’s breath before and during swallowing, followed by an immediate cough after swallowing. The maneuver aims to safeguard against aspiration and clear any post-swallowing residue.
References
- Dziewas, R.; Allescher, H.D.; Aroyo, I.; Bartolome, G.; Beilenhoff, U.; Bohlender, J.; Breitbach-Snowdon, H.; Fheodoroff, K.; Glahn, J.; Heppner, H.J.; et al. Diagnosis and treatment of neurogenic dysphagia—S1 guideline of the German Society of Neurology. Neurol. Res. Pract. 2021, 3, 23.
- Willig, T.N.; Paulus, J.; Lacau Saint Guily, J.; Beon, C.; Navarro, J. Swallowing problems in neuromuscular disorders. Arch. Phys. Med. Rehabil. 1994, 75, 1175–1181.
- Panebianco, M.; Marchese-Ragona, R.; Masiero, S.; Restivo, D.A. Dysphagia in neurological diseases: A literature review. Neurol. Sci. 2020, 41, 3067–3073.
- Lápez-Liria, R.; Parra-Egeda, J.; Vega-Ramírez, F.A.; Aguilar-Parra, J.M.; Trigueros-Ramos, R.; Morales-Gázquez, M.J.; Rocamora-Pérez, P. Treatment of Dysphagia in Parkinson’s Disease: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4104.
- Lee, J.; Madhavan, A.; Krajewski, E.; Lingenfelter, S. Assessment of dysarthria and dysphagia in patients with amyotrophic lateral sclerosis: Review of the current evidence. Muscle Nerve 2021, 64, 520–531.
- Kim, J.S.; Youn, J.; Suh, M.K.; Kim, T.E.; Chin, J.; Park, S.; Cho, J.W. Cognitive and Motor Aspects of Parkinson’s Disease Associated with Dysphagia. Can. J. Neurol. Sci. 2015, 42, 395–400.
- Matsuo, K.; Palmer, J.B. Anatomy and physiology of feeding and swallowing: Normal and abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707.
- Lee, E.; Kim, G.J.; Ryu, H.; Jung, K.I.; Yoo, W.K.; Ohn, S.H. Pharyngeal Structure and Dysphagia in Patients with Parkinson’s Disease and Related Disorders. Dysphagia 2023.
- Suttrup, I.; Suttrup, J.; Suntrup-Krueger, S.; Siemer, M.L.; Bauer, J.; Hamacher, C.; Oelenberg, S.; Domagk, D.; Dziewas, R.; Warnecke, T. Esophageal dysfunction in different stages of Parkinson’s disease. Neurogastroenterol. Motil. 2017, 29, e12915.
- Labeit, B.; Berkovich, E.; Claus, I.; Roderigo, M.; Schwake, A.L.; Izgelov, D.; Mimrod, D.; Ahring, S.; Oelenberg, S.; Muhle, P.; et al. Dysphagia for medication in Parkinson’s disease. NPJ Parkinsons Dis. 2022, 8, 156.
- Qayyum, A.; Mierzwa, K.; See, M.; Sharma, A.; Montgommery, P.Q. Laser arytenoidectomy for bilateral vocal fold palsy in Parkinson’s disease. J. Laryngol. Otol. 2005, 119, 831–833.
- Lee, D.H.; Lim, S.C.; Lee, J.K. Bilateral vocal cord paralysis in a patient with Parkinson’s disease. B-ENT 2012, 8, 141–142.
- Katoh, M.; Ueha, R.; Sato, T.; Sugasawa, S.; Goto, T.; Yamauchi, A.; Yamasoba, T. Choice of Aspiration Prevention Surgery for Patients With Neuromuscular Disorders: Report of Three Cases. Front. Surg. 2019, 6, 66.
- Calandra-Buonaura, G.; Alfonsi, E.; Vignatelli, L.; Benarroch, E.E.; Giannini, G.; Iranzo, A.; Low, P.A.; Martinelli, P.; Provini, F.; Quinn, N.; et al. Dysphagia in multiple system atrophy consensus statement on diagnosis, prognosis and treatment. Parkinsonism Relat. Dis. 2021, 86, 124–132.
- Tsuchiya, K.; Ueha, R.; Suzuki, S.; Goto, T.; Sato, T.; Nito, T.; Yamasoba, T. Heightened risk of early vocal fold motion impairment onset and dysphagia in the parkinsonian variant of multiple system atrophy: A comparative study. Clin. Park. Relat. Disord. 2020, 3, 100037.
- Ueha, R.; Goto, T.; Sato, T.; Nativ-Zeltzer, N.; Shen, S.C.; Nito, T.; Belafsky, P.C.; Yamasoba, T. High Resolution Manofluorographic Study in Patients With Multiple System Atrophy: Possible Early Detection of Upper Esophageal Sphincter and Proximal Esophageal Abnormality. Front. Med. 2018, 5, 286.
- Ueha, R.; Sato, T.; Goto, T.; Yamauchi, A.; Nativ-Zeltzer, N.; Mitsui, J.; Belafsky, P.C.; Yamasoba, T. Esophageal Dysmotility is Common in Patients With Multiple System Atrophy. Laryngoscope 2021, 131, 832–838.
- Gandor, F.; Vogel, A.; Claus, I.; Ahring, S.; Gruber, D.; Heinze, H.J.; Dziewas, R.; Ebersbach, G.; Warnecke, T. Laryngeal Movement Disorders in Multiple System Atrophy: A Diagnostic Biomarker? Mov. Disord. 2020, 35, 2174–2183.
- Ueha, R.; Maeda, E.; Ino, K.; Shimizu, T.; Sato, T.; Goto, T.; Yamasoba, T. Sleep-Induced Glottis Closure in Multiple System Atrophy Evaluated by Four-Dimensional Computed Tomography. Front. Med. 2020, 7, 132.
- Ueha, R.; Nito, T.; Sakamoto, T.; Yamauchi, A.; Tsunoda, K.; Yamasoba, T. Post-operative swallowing in multiple system atrophy. Eur. J. Neurol. 2016, 23, 393–400.
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; van den Berg, L.H. Amyotrophic lateral sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071.
- Ruoppolo, G.; Schettino, I.; Frasca, V.; Giacomelli, E.; Prosperini, L.; Cambieri, C.; Roma, R.; Greco, A.; Mancini, P.; De Vincentiis, M.; et al. Dysphagia in amyotrophic lateral sclerosis: Prevalence and clinical findings. Acta Neurol. Scand. 2013, 128, 397–401.
- Rugaitiene, M.; Damuleviciene, G.; Lesauskaite, V.; Uloziene, I. Oropharyngeal Dysphagia as the Main Expression of Amyotrophic Lateral Sclerosis. Medicina 2022, 58, 647.
- Taniguchi, H.; Nakayama, H.; Hori, K.; Nishizawa, M.; Inoue, M.; Shimohata, T. Esophageal Involvement in Multiple System Atrophy. Dysphagia 2015, 30, 669–673.
- Onesti, E.; Schettino, I.; Gori, M.C.; Frasca, V.; Ceccanti, M.; Cambieri, C.; Ruoppolo, G.; Inghilleri, M. Dysphagia in amyotrophic lateral sclerosis: Impact on Patient Behavior, Diet adaptation, and riluzole Management. Front. Neurol. 2017, 8, 94.
- Soga, T.; Suzuki, N.; Kato, K.; Kawamoto-Hirano, A.; Kawauchi, Y.; Izumi, R.; Toyoshima, M.; Mitsuzawa, S.; Shijo, T.; Ikeda, K.; et al. Long-term outcomes after surgery to prevent aspiration for patients with amyotrophic lateral sclerosis. BMC Neurol. 2022, 22, 94.
- Kaneoka, A.; Ueha, R.; Nagatomo, M.; Matsunaga, A.; Umezaki, S.; Inokuchi, H.; Ogata, T. Esophageal Speech for a Patient with Amyotrophic Lateral Sclerosis Who Underwent a Central-part Laryngectomy to Prevent Aspiration: A Case Report. Prog. Rehabil. Med. 2022, 7, 20220064.
- Vásquez-Correa, J.C.; Orozco-Arroyave, J.R.; Bocklet, T.; Nöth, E. Towards an automatic evaluation of the dysarthria level of patients with Parkinson’s disease. J. Commun. Disord. 2018, 76, 21–36.
- Erriu, M.; Pili, F.M.; Cadoni, S.; Garau, V. Diagnosis of Lingual Atrophic Conditions: Associations with Local and Systemic Factors. A Descriptive Review. Open Dent. J. 2016, 10, 619–635.
- Kaindlstorfer, C.; Granata, R.; Wenning, G.K. Tremor in Multiple System Atrophy—A review. Tremor Other Hyperkinet Mov. 2013, 3, 1–20.
- Fabbri, M.; Abreu, L.; Santos, T.; Ferreira, J.J. Resting and Reemergent Tongue Tremor as Presenting Symptoms of Parkinson’s Disease. Mov. Disord. Clin. Pract. 2017, 4, 273–274.
- Yaguchi, H.; Sakuta, K.; Mukai, T.; Miyagawa, S. Fiberoptic laryngoscopic neurological examination of amyotrophic lateral sclerosis patients with bulbar symptoms. J. Neurol. Sci. 2022, 440, 120325.
- Glade, R.S.; Deal, R. Diagnosis and Management of Velopharyngeal Dysfunction. Oral. Maxillofac. Surg. Clin. N. Am. 2016, 28, 181–188.
- Van der Graaff, M.M.; Grolman, W.; Westermann, E.J.; Boogaardt, H.C.; Koelman, H.; van der Kooi, A.J.; Tijssen, M.A.; de Visser, M. Vocal Cord Dysfunction in Amyotrophic Lateral Sclerosis. Arch. Neurol. 2009, 66, 1329–1333.
- Bahk, J.; Yang, W.D.; Fishman, J. Bilateral vocal cord paralysis in Miller Fisher syndrome/Guillain-Barre overlap syndrome and a review of previous case series. BMJ Case Rep. 2021, 14, e240386.
- Logemann, J.A. Approaches to management of disordered swallowing. Baillieres Clin. Gastroenterol. 1991, 5, 269–280.
- Gandhi, P.; Peladeau-Pigeon, M.; Simmons, M.; Steele, C.M. Exploring the Efficacy of the Effortful Swallow Maneuver for Improving Swallowing in People With Parkinson Disease-A Pilot Study. Arch. Rehabil. Res. Clin. Transl. 2023, 5, 100276.
- Cosentino, G.; Todisco, M.; Giudice, C.; Tassorelli, C.; Alfonsi, E. Assessment and treatment of neurogenic dysphagia in stroke and Parkinson’s disease. Curr. Opin. Neurol. 2022, 35, 741–752.
- Brent, J.R.; Franz, C.K.; Coleman, J.M., 3rd; Ajroud-Driss, S. ALS: Management Problems. Neurol. Clin. 2020, 38, 565–575.
- Masuda, H.; Ueha, R.; Sato, T.; Goto, T.; Koyama, M.; Yamauchi, A.; Kaneoka, A.; Suzuki, S.; Yamasoba, T. Risk Factors for Aspiration Pneumonia After Receiving Liquid-Thickening Recommendations. Otolaryngol. Head Neck Surg. 2022, 167, 125–132.
- Cheng, I.; Hamad, A.; Sasegbon, A.; Hamdy, S. Advances in the Treatment of Dysphagia in Neurological Disorders: A Review of Current Evidence and Future Considerations. Neuropsychiatr. Dis. Treat. 2022, 18, 2251–2263.
- Hoffman, M.R.; Ciucci, M.R.; Mielens, J.D.; Jiang, J.J.; McCulloch, T.M. Pharyngeal swallow adaptations to bolus volume measured with high-resolution manometry. Laryngoscope 2010, 120, 2367–2373.
- Kao, T.H.; Perry, B.J. The Current State and Future Directions of Swallowing Care in Amyotrophic Lateral Sclerosis. Curr. Phys. Med. Rehabil. Rep. 2023, 11, 199–211.
- Alghadir, A.H.; Zafar, H.; Al-Eisa, E.S.; Iqbal, Z.A. Effect of posture on swallowing. Afr. Health Sci. 2017, 17, 133–137.
- Yoshikawa, M.; Nagakawa, K.; Tanaka, R.; Yamawaki, K.; Mori, T.; Hiraoka, A.; Higa, C.; Nishikawa, Y.; Yoshida, M.; Tsuga, K. Improper sitting posture while eating adversely affects maximum tongue pressure. J. Dent. Sci. 2021, 16, 467–473.
- Nakamura, T.; Fujishima, I. Usefulness of ice massage in triggering the swallow reflex. J. Stroke Cerebrovasc. Dis. 2013, 22, 378–382.
- Burkhead, L.M.; Sapienza, C.M.; Rosenbek, J.C. Strength-training exercise in dysphagia rehabilitation: Principles, procedures, and directions for future research. Dysphagia 2007, 22, 251–265.
- Yoshida, M.; Groher, M.E.; Crary, M.A.; Mann, G.C.; Akagawa, Y. Comparison of surface electromyographic (sEMG) activity of submental muscles between the head lift and tongue press exercises as a therapeutic exercise for pharyngeal dysphagia. Gerodontology 2007, 24, 111–116.
- Sze, W.P.; Yoon, W.L.; Escoffier, N.; Rickard Liow, S.J. Evaluating the Training Effects of Two Swallowing Rehabilitation Therapies Using Surface Electromyography—Chin Tuck Against Resistance (CTAR) Exercise and the Shaker Exercise. Dysphagia 2016, 31, 195–205.
- Pitts, T.; Bolser, D.; Rosenbek, J.; Troche, M.; Okun, M.S.; Sapienza, C. Impact of expiratory muscle strength training on voluntary cough and swallow function in Parkinson disease. Chest 2009, 135, 1301–1308.
- Reyes, A.; Ziman, M.; Nosaka, K. Respiratory muscle training for respiratory deficits in neurodegenerative disorders: A systematic review. Chest 2013, 143, 1386–1394.
- Heijnen, B.J.; Speyer, R.; Baijens, L.W.J.; Bogaardt, H.C.A. Neuromuscular electrical stimulation versus traditional therapy in patients with Parkinson’s disease and oropharyngeal dysphagia: Effects on quality of life. Dysphagia 2012, 27, 336–345.
- Schindler, A.; Pizzorni, N.; Cereda, E.; Cosentino, G.; Avenali, M.; Montomoli, C.; Abbruzzese, G.; Antonini, A.; Barbiera, F.; Benazzo, M.; et al. Consensus on the treatment of dysphagia in Parkinson’s disease. J. Neurol. Sci. 2021, 430, 120008.
- Balou, M.; Herzberg, E.G.; Kamelhar, D.; Molfenter, S.M. An intensive swallowing exercise protocol for improving swallowing physiology in older adults with radiographically confirmed dysphagia. Clin. Interv. Aging 2019, 14, 283–288.
- Chen, C.H.; Lin, C.Y.; Chen, C.L.; Chen, K.T.; Lee, C.; Yu, Y.H.; Shih, C.Y. Long-Term Effectiveness of Physical Exercise-Based Swallowing Interventions for Older Adults with Dementia in a Day-Care Center. Healthcare 2023, 11, 1262.
- Bahia, M.M.; Lowell, S.Y. A Systematic Review of the Physiological Effects of the Effortful Swallow Maneuver in Adults With Normal and Disordered Swallowing. Am. J. Speech Lang. Pathol. 2020, 29, 1655–1673.
- Drulia, T.C.; Ludlow, C.L. Relative Efficacy of Swallowing versus Non-swallowing Tasks in Dysphagia Rehabilitation: Current Evidence and Future Directions. Curr. Phys. Med. Rehabil. Rep. 2013, 1, 242–256.
- Kunieda, K.; Sugiyama, J.; Nomoto, A.; Ohno, T.; Shigematsu, T.; Fujishima, I. Compensatory swallowing methods in a patient with dysphagia due to lateral medullary syndrome-vacuum and prolonged swallowing: A case report. Medicine 2022, 101, e28524.
- Huckabee, M.L.; Flynn, R.; Mills, M. Expanding Rehabilitation Options for Dysphagia: Skill-Based Swallowing Training. Dysphagia 2023, 38, 756–767.
- Saconato, M.; Chiari, B.M.; Lederman, H.M.; Gonçalves, M.I. Effectiveness of Chin-tuck Maneuver to Facilitate Swallowing in Neurologic Dysphagia. Int. Arch. Otorhinolaryngol. 2016, 20, 13–17.
- Logemann, J.A.; Kahrilas, P.J.; Kobara, M.; Vakil, N.B. The Benefit of Head Rotation on Pharyngoesophageal Dysphagia. Arch. Phys. Med. Rehabil. 1989, 70, 767–771.
- Seo, M.; Park, J.W. Head rotation as an effective compensatory technique for dysphagia caused by unilateral cervical osteophytes. J. Int. Med. Res. 2022, 50, 1–9.
- Chaudhuri, G.; Hildner, C.D.; Brady, S.; Hutchins, B.; Aliga, N.; Abadilla, E. Cardiovascular effects of the supraglottic and super-supraglottic swallowing maneuvers in stroke patients with dysphagia. Dysphagia 2002, 17, 19–23.
More
Information
Subjects:
Clinical Neurology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
599
Revisions:
2 times
(View History)
Update Date:
16 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




