
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hyeuk Jin Han | -- | 2322 | 2024-01-12 03:12:21 | | | |
| 2 | Jessie Wu | -5 word(s) | 2317 | 2024-01-12 04:03:04 | | |
Video Upload Options
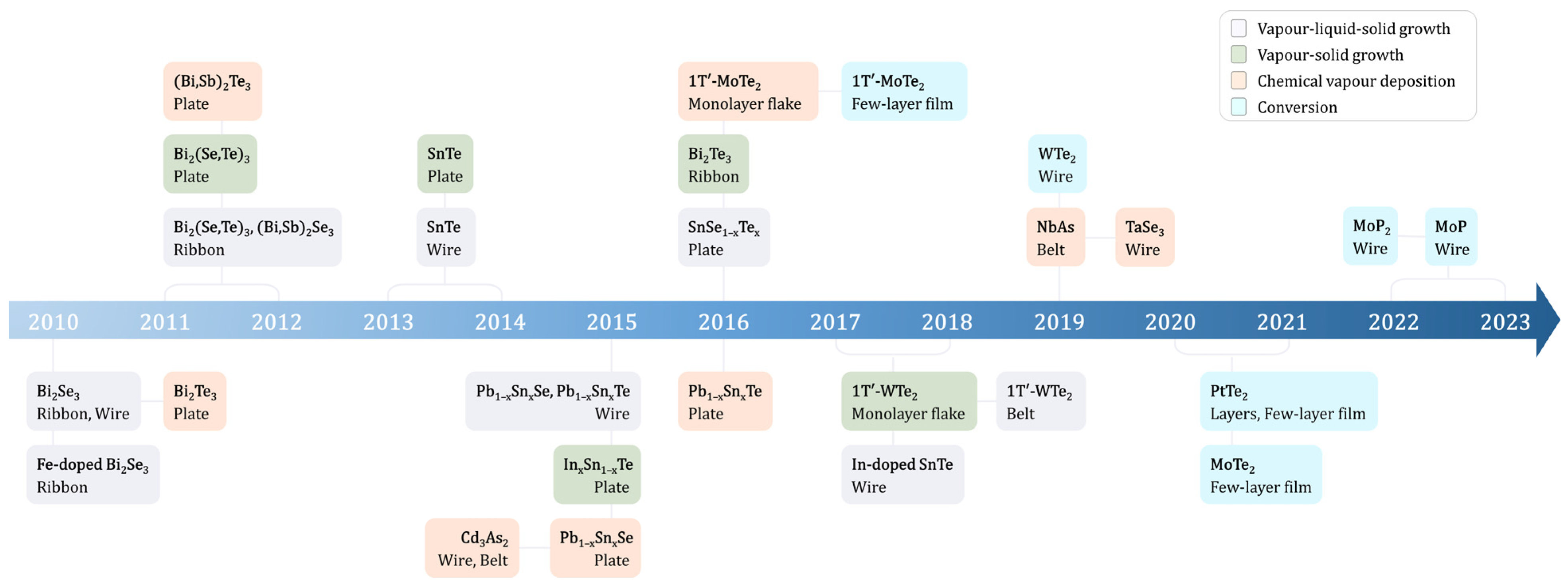
The discovery of topological materials has opened up new areas in condensed matter physics. These materials are noted for their distinctive electronic properties, unlike conventional insulators and metals. This discovery has not only spurred new research areas but also offered innovative approaches to electronic device design. A key aspect of these materials is now that transforming them into nanostructures enhances the presence of surface or edge states, which are the key components for their unique electronic properties. Synthesis methods are discussed, including vapor–liquid–solid (VLS) growth, chemical vapor deposition (CVD), and chemical conversion techniques. Moreover, the scaling down of topological nanomaterials has revealed new electronic and magnetic properties due to quantum confinement.
1. Introduction
2. Synthesis Methodologies
References
- Liu, P.; Williams, J.R.; Cha, J.J. Topological Nanomaterials. Nat. Rev. Mater. 2019, 4, 479–496.
- Han, H.J.; Liu, P.; Cha, J.J. 1D Topological Systems for Next-Generation Electronics. Matter 2021, 4, 2596–2598.
- König, M.; Wiedmann, S.; Brüne, C.; Roth, A.; Buhmann, H.; Molenkamp, L.W.; Qi, X.-L.; Zhang, S.-C. Quantum Spin Hall Insulator State in HgTe Quantum Wells. Science 2007, 318, 766–770.
- Kane, C.L.; Mele, E.J. Z2 Topological Order and the Quantum Spin Hall Effect. Phys. Rev. Lett. 2005, 95, 146802.
- Fu, L.; Kane, C.L. Time Reversal Polarization and a Z2 Adiabatic Spin Pump. Phys. Rev. B 2006, 74, 195312.
- Qi, X.-L.; Zhang, S.-C. Topological Insulators and Superconductors. Rev. Mod. Phys. 2011, 83, 1057–1110.
- Armitage, N.P.; Mele, E.J.; Vishwanath, A. Weyl and Dirac Semimetals in Three-Dimensional Solids. Rev. Mod. Phys. 2018, 90, 15001.
- Gilbert, M.J. Topological Electronics. Commun. Phys. 2021, 4, 70.
- He, Q.L.; Hughes, T.L.; Armitage, N.P.; Tokura, Y.; Wang, K.L. Topological Spintronics and Magnetoelectronics. Nat. Mater. 2022, 21, 15–23.
- Breunig, O.; Ando, Y. Opportunities in Topological Insulator Devices. Nat. Rev. Phys. 2022, 4, 184–193.
- Heremans, J.P.; Cava, R.J.; Samarth, N. Tetradymites as Thermoelectrics and Topological Insulators. Nat. Rev. Mater. 2017, 2, 17049.
- Tian, W.; Yu, W.; Shi, J.; Wang, Y. The Property, Preparation and Application of Topological Insulators: A Review. Materials 2017, 10, 814.
- Mishra, S.K.; Satpathy, S.; Jepsen, O. Electronic Structure and Thermoelectric Properties of Bismuth Telluride and Bismuth Selenide. J. Phys. Condens. Matter 1997, 9, 461.
- Xu, J.-L.; Sun, Y.-J.; He, J.-L.; Wang, Y.; Zhu, Z.-J.; You, Z.-Y.; Li, J.-F.; Chou, M.M.C.; Lee, C.-K.; Tu, C.-Y. Ultrasensitive Nonlinear Absorption Response of Large-Size Topological Insulator and Application in Low-Threshold Bulk Pulsed Lasers. Sci. Rep. 2015, 5, 14856.
- Yang, J.; Yu, W.; Pan, Z.; Yu, Q.; Yin, Q.; Guo, L.; Zhao, Y.; Sun, T.; Bao, Q.; Zhang, K. Ultra-Broadband Flexible Photodetector Based on Topological Crystalline Insulator SnTe with High Responsivity. Small 2018, 14, 1802598.
- Hsieh, D.; Xia, Y.; Wray, L.; Qian, D.; Pal, A.; Dil, J.H.; Osterwalder, J.; Meier, F.; Bihlmayer, G.; Kane, C.L.; et al. Observation of Unconventional Quantum Spin Textures in Topological Insulators. Science 2009, 323, 919–922.
- Tian, J.; Miotkowski, I.; Hong, S.; Chen, Y.P. Electrical Injection and Detection of Spin-Polarized Currents in Topological Insulator Bi2Te2Se. Sci. Rep. 2015, 5, 14293.
- Pesin, D.; MacDonald, A.H. Spintronics and Pseudospintronics in Graphene and Topological Insulators. Nat. Mater. 2012, 11, 409–416.
- Ando, Y.; Hamasaki, T.; Kurokawa, T.; Ichiba, K.; Yang, F.; Novak, M.; Sasaki, S.; Segawa, K.; Ando, Y.; Shiraishi, M. Electrical Detection of the Spin Polarization Due to Charge Flow in the Surface State of the Topological Insulator Bi1.5Sb0.5Te1.7Se1.3. Nano Lett. 2014, 14, 6226–6230.
- Tian, J.; Hong, S.; Miotkowski, I.; Datta, S.; Chen, Y.P. Observation of Current-Induced, Long-Lived Persistent Spin Polarization in a Topological Insulator: A Rechargeable Spin Battery. Sci. Adv. 2023, 3, e1602531.
- Fan, Y.; Kou, X.; Upadhyaya, P.; Shao, Q.; Pan, L.; Lang, M.; Che, X.; Tang, J.; Montazeri, M.; Murata, K.; et al. Electric-Field Control of Spin–Orbit Torque in a Magnetically Doped Topological Insulator. Nat. Nanotechnol. 2016, 11, 352–359.
- Kandala, A.; Richardella, A.; Rench, D.W.; Zhang, D.M.; Flanagan, T.C.; Samarth, N. Growth and Characterization of Hybrid Insulating Ferromagnet-Topological Insulator Heterostructure Devices. Appl. Phys. Lett. 2013, 103, 202409.
- Yao, J.; Shao, J.; Wang, Y.; Zhao, Z.; Yang, G. Ultra-Broadband and High Response of the Bi2Te3–Si Heterojunction and Its Application as a Photodetector at Room Temperature in Harsh Working Environments. Nanoscale 2015, 7, 12535–12541.
- Xia, Y.; Qian, D.; Hsieh, D.; Wray, L.; Pal, A.; Lin, H.; Bansil, A.; Grauer, D.; Hor, Y.S.; Cava, R.J.; et al. Observation of a Large-Gap Topological-Insulator Class with a Single Dirac Cone on the Surface. Nat. Phys. 2009, 5, 398–402.
- Chen, Y.L.; Analytis, J.G.; Chu, J.-H.; Liu, Z.K.; Mo, S.-K.; Qi, X.L.; Zhang, H.J.; Lu, D.H.; Dai, X.; Fang, Z.; et al. Experimental Realization of a Three-Dimensional Topological Insulator, Bi2Te3. Science 2009, 325, 178–181.
- Arakane, T.; Sato, T.; Souma, S.; Kosaka, K.; Nakayama, K.; Komatsu, M.; Takahashi, T.; Ren, Z.; Segawa, K.; Ando, Y. Tunable Dirac Cone in the Topological Insulator Bi2−xSbxTe3-ySey. Nat. Commun. 2012, 3, 636.
- Nayak, J.; Fecher, G.H.; Ouardi, S.; Shekhar, C.; Tusche, C.; Ueda, S.; Ikenaga, E.; Felser, C. Temperature-Induced Modification of the Dirac Cone in the Tetradymite Topological Insulator Bi2Te2Se. Phys. Rev. B 2018, 98, 75206.
- Xu, Y.; Miotkowski, I.; Liu, C.; Tian, J.; Nam, H.; Alidoust, N.; Hu, J.; Shih, C.-K.; Hasan, M.Z.; Chen, Y.P. Observation of Topological Surface State Quantum Hall Effect in an Intrinsic Three-Dimensional Topological Insulator. Nat. Phys. 2014, 10, 956–963.
- Cen, C.; Thiel, S.; Hammerl, G.; Schneider, C.W.; Andersen, K.E.; Hellberg, C.S.; Mannhart, J.; Levy, J. Nanoscale Control of an Interfacial Metal–Insulator Transition at Room Temperature. Nat. Mater. 2008, 7, 298–302.
- Annadi, A.; Cheng, G.; Lee, H.; Lee, J.-W.; Lu, S.; Tylan-Tyler, A.; Briggeman, M.; Tomczyk, M.; Huang, M.; Pekker, D.; et al. Quantized Ballistic Transport of Electrons and Electron Pairs in LaAlO3/SrTiO3 Nanowires. Nano Lett. 2018, 18, 4473–4481.
- Kumar, N.; Guin, S.N.; Manna, K.; Shekhar, C.; Felser, C. Topological Quantum Materials from the Viewpoint of Chemistry. Chem. Rev. 2020, 121, 2780–2815.
- Shekhar, C.; Nayak, A.K.; Sun, Y.; Schmidt, M.; Nicklas, M.; Leermakers, I.; Zeitler, U.; Skourski, Y.; Wosnitza, J.; Liu, Z.; et al. Extremely Large Magnetoresistance and Ultrahigh Mobility in the Topological Weyl Semimetal Candidate NbP. Nat. Phys. 2015, 11, 645–649.
- Lv, B.Q.; Feng, Z.-L.; Xu, Q.-N.; Gao, X.; Ma, J.-Z.; Kong, L.-Y.; Richard, P.; Huang, Y.-B.; Strocov, V.N.; Fang, C.; et al. Observation of Three-Component Fermions in the Topological Semimetal Molybdenum Phosphide. Nature 2017, 546, 627–631.
- Kumar, N.; Sun, Y.; Xu, N.; Manna, K.; Yao, M.; Süss, V.; Leermakers, I.; Young, O.; Förster, T.; Schmidt, M.; et al. Extremely High Magnetoresistance and Conductivity in the Type-II Weyl Semimetals WP2 and MoP2. Nat. Commun. 2017, 8, 1642.
- Hsin, C.-L.; Yu, S.; Wu, W.-W. Cobalt Silicide Nanocables Grown on Co Films: Synthesis and Physical Properties. Nanotechnology 2010, 21, 485602.
- Lien, S.-W.; Garate, I.; Bajpai, U.; Huang, C.-Y.; Hsu, C.-H.; Tu, Y.-H.; Lanzillo, N.; Bansil, A.; Chang, T.-R.; Liang, G.; et al. Unconventional Resistivity Scaling in Topological Semimetal CoSi. NPJ Quantum Mater. 2023, 8, 3.
- Schmitt, A.L.; Zhu, L.; Schmeiβer, D.; Himpsel, F.J.; Jin, S. Metallic Single-Crystal CoSi Nanowires via Chemical Vapor Deposition of Single-Source Precursor. J. Phys. Chem. B 2006, 110, 18142–18146.
- Zhang, C.; Ni, Z.; Zhang, J.; Yuan, X.; Liu, Y.; Zou, Y.; Liao, Z.; Du, Y.; Narayan, A.; Zhang, H.; et al. Ultrahigh Conductivity in Weyl Semimetal NbAs Nanobelts. Nat. Mater. 2019, 18, 482–488.
- Liu, P.; Han, H.J.; Wei, J.; Hynek, D.; Hart, J.L.; Han, M.G.; Trimble, C.J.; Williams, J.; Zhu, Y.; Cha, J.J. Synthesis of Narrow SnTe Nanowires Using Alloy Nanoparticles. ACS Appl. Electron. Mater. 2021, 3, 184–191.
- Park, K.; Jung, M.; Kim, D.; Bayogan, J.R.; Lee, J.H.; An, S.J.; Seo, J.; Seo, J.; Ahn, J.-P.; Park, J. Phase Controlled Growth of Cd3As2 Nanowires and Their Negative Photoconductivity. Nano Lett. 2020, 20, 4939–4946.
- De Yoreo, J.J.; Vekilov, P.G. Principles of Crystal Nucleation and Growth. Rev. Mineral. Geochem. 2003, 54, 57–93.
- Zhang, Z.; Lagally, M.G. Atomistic Processes in the Early Stages of Thin-Film Growth. Science 1997, 276, 377–383.
- Jin, G.; Lee, C.-S.; Okello, O.F.N.; Lee, S.-H.; Park, M.Y.; Cha, S.; Seo, S.-Y.; Moon, G.; Min, S.Y.; Yang, D.-H.; et al. Heteroepitaxial van Der Waals Semiconductor Superlattices. Nat. Nanotechnol. 2021, 16, 1092–1098.
- Alegria, L.; Yao, N.; Petta, J. MOCVD Synthesis of Compositionally Tuned Topological Insulator Nanowires. Phys. Status Solidi RRL. 2014, 8, 991–996.
- Jin, G.; Lee, C.S.; Liao, X.; Kim, J.; Wang, Z.; Okello, O.F.N.; Park, B.; Park, J.; Han, C.; Heo, H.; et al. Atomically Thin Three-Dimensional Membranes of van Der Waals Semiconductors by Wafer-Scale Growth. Sci. Adv. 2019, 5, eaaw3180.
- Brahlek, M.; Lapano, J.; Lee, J.S. Topological Materials by Molecular Beam Epitaxy. J. Appl. Phys. 2020, 128, 210902.
- Molinari, A.; Balduini, F.; Rocchino, L.; Wawrzyńczak, R.; Sousa, M.; Bui, H.; Lavoie, C.; Stanic, V.; Jordan-Sweet, J.; Hopstaken, M.; et al. Disorder-Induced Magnetotransport Anomalies in Amorphous and Textured Co1−xSix Semimetal Thin Films. ACS Appl. Electron. Mater. 2023, 5, 2624–2637.
- Yanez-Parreno, W.; Huang, Y.-S.; Ghosh, S.; Islam, S.; Steinebronn, E.; Richardella, A.; Mkhoyan, K.; Samarth, N. Thin Film Growth of the Weyl Semimetal NbAs. arXiv 2023, arXiv:2304.13959.
- Bedoya-Pinto, A.; Pandeya, A.K.; Liu, D.; Deniz, H.; Chang, K.; Tan, H.; Han, H.; Jena, J.; Kostanovskiy, I.; Parkin, S.S.P. Realization of Epitaxial NbP and TaP Weyl Semimetal Thin Films. ACS Nano 2020, 14, 4405–4413.
- Sohn, S.; Jung, Y.; Xie, Y.; Osuji, C.; Schroers, J.; Cha, J.J. Nanoscale Size Effects in Crystallization of Metallic Glass Nanorods. Nat. Commun. 2015, 6, 8157.
- Pilidi, A.; Speliotis, T.; Litsardakis, G. Structural and Magnetotransport Characterization of Magnetron Sputtered Co-Doped Bi2Te3 Thin Films. J. Magn. Magn. Mater. 2020, 511, 166971.
- Fujiwara, K.; Ikeda, J.; Shiogai, J.; Seki, T.; Takanashi, K.; Tsukazaki, A. Ferromagnetic Co3Sn2S2 Thin Films Fabricated by Co-Sputtering Technique. Jpn. J. Appl. Phys. 2019, 58, 050912.
- Xu, Y.; Zhao, J.; Yi, C.; Wang, Q.; Yin, Q.; Wang, Y.; Hu, X.; Wang, L.; Liu, E.; Xu, G.; et al. Electronic Correlations and Flattened Band in Magnetic Weyl Semimetal Candidate Co3Sn2S2. Nat. Commun. 2020, 11, 3985.
- Han, H.J.; Kumar, S.; Jin, G.; Ji, X.; Hart, J.L.; Hynek, D.J.; Sam, Q.P.; Hasse, V.; Felser, C.; Cahill, D.G.; et al. Topological Metal MoP Nanowire for Interconnect. Adv. Mater. 2023, 2208965, 2208965.
- Jin, G.; Han, H.J.; Hart, J.L.; Sam, Q.P.; Kiani, M.T.; Hynek, D.J.; Hasse, V.; Felser, C.; Cha, J.J. Vapor Phase Synthesis of Topological Semimetal MoP2 Nanowires and Their Resistivity. Appl. Phys. Lett. 2022, 121, 113105.
- Kiani, M.T.; Sam, Q.P.; Jin, G.; Pamuk, B.; Han, H.J.; Hart, J.L.; Stauff, J.R.; Cha, J.J. Nanomolding of Metastable Mo4P3. Matter 2023, 6, 1894–1902.
- Guo, Y.; Lin, Y.; Xie, K.; Yuan, B.; Zhu, J.; Shen, P.-C.; Lu, A.-Y.; Su, C.; Shi, E.; Zhang, K.; et al. Designing Artificial Two-Dimensional Landscapes via Atomic-Layer Substitution. Proc. Natl. Acad. Sci. USA 2021, 118, e2106124118.
- Shi, J.; Haowei, X.; Heide, C.; HuangFu, C.; Xia, C.; Quesada, F.; Shen, H.; Zhang, T.; Yu, L.; Johnson, A.; et al. Giant Room-Temperature Nonlinearities from a Monolayer Janus Topological Semiconductor. Nat. Commun. 2023, 14, 4953.
- Hynek, D.; Onder, E.; Hart, J.; Jin, G.; Wang, M.; Singhania, R.; Davis, B.; Strandwitz, N.; Cha, J. Substrate Effects on Growth Dynamics of WTe2 Thin Films. Adv. Mater. Interfaces 2023, 10, 2202397.




