1. Introduction
Batteries are becoming increasingly essential in modern society to power many devices, including smartphones, laptops, electric cars, and renewable energy grids. As the demand for portable electronics and electric vehicles (EVs) continues to rise, the need for high-performing, long-lasting, and safe batteries is becoming more pressing. Advances in battery technology can significantly impact how we live and work, from enabling sustainable energy to reducing our reliance on fossil fuels. When John B. Goodenough and his team published the famous paper “A new cathode material for batteries of high energy density” in 1980
[1], they could not have foreseen the far-reaching consequences of their work. Since then, lithium-ion batteries (LIBs) have established themselves as the leading technology in the global battery market due to their superior energy density, extended cycle life, and low self-discharge rates. They are employed in various applications, such as smartphones, laptops, electric vehicles, and renewable energy storage systems
[2]. Furthermore, they have experienced a significant decline in cost, with Bloomberg NEF’s 2021 battery price survey reporting an 89% reduction in prices since 2010 and an increase in installed capacity values (with a global installed capacity of over 800 GWh as of 2020), which highlights the rapid progress of battery technology in recent years
[3][4].
Unfortunately, LIBs suffer from safety concerns related to their potential for thermal runaway and fire, especially when overcharged or exposed to high temperatures, as well as relatively long charging times
[5]. Therefore, intensified research in battery technologies is inevitable. Among upcoming and highly promising battery technologies is the so-called solid-state battery (SSB), a novel battery technology that is vital in shaping the future of energy and sustainability. By using solid electrolytes instead of liquid ones, SSBs differ significantly from LIBs due to their enhanced safety, higher energy density, and longer lifespan
[6][7][8][9][10]. These unique attributes make SSBs appealing for applications with specific requirements.
One such area is the transportation industry, encompassing EVs and aerospace. EVs, a prominent sector, stand to benefit significantly from SSBs, driving substantial investment and research in this direction. Although LIBs currently dominate electric battery vehicles, SSBs offer distinct advantages, notably fast charging and improved safety. Solid electrolytes eliminate the risk of electrolyte leakage or vaporization and mitigate the potential for flammable organic solvents. They also prevent side reactions between electrodes and electrolytes that could lead to dendrite formation. Moreover, the higher energy density of SSBs can extend the range of electric vehicles, enhancing their viability for long-distance travel. Major companies like Toyota, Honda, Nissan, Ford, BMW, and Volkswagen have actively pursued SSB development for electric vehicles.
SSBs have already found utility in aerospace applications due to their lighter weight, compactness, and higher energy density. These attributes make them suitable for energy storage in spacecraft. The safety features of SSBs make them particularly appealing for this application, in contrast to conventional LIBs, which are lighter and more compact but often have lower safety levels. Solid electrolytes enable SSBs to withstand extreme temperatures in space environments. Certain SSBs, such as lithium-air batteries, can function at temperatures as low as −73 °C
[11], while others, like lithium-oxygen batteries, can operate at temperatures up to 120 °C
[12]. In addition to the transport sector, there is a growing demand for batteries offering the advantages provided by SSBs in various industries, such as medical devices and consumer electronics. These sectors find SSBs to be compelling choices for their specific needs. The multiple applications discussed underscore the potential of SSBs and their significance for the future.
2. Solid-State Electrolyte Materials
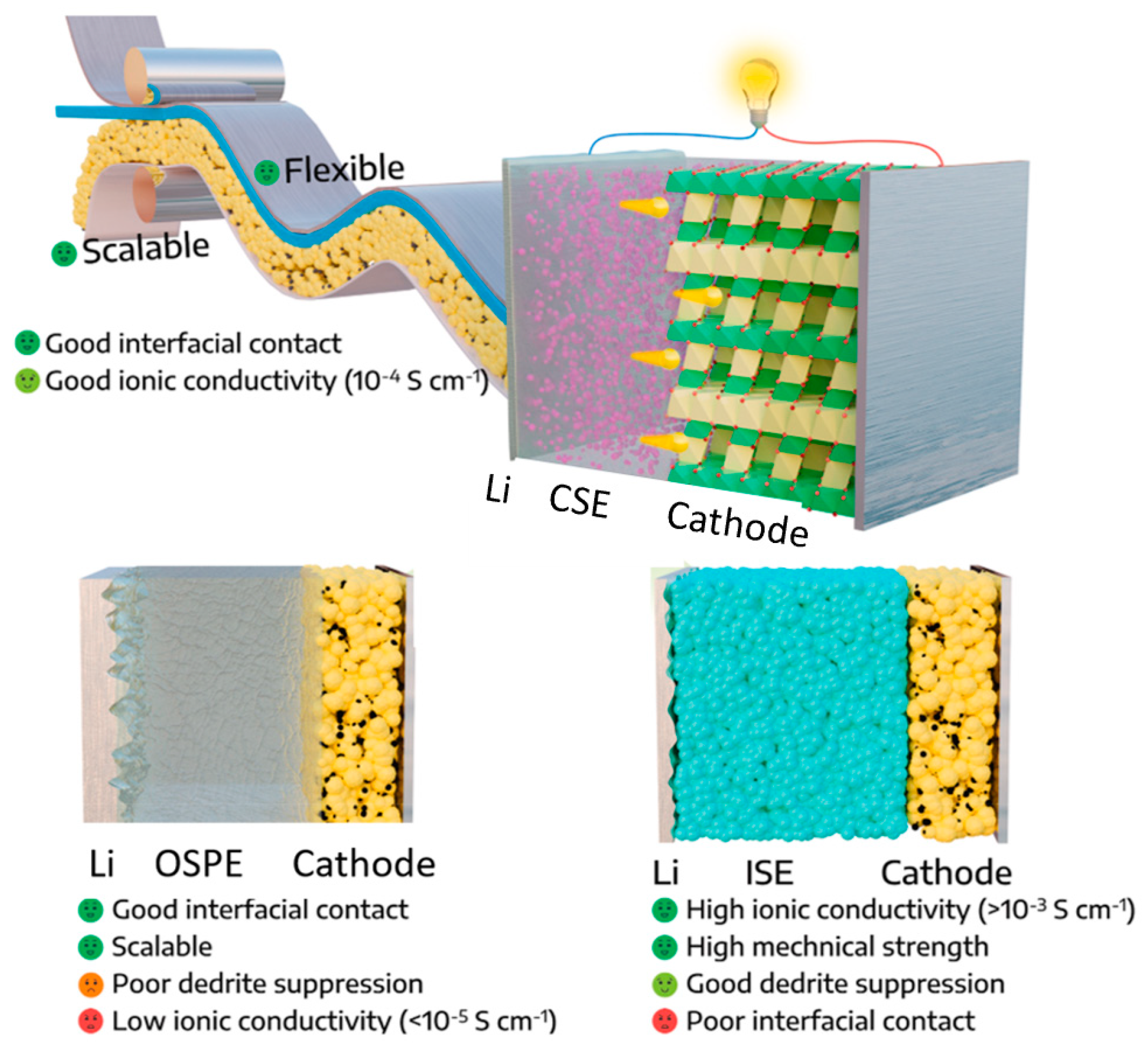
SSBs are an emerging technology that has the potential to revolutionize the energy storage industry. Unlike traditional LIBs, which use a liquid electrolyte to transport ions between the cathode and anode, SSBs use a solid-state electrolyte (SSE) to perform the same transport function. As shown in
Figure 1, SSEs used in rechargeable batteries can be divided into three categories based on chemical composition: inorganic solid ceramic electrolytes, organic solid polymer electrolytes, and solid composite electrolytes, a combination of the first two material classes
[7][13][14].
Figure 1. Comparison of the structure and properties of inorganic solid electrolytes (ISEs), organic solid polymer electrolytes (OSPEs), and composite solid electrolytes (CSEs)
[15]. Reprinted with permission from Elsevier.
Inorganic solid electrolytes (ISEs) are typically made from lithium ceramics such as lithium aluminum titanium phosphate (LATP). They offer high ion conductivity and thermal stability but can be brittle and difficult to manufacture
[7].
Organic solid polymer electrolytes (OSPEs) are made from polymers such as polyethylene oxide (PEO) or polyvinylidene fluoride (PVDF). They offer good mechanical flexibility and processability, but lower ion conductivity than inorganic solid ceramic electrolytes
[8].
Composite solid electrolytes (CSEs) combine inorganic ceramic materials with organic polymers to achieve high ion conductivity and good mechanical properties. They can be designed to have specific properties by varying the composition and structure of the materials.
There are critical factors for the success of SSEs in SSBs. In essence, optimal SSEs should exhibit characteristics such as extremely low electronic conductivity (<10
−10 S cm
−1) coupled with high Li
+ conductivity (>10
−3 S cm
−1)
[16]. Furthermore, they should demonstrate favorable chemical compatibility with electrodes, a broad electrochemical stability range, and exceptional thermal stability
[17]. Researchers are working on various strategies to improve Li
+ conductivity, such as optimizing the microstructure of the materials, incorporating dopants, and using hybrid materials
[11][12][17][18].
2.1. Inorganic Solid Electrolytes
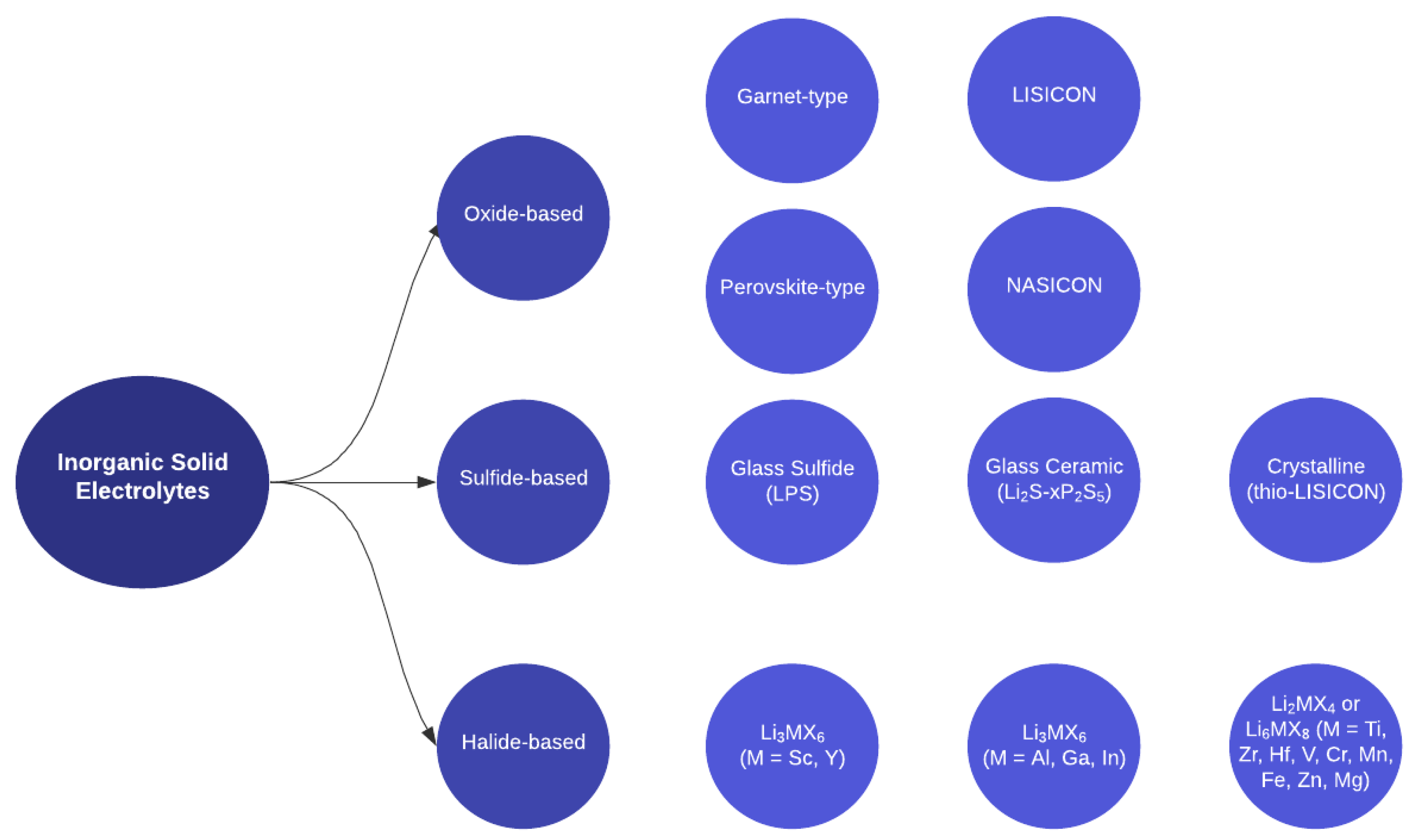
Inorganic solid electrolytes (ISEs) are a class of ceramic materials that exhibit high ionic conductivity for lithium (Li), sodium (Na), or other alkali metal ions and can, therefore, provide a stable and efficient transport medium for ion flow between the anode and cathode in a battery
[14]. While the use of ISEs is still relatively new and requires further research and development, it holds great potential to advance the field of energy storage and pave the way for safer, more efficient, and environmentally friendly batteries. Based on anion chemistry, ISEs are divided into three classes: oxide-based, sulfide-based, and halide-based
[19].
Figure 2 illustrates further sub-divisions within these divisions. Each class of materials has unique advantages and limitations that make them suitable for different battery applications.
Figure 2. Schematic representation of inorganic solid electrolyte material classes.
2.2. Organic Solid Polymer Electrolytes (OSPEs)
Polymer electrolytes have emerged as a promising alternative to traditional ISEs in SSBs due to their unique properties and potential advantages. Unlike inorganic electrolytes, polymer electrolytes are made of organic polymers that can be designed to have high ionic conductivity, good thermal stability, and mechanical flexibility. Additionally, polymer electrolytes can reduce the interface resistance between the electrodes and the electrolyte, improving battery performance. Furthermore, polymer electrolytes can be processed using cost-effective and scalable methods, making them attractive for large-scale manufacturing. Despite these promising characteristics, polymer electrolytes face challenges related to low ionic conductivity, chemical stability, and mechanical strength
[20][21]. Therefore, ongoing research is focused on developing new polymer materials and optimizing the properties of existing ones to overcome these limitations and unlock the full potential of polymer electrolytes in SSBs.
PVDF is a type of polymer material that is sometimes used as an electrolyte in SSBs, particularly in combination with Li salts such as lithium bis(trifluoromethanesulfonyl)imide (LiTFSI)
[20][22]. PVDF is a polymer composed of carbon, hydrogen, fluorine, and sometimes other elements such as oxygen or chlorine. Its repeating unit is CH
2CF
2, and the polymer chain can be linear or branched depending on the specific polymerization process used. PVDF can be synthesized through a process known as polymerization, in which monomers such as vinylidene fluoride are reacted in the presence of a catalyst and/or initiator to form a polymer chain
[23]. The resulting PVDF polymer can be further processed into various forms, such as films, fibers, or powders.
To use PVDF as an electrolyte in SSBs, the polymer is typically combined with a Li salt such as LiTFSI. The PVDF/LiTFSI mixture can be dissolved in a solvent such as acetonitrile or propylene carbonate to form a gel or polymer electrolyte. The resulting electrolyte can be cast into films or other shapes and incorporated into the battery design
[23]. PVDF-based electrolytes have some advantages over other types of solid electrolytes. They can have relatively high ionic conductivity and good mechanical properties, which can improve the overall performance and stability of the battery
[8]. However, PVDF-based electrolytes can also have drawbacks, such as limited electrochemical stability and potential reactivity with Li metal anodes
[21]. As a result, PVDF-based electrolytes may be more suitable for specific battery designs or applications rather than being a universal solution.
PEO is a polymer material commonly used as an electrolyte in SSBs, particularly in combination with Li salts such as LiTFSI. PEO is a polymer composed of carbon, hydrogen, and oxygen. Its repeating unit is CH
2CH
2O, and the polymer chain can be linear or branched depending on the specific polymerization process used
[21]. PEO-based electrolytes have some advantages over other types of solid electrolytes. They can have relatively high ionic conductivity and good mechanical properties, which can enhance the overall efficiency and durability of the battery. PEO-based electrolytes also have good compatibility with Li metal anodes, which can reduce the risk of dendrite formation and improve the overall safety of the battery
[22]. In addition, PEO-based electrolytes can be relatively low-cost and easy to manufacture compared to other solid electrolytes
[24]. However, PEO-based electrolytes can also have some drawbacks. They can have limited electrochemical stability and be prone to degradation over time, particularly in the presence of moisture or other contaminants. In addition, PEO-based electrolytes can be relatively sensitive to temperature and may require careful control of the operating conditions to maintain their performance
[22][24].
Poly(acrylonitrile) (PAN) is a polymer that has been investigated as a potential electrolyte material for SSBs. PAN-based polymer electrolytes have been shown to have high ionic conductivity and good mechanical properties, which make them attractive for use in SSBs
[24]. The composition of PAN-based polymer electrolytes typically involves mixing PAN with a Li salt and a plasticizer, which helps improve the polymer electrolyte’s ionic conductivity. The Li salt dissociates in the polymer matrix to form free Li
+, which is responsible for the charge transport within the electrolyte.
PAN-based polymer electrolytes can be manufactured using cost-effective and scalable methods, such as solution casting or electrospinning. Solution casting involves dissolving PAN, the Li salt, and the plasticizer in a solvent and casting the resulting solution into a thin film. Electrospinning consists of using an electric field to spin a polymer solution into nanofibers, which can be used to form a three-dimensional network that enhances the mechanical strength and ionic conductivity of the electrolyte
[25]. One advantage of PAN-based polymer electrolytes is their high ionic conductivity, which can be attributed to the dissociation of the Li salt and the plasticizer’s ability to increase the mobility of the Li
+. PAN-based polymer electrolytes also have good mechanical properties, such as high elasticity and tensile strength, which make them resistant to deformation and cracking during battery operation.
Overall, PAN-based polymer electrolytes show promise as a potential electrolyte material for SSBs. Ongoing research is focused on optimizing the composition and processing of PAN-based polymer electrolytes to improve their ionic conductivity, mechanical properties, and stability over the battery’s lifetime.
2.3. Composite Solid Electrolytes (CSEs)
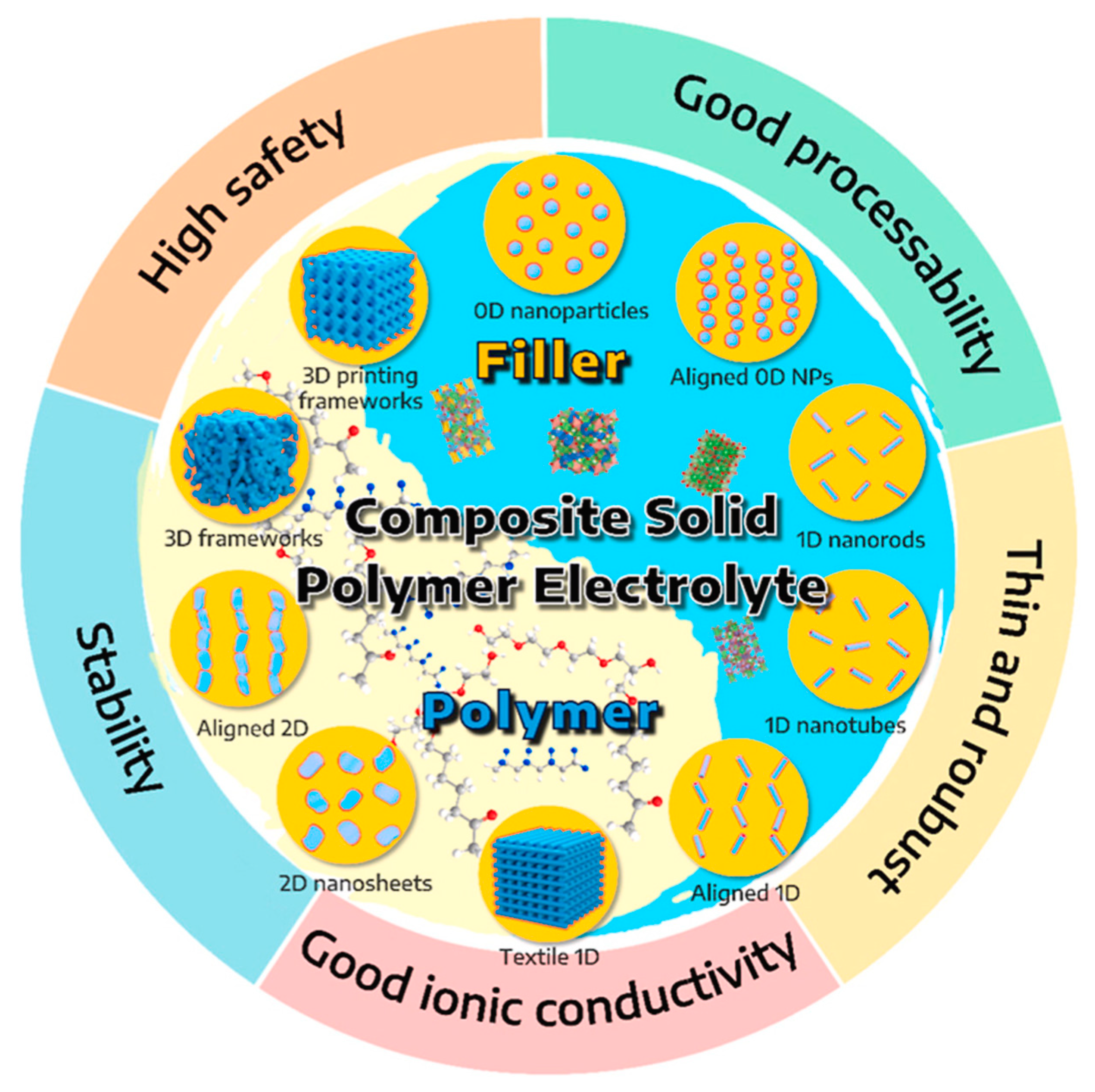
While some studies have concentrated on either inorganic solid ceramic electrolytes or organic solid polymer electrolytes, there is a rising trend of research attention towards CSEs. These electrolytes merge the strengths of both inorganic and organic solid electrolytes while eliminating their drawbacks. In CSEs, inorganic ceramic electrolytes function primarily as fillers to improve mechanical strength and ionic conductivity
[25]. Some examples of these fillers and the overall advantages of CSEs are shown in
Figure 3.
Figure 3. Illustration depicting the structure of fillers and template polymers used in developing CSEs. The graphic also highlights the overarching benefits of using CSEs
[15]. Reprinted with permission from Elsevier.
The purpose of adding inorganic fillers into the polymer matrix is to improve mechanical strength, increase ionic conductivity, and improve stability. Recent research includes the investigation of various morphologies such as 0D nanoparticles, 1D nanowires, 2D nanosheets, and 3D frameworks. Depending on the Li ion conductivity, the inorganic fillers can be classified into two categories: passive and active.
Passive inorganic fillers are commonly used in polymer composite SSEs to improve their mechanical and thermal properties. These fillers do not participate in the ionic conduction process but rather act as a supporting material to enhance the overall performance of the composite electrolyte. Inert fillers are mainly oxide ceramics with a spherical particle shape, like Al
2O
3, silica (SiO
2), and TiO
2 [25][26].
One common passive filler used in polymer composite SSEs is SiO2, known for its excellent mechanical properties and thermal stability. Adding SiO2 nanoparticles to polymer electrolytes has been shown to improve their mechanical strength, modulus, and thermal stability while maintaining their high ionic conductivity. In terms of electrochemical properties, studies have shown that adding SiO2 nanoparticles can improve the ionic conductivity of polymer electrolytes. This is thought to be due to the increase in the number of ion-conducting pathways within the composite electrolyte, as well as the improved interfacial contact between the polymer matrix and the SiO2 nanoparticles.
A recent study found that by adding SiO
2 nanotubes to PEO/LiTFSI, the ionic conductivity increased from 6.13 × 10
−8 S cm
−1 to 4.35 × 10
−4 S cm
−1 at 30 °C
[27]. They proposed that the interaction between the SiO
2 nanotubes and the composite contributed to the efficient transport of Li
+. Additionally, the assembled cell showed good cycle life. Other passive fillers that have been investigated include Al
2O
3, magnesium oxide (MgO), and titanium dioxide (TiO
2). These fillers have been shown to improve the mechanical and thermal properties of the polymer electrolytes while maintaining their high ionic conductivity
[28].
Incorporating passive inorganic fillers into polymer composite SSEs can provide several benefits, such as improved mechanical strength, better thermal stability, and increased resistance to deformation and cracking. These benefits can make the composite electrolytes more durable and longer lasting in demanding applications, such as high-performance LIBs or supercapacitors.
Active fillers contain Li ions in their composition and are used in polymer composite SSEs to enhance their ionic conductivity by providing a continuous pathway for ion transport. These fillers are typically ceramic materials with high ionic conductivity and can act as an active component in the composite electrolyte.
One of the most common active inorganic fillers used in polymer composite SSEs is Li-ion conducting ceramics, such as LLZO, LATP, and lithium phosphorus oxynitride (LiPON). These materials have high ionic conductivity and can provide a continuous pathway for ion transport in the composite electrolyte, resulting in higher overall ionic conductivity. Recent research has shown that incorporating LLZO into polymer electrolytes can significantly improve their ionic conductivity. For example, one study found that a composite electrolyte containing 30 wt.% LLZO achieved an ionic conductivity of 2.2 × 10
−4 S cm
−1 at room temperature, much higher than that of the pure polymer electrolyte. Researchers have also investigated the effect of different types of LLZO particles on the performance of composite electrolytes and found that smaller LLZO particles with a higher surface area led to higher ionic conductivity
[29]. Recent studies have found that LATP can improve the ionic conductivity of polymer electrolytes, as well as their thermal stability and mechanical strength. For example, one study found that a composite electrolyte containing a porous LATP framework was able to serve as a physical barrier to suppress the growth of Li dendrites and showed an ionic conductivity of 7.47 × 10
−4 S cm
−1 at 60 °C, which is higher than that of PEO (1.0 × 10
−4 S cm
−1) at RT
[30].
NASICON is an active inorganic filler material with great potential for improving the ionic conductivity of polymer composite SSEs. Recent research has focused on optimizing the use of NASICON as an active filler material. One study found that incorporating NASICON into a polymer electrolyte significantly improved its ionic conductivity and mechanical properties. The researchers found that the optimal NASICON content was 20 wt.%, which resulted in a polymer electrolyte with a high ionic conductivity of 1.44 × 10
−3 S cm
−1 and good mechanical strength. Another study investigated the effect of sodium doping on the ionic conductivity of NASICON-based SSEs. The researchers found that increasing the amount of sodium doping led to an increase in the ionic conductivity of the material. They also found that the addition of NASICON improved the thermal stability of the polymer electrolyte, making it more suitable for high-temperature applications
[31].
Other active fillers that have been investigated include sulfides, oxides, and nitrides, such as Li
2S, lithium nitride (Li
3N), and lithium magnesium oxide (LiMg
0.05O). These materials have also been shown to improve the ionic conductivity of the polymer electrolyte and enhance its overall performance. Compared with passive fillers, active fillers have a stronger enhancement effect on the ionic conductivity of SPEs. This is mainly due to the intrinsic high bulk ionic conductivity of active ceramics
[26]. Examples of some CSEs, along with their ionic conductivity, are shown in
Table 1.
Table 1. Ionic conductivity of typical inorganic filler-based composite solid electrolytes.
| Compound |
Ionic Conductivity/S cm−1 at T/°C |
Source |
| SiO2/PPC/LiTFSI |
8.5 × 10−4/60 °C |
[32] |
| SiO2 NTs/PEO/LiTFSI |
4.35 × 10−4/30 °C |
[27] |
| SiO2 NFs/PEO-LiTFSI-SN |
1.3 × 10−4/30 °C |
[33] |
| LLZO/PEO/LiTFSI/PEGDME |
4.7 × 10−4/60 °C |
[34] |
| LLZO NWs/PEO/LiTFSI |
2.39 × 10−4/RT |
[35] |
| Li/LATP-3D/LiFePO4 |
7.47 × 10−4/60 °C |
[30] |
| LLZAO-PEO/LiClO4 |
2.25 × 10−5/30 °C |
[29] |
| LLTO/PEO |
3.31 × 10−4/RT |
[36] |
| LLTO/PAN-PVDF |
1.43 × 10−3/RT |
[37] |
3. Electrode Materials for SSBs
3.1. Anode
The anode is the battery’s negative electrode and is responsible for releasing electrons during the discharge process. In SSBs, the anode is typically made of a Li-containing material, such as Li metal, lithium titanium oxide (Li
4Ti
5O
12), or lithium silicon (LiSi). These materials are chosen because they have high energy densities and good stability but can also be prone to degradation over time
[38]. Substituting the traditional graphite anode with Li metal presents a promising avenue. Indeed, according to Jie Xiao
[39], this could amplify the energy density of batteries by a factor of approximately 1.5. This transition, however, sets in motion a series of chemical alterations within the liquid electrolyte upon encountering Li metal and is, therefore, only possible with a solid electrolyte. Indeed, these transformations give rise to the creation of hazardous organic salts, which subsequently precipitate and evolve into the infamous structures known as Li dendrites. These dendritic formations can grow and extend, ultimately piercing through the separator that keeps the battery’s components isolated. This breach in the separator leads to short circuits within the battery, thereby introducing a significant safety concern
[39].
Nonetheless, the utilization of solid electrolytes brings forth a set of distinctive advantages. These SSEs possess characteristics that render them non-volatile and non-flammable, making the battery safer. Moreover, they exhibit an extended range of electrochemical stability, allowing for more efficient battery operation. Critically, they offer the capability to effectively inhibit the progress of Li dendrites. Indeed, Pilgun et al.
[40] showed that by preventing these dendrites from penetrating the separator, the solid electrolytes play a pivotal role in enhancing the overall safety of the battery.
Thus, SSEs allow the employment of metallic Li as a negative electrode. Currently, most SSBs opt for a Li metal anode due to its promising theoretical capacity, lightweight nature, and low electrochemical potential. However, Li has drawbacks like high reactivity, susceptibility to oxidation, and limited availability compared to potential substitutes. Moreover, there have been instances of Li dendrite formation, even when using certain SSEs, which poses safety concerns for the battery. Consequently, researchers are actively exploring alternative anode materials such as Si, S, metallic alloys, tin (Sn), Ti, and carbon-based substances
[41].
Li metal is commonly used as an anode in SSBs due to its high theoretical capacity, lightweight nature, and low electrode potential. However, it also has drawbacks related to its high reactivity, susceptibility to oxidation, and dendrite formation, posing safety concerns. Researchers have improved the electrochemical performance of these systems by using graphite coatings, facilitating three-dimensional Li-ion transport on the graphite’s surface and enhancing mechanical properties
[42].
Another option is lithium titanate (LTO, Li
4Ti
5O
12) anodes. They are known for their safety and cycle life and, although not offering the high energy density of Li metal, they are stable and dependable. Garnet-structured SSEs are gaining attention, but their lithiophobic nature creates interface resistance. A composite anode material comprising Li metal and LTO has been proposed to address this challenge
[43].
Silicon stands out as a highly promising anode material for batteries due to its exceptional theoretical capacity, cost-effectiveness, and stability in air. It has garnered significant attention for use in EVs and SSBs, addressing concerns related to energy storage and driving range limitations in the EV industry
[44]. However, Si faces inherent challenges, notably substantial volume expansion and pulverization during charge and discharge cycles. Researchers are actively exploring innovative solutions to mitigate these issues, aiming to enhance the performance and longevity of Si-based battery systems
[44]. In the context of SSBs, integrating Si-based negative electrodes is a key focus to align with advanced electrolyte technologies and establish stable battery operation
[44]. Nevertheless, the relatively low ionic conductivity of solid electrolytes and the substantial resistance encountered at the electrode-electrolyte interface present challenges that affect overall battery performance. The appeal of Si as an anode material in ASSBs stems from its ready availability, non-toxic nature, and remarkable theoretical capacity, making it a competitive candidate in the pursuit of next-generation battery technology
[45]. However, it is essential to acknowledge certain limitations associated with Si-based anodes, including concerns related to mechanical integrity, limited electrical conductivity, and cycling lifespan
[46][47]. These challenges underscore the ongoing efforts in material modification and engineering to harness Si’s full potential while addressing its drawbacks in advanced battery systems.
Lithium silicides (Li-Si alloys) and sulfur are other potential anode materials. They offer higher energy density but face challenges such as low electrical conductivity. To enhance the energy storage capability, researchers have explored heteroatom doping, including S, P, nitrogen, oxygen, and boron doping. Among these, S doping has garnered significant attention. S atoms have a larger covalent radius, which expands the interlayer spacing of carbon materials, thereby increasing the number of active sites available for sodium storage
[48].
Certain SSBs are exploring metallic alloys like lithium-aluminum (Li-Al) and lithium-tin (Li-Sn), striking a balance between capacity and stability
[49]. In a study by Zhang et al., a polymer electrolyte reinforced with polyacrylonitrile (PAN) fibers and a protective Li-Sn alloy layer on the Li anode has significantly extended the cycle lifespan of room-temperature SSBs. The Li-Sn alloy layer acted as a passivation layer, preventing unwanted reactions between metallic Li and the solid polymer electrolyte (SPE), and enhancing compatibility and stability
[50]. Alloy anodes in SSBs offer mechanical advantages over other materials, avoiding issues like short-circuiting and stabilizing the solid-electrolyte interphase, thus advancing the development of efficient and reliable SSBs
[51].
Finally, silver-carbon composite interlayers have shown potential for enabling Li-free cycling in SSBs. During battery charge, Li intercalates into graphite and reacts with Ag to form Li-Ag alloys. Discharge proceeds through Li-deficient Li-Ag phases. Higher charging rates delay Li-Ag phase formation, resulting in more Li metal deposition
[52].
3.2. Cathode
The cathode is the positive electrode of the battery, responsible for accepting electrons during the discharge process. In SSBs, the cathode is typically made of a Li-containing material, such as lithium cobalt oxide (LiCoO
2), lithium iron phosphate (LiFePO
4), or lithium nickel manganese cobalt oxide (LiNiMnCoO
2). These materials are chosen because they have high energy densities, good stability, and relatively low cost
[38], but can only be properly used in non-reactive electrolytes. Indeed, some high-energy cathode materials, such as those based on nickel or manganese, can be sensitive to the electrolyte’s chemistry. SSEs can provide a more stable interface with these cathode materials, reducing unwanted side reactions and enhancing the overall efficiency and cycle life of the battery
[53]. Moreover, cathodes that interact directly with air or oxygen, such as lithium-air (Li-O
2) or sodium-oxygen (Na-O
2) batteries, can benefit from SSEs that prevent the infiltration of moisture and contaminants from the air
[54][55]. It extends the lifespan of the cathode by inhibiting dendrite growth and unwanted reactions. The wider electrochemical stability window allows higher voltage operation, potentially boosting energy density. Additionally, the SSE prevents cathode-electrolyte reactions, preserving cathode capacity. Its stability enhances safety for thermally sensitive cathodes, and its interface with the cathode improves overall electrochemical performance. Overall, SSEs provide flexibility in cathode selection, minimize self-discharge, and positively impact the cathode’s stability and performance over time
[14].
However, the primary determinant of battery energy density remains the selection of cathode materials. This choice significantly impacts the potential energy storage within the battery. To achieve heightened energy density, it becomes crucial to diminish the resistance at the interface connecting the electrolyte and the electrode
[56].
Unlike traditional LIBs, SSBs function in a distinct manner where the movement of Li
+ occurs through the solid portions, encompassing both bulk and solid-to-solid interfaces. Consequently, optimizing the connections between SSEs and cathode materials becomes essential to establish efficient pathways for Li
+ transport within the electrode
[57]. The decision regarding the cathode material is thus heavily influenced by the chosen electrolyte. Their compositions must often be closely aligned to prevent additional complications at the interface.
For ISEs, cathode materials rich in nickel (Ni) have recently been used in LIBs due to their ability to hold high reversible capacities exceeding 200 mA g
−1. Unfortunately, the applicability of these cathode materials in SSBs is limited due to their relatively low density, which can lead to particle cracking and loss of Li-ion transport pathways. This, in turn, results in notable performance degradation during the cycling of SSBs
[58]. To address this issue, researchers have explored various structural adjustments to enhance the mechanical strength and density of cathode materials, aiming to make them compatible with SSBs. In this context, cathode materials with a single-crystalline structure show promise due to their cohesive form, absence of defects in their microstructure, and excellent particle hardness
[56].
Research findings indicate that the interfacial resistance at the solid-solid junctions between the lithium phosphorus sulfur chloride (LPSCl) solid electrolyte and cathode materials can be effectively lowered by modifying the structure of Ni-rich cathode materials to adopt a single-crystalline form
[56]. An effective technique to mitigate interfacial impedance involves applying a coating of Li ionic conductors onto oxide cathodes. This method has shown improvements in initial charge-discharge capacity and rate performance. For instance, LiNbO
3-coated Ni-rich LiNi
0.8Co
0.1Mn
0.1O
2 (NCM811) cathode has exhibited noteworthy electrochemical enhancements in SSBs operating at 35 °C and 60 °C
[59]. However, challenges remain regarding cycle performance enhancement for oxide cathodes, primarily due to issues like incomplete coating with thin inactive buffer layers or decreased electronic conductivity and specific capacity with thicker inactive buffer layers. To address this, constructing a core-shell architecture for Ni-rich oxide cathode materials proves effective, where the Ni-rich core contributes to high capacity, and the Ni-low shell ensures stable interactions with sulfide electrolytes, allowing for complete coating
[58][59].
Zhang et al.
[60] demonstrated that a solid-state lithium metal battery using a ceramic-based composite solid electrolyte and a LiNi
0.5Co
0.2Mn
0.3O
2 (NCM523)-based composite cathode yielded superior performance. Yu et al.
[61] proposed hexaazatriphenylene (HATN)-based organic materials as suitable cathodes for quasi-solid-state lithium-organic batteries. Combining these organic cathodes with a gel polymer electrolyte modified with a succinonitrile plasticizer resulted in improved electrochemical performance.
Moreover, addressing resistance at the electrolyte-electrode interface is crucial to elevate energy density. Unlike conventional LIBs, SSBs function uniquely, necessitating optimized connections between solid electrolytes and cathodes for efficient Li ion transport. The choice of cathode material is intricately tied to the electrolyte, requiring compositional alignment to prevent complications. While ISEs have succeeded with nickel-rich cathodes in LIBs, their potential in SSBs is hindered by density-related challenges, prompting innovative solutions such as single-crystalline structures. Research shows that modifying cathode structures can reduce interfacial resistance, and strategies like Li ionic conductor coatings on oxide cathodes can significantly enhance performance. Organic-based cathodes, as exemplified by HATN, are especially promising when combined with advanced electrolyte modifications.
4. Additive Manufacturing of SSBs
SSBs are safer and better than LIBs and can be produced at a commercial scale by additive manufacturing, also known as 3-dimensional (3D) printing. Researchers and engineers have been exploring the integration of 3D printing techniques to fabricate SSBs, aiming to enhance their efficiency, energy density, and overall performance. The use of 3D printing in the fabrication of SSBs offers advantages such as intricate design possibilities, improved manufacturing precision, and the ability to create complex internal structures that enhance battery performance
[62]. Researchers have investigated various 3D printing technologies, including selective laser sintering, stereolithography (SLA), and roll-to-roll printing, to create intricate solid electrolyte structures and optimize the overall battery architecture
[63].
For electrode materials, conventional thin film fabrication methods, including sol-gel techniques, electron beam evaporation, and chemical vapor deposition, are complex and expensive, often leading to undesired side reactions that reduce the efficiency of LIBs. Inkjet printing, as reported by Zhao et al., offers a simpler approach, demonstrating improved electrochemical efficiency for SnO
2 and LiCoO
2 thin film electrodes
[64]. Substituting conventional carbon black with surface-modified carbon in the ink further enhances electrochemical properties. Utilizing the direct ink writing (DIW) technique, planners and a 3D-patterned LiMn
2O
4 cathode were constructed, incorporating carbon black, PVDF, and N-methyl-2-pyrrolidone
[65]. The resultant cell exhibited superior specific capacity and rate capability when compared to conventional flat electrodes.
Printing techniques are also being employed for the fabrication of SSEs. Delannoy et al. investigated LIB construction incorporating porous electrodes, where the SSE was fabricated using inkjet printing with a silica-based chemical solution as ink. The inkjet-printed electrolyte exhibited comparable electrochemical performance to the physically vapor-deposited counterpart
[66]. In parallel, diverse 3D-printable LATP-based inks were developed through the DIW technique, enabling the creation of arbitrary shapes (L, T, and +) and achieving higher conductivities (up to 4.24 × 10
−4 S cm
−1). These inks were employed in the construction of ceramic SSEs. LATP-based electrolytes were directly printed on LiFePO
4 cathodes, demonstrating a high discharge capacity of 150 mA h g
−1 at 0.5 °C
[67]. In a noteworthy contribution to overcoming the obstacle of poor interfacial contact within SSBs, a recent study reports the use of SPEs fabricated through SLA 3D printing for SSBs. The SLA-printed OSPE demonstrates an impressive ionic conductivity of 3.7 × 10
−4 S cm
−1 at 25 °C, as well as reduced interfacial impedance, outperforming structures utilizing conventional structure-free OSPE
[68].
The integration of 3D printing can potentially streamline the manufacturing process of SSBs, reducing production costs and making these advanced energy storage devices more commercially viable
[62]. As research and development in this field continue, it is anticipated that further breakthroughs will be made, pushing the boundaries of both 3D printing and SSB technology.