Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | David James | -- | 3865 | 2024-01-11 05:39:40 | | | |
| 2 | Camila Xu | Meta information modification | 3865 | 2024-01-11 06:20:30 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
James, D.G. Monarch Population Dynamics in Western North America. Encyclopedia. Available online: https://encyclopedia.pub/entry/53715 (accessed on 07 February 2026).
James DG. Monarch Population Dynamics in Western North America. Encyclopedia. Available at: https://encyclopedia.pub/entry/53715. Accessed February 07, 2026.
James, David G.. "Monarch Population Dynamics in Western North America" Encyclopedia, https://encyclopedia.pub/entry/53715 (accessed February 07, 2026).
James, D.G. (2024, January 11). Monarch Population Dynamics in Western North America. In Encyclopedia. https://encyclopedia.pub/entry/53715
James, David G.. "Monarch Population Dynamics in Western North America." Encyclopedia. Web. 11 January, 2024.
Copy Citation
The Monarch butterfly, Danaus plexippus (Linnaeus, 1758), in western North America appears to be undergoing a period of flux in terms of population size and ecology.
population dynamics
stressors
neonicotinoids
climate change
habitat loss
1. Introduction
The Monarch butterfly, Danaus plexippus (Linnaeus, 1758), in western North America appears to be undergoing a period of flux in terms of population size and ecology. From a historical high of an estimated three to ten million butterflies overwintering annually along the California coast in the 1980s, the population fell precipitously to about 200,000–400,000 overwintering butterflies at the beginning of the 21st century [1]. For 16 years (2001–2017), winter populations ranged from about 58,000–300,000 butterflies before another substantial decline to around 30,000 butterflies occurred in 2018. The smallest overwintering population of monarchs ever recorded in western North America occurred in 2020 (1899), before a remarkable rebound to 247,246 in 2021 and 335,479 in 2022 [https://www.westernmonarchcount.org/ accessed on 2 January 2024].
2. Monarch Population Dynamics in Western North America (1980s–2022)
2.1. Monitoring
In common with the eastern North American population of monarchs, the size of aggregated overwintering populations has been used to provide an estimate of the population size of western North American monarchs [1][2]. However, unlike the eastern population, most of which overwinter in a single geographic area in Mexico [3], the western population overwinters at almost 400 widely separated sites along 1100 km of the California coastline [4][5]. During the 1980–1990s, estimates were made at small numbers (1–40) of overwintering sites as part of scientific studies [6][7] or limited citizen scientist efforts [1]. In 1997, a more organized count conducted by citizen scientists was established by Walt Sakai, Dennis Frey, Mia Monroe, and David Marriott [8]. They proposed to conduct a count over a three-week period every Thanksgiving at 100 or more overwintering sites. The Western Monarch Thanksgiving Count (WMTC) was adopted by the Xerces Society for Invertebrate Conservation in 2000 and since 2016 has included counts from 253–272 overwintering sites annually. Citizen scientists are trained annually to count monarchs roosting at overwintering sites, but the numbers are estimates at best because of the density of individuals in clusters. Nevertheless, these ‘counts’ are more accurate than the estimates of overwintering monarchs in Mexico derived from the number of hectares that they occupy [9]. Ultimately, the WMTC provides with a proxy for the size of the western monarch population since at least 1997. Less confidence can be applied to pre-1997 estimates, but they still provide a useful ‘snapshot’ of monarch populations during the 1980–1990s.
2.2. The Big Decline Post-1997
Schultz et al. [1] estimated that the monarch overwintering population at the turn of the century had fallen by 97% of its average historic abundance in the 1980s from about 3–10 million to 200,000–400,000 butterflies. Thereafter, for 16 years until 2017, the overwintering population fluctuated within a range of 100,000–300,000 individuals (Figure 1). The sudden drop and consistent lower overwintering numbers after 1997 suggest a major detrimental change within the ecology of western monarchs occurred at that time. A 67% drop in the eastern North American overwintering population in Mexico occurred one year before the 55% drop in the western population, suggesting some commonality of causative factors. Many monarch scientists believe that the rapid increase in the use of genetically-modified herbicide-resistant crops, primarily soybean and corn, over vast areas of the eastern US from 1996 onwards was a prime driver of the decline in overwintering populations in Mexico [10]. For the first time, weeds could be sprayed without affecting crops, resulting in large cropland areas, particularly in the mid-west, devoid of weeds, including milkweed [11]. While herbicide-resistant crops were also used in the western US, croplands occupy a much smaller area west of the Rocky Mountains [12].
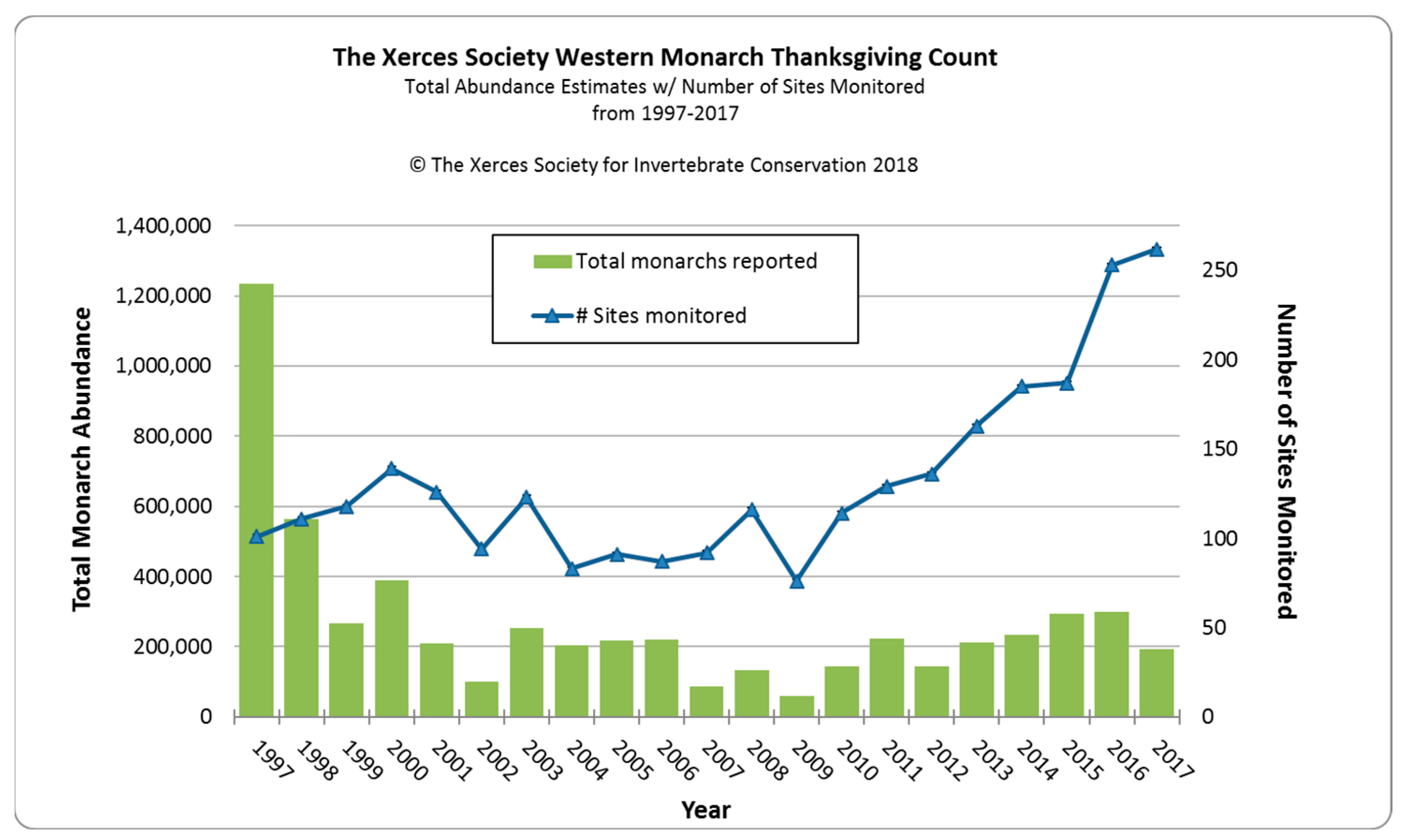
Figure 1. Western North American overwintering populations of monarch butterflies for 1997–2017 as assessed by annual Western Monarch Thanksgiving Counts (The Xerces Society for Invertebrate Conservation).
2.3. Neonicotinoids as the Potential Primary Driver of the Western Monarch Decline Post-1997
The major stressors on monarch populations in North America are widely considered to be habitat loss, climate change, and increased use of pesticides [13]. Habitat loss is considered to be an important driver of monarch decline in North America [14], but its impact is likely to be relatively progressive over time and not sudden. Similarly, any adverse impact of climate change on monarch ecology would likely be progressive and not sudden. While the growth in the use of synthetic pesticides has also been progressive since the 1950s, a new class of pesticides, the neonicotinoids, emerged in the early 1990s to rapidly become the most widely used insecticides in the world [15]. Neonicotinoid use in North America increased dramatically from 1994–2011 [16], coinciding with a 55–67% decline in the size of monarch overwintering populations. Today, neonicotinoids still maintain the largest global share of the insecticide market (24%) [17].
While any association between monarch population decline and the rise of neonicotinoid use in the late 1990s remains correlative, there is now much field and laboratory evidence for lethal and harmful sub-lethal effects from this class of insecticides on beneficial insects, including butterflies [18][19][20]. Correlative associations have also been reported for population declines of butterfly faunas in Great Britain and California and the increased use of neonicotinoid insecticides [21][22]. While most studies on the sub-lethal impacts of neonicotinoids have focused on honey bees [23][24], an increasing number have looked at butterflies [25]. Sublethal impacts of field-realistic levels of the neonicotinoids, imidacloprid, and clothianidin on monarch larvae were reported by Pecenka and Lundgren [26] and Krischik et al. [27]. Clothianidin at levels of 0.5–5.0 ppb reduced the weight and body length of first instar larvae. It also increased the period spent as first instar larvae and resulted in 50% mortality [26]. Krischick et al. [27] showed imidacloprid at 15 ppb significantly reduced survival of early instar monarch larvae. Arrested pupal ecdysis occurred in 60–82% of monarch larvae exposed to neonicotinoids as late instar larvae [28][29].
Neonicotinoids are highly systemic compounds readily transported through the vascular system of plants, poisoning herbivores whether they feed on stems, leaves, flowers, or seeds [30]. A likely important route of neonicotinoid exposure to pollinators like butterflies is dissemination through nectar and pollen [31]. In addition to crop flowers being contaminated with neonicotinoids, the mobility and longevity of these compounds in soil and water [32][33] means that residues can also be found in non-target plants at distances from crops [34]. Surveys of streams in agricultural and urban areas in the US have found neonicotinoid residues widespread in surface waters [35][36][37], and they have also been found in snow and spring meltwater in Canadian prairie wetlands [38]. Neonicotinoid insecticide residue levels in crop nectar and pollen differ considerably according to the amount applied to crops and landscapes as well as the method of application. Seed treatments result in relatively low levels of nectar and pollen, usually less than 10 ppb [31][34]. Residues in pollen and nectar from crops treated with foliar applications range from 10–100 ppb [27]. The greatest neonicotinoid residues (1000–4500 ppb) are found in nectar and pollen from landscape trees and plants treated with soil drenches [27]. Another less-researched, but likely source of high levels of neonicotinoid contamination of pollen and nectar, are home garden plants [39], which may receive recommended application rates > 40 times greater than those used in agricultural systems. Garden plants propagated in nurseries receive even higher rates of application and may contain neonicotinoid residues in nectar and pollen of up to 45,000 ppb [27]. Recent research indicates that plant species vary in the efficiency of uptake of neonicotinoids. Milkweeds (two species) had a low uptake (<0.5%) compared to Red Clover (50%) [40].
A brief laboratory study assessed the impact of the neonicotinoid, imidacloprid, provided as adult nourishment on adult monarch longevity [41]. Imidacloprid at 23.5 ppb, a field-realistic rate reported from wild nectar and pollen, was fed ad libitum to newly-eclosed monarchs in a sugar-based diet for 22 days. Treated monarchs showed reduced longevity, with 78.8% mortality by day 22, compared to 20% in untreated monarchs. In a similar study, imidacloprid/syrup, force-fed at 15 and 30 ppb, had no effect on the survival of monarchs [27]. In addition, free-ranging monarchs in small mesh cages allowed to feed on flowers containing 6030 ppb or 10,400 ppb also showed no difference in survival and fecundity from non-exposed butterflies [27]. However, the authors noted that monarchs may not have been able to forage adequately in the small cages. Uncontaminated 30% honey water sponges were also supplied as food, and monarchs may have fed less on or avoided the neonicotinoid-contaminated flowers. More recently, Prouty et al. [42] concluded that adult monarchs show high tolerance to field-realistic levels of neonicotinoid insecticides. However, these authors held monarchs for only 10 days during exposure to imidacloprid, which is insufficient to show an impact on medium- to long-term survival, judging from the results of James [41]. Similarly, Krishnan et al. [28] held neonicotinoid-treated monarchs for just four days to assess mortality. Mortality in monarchs exposed to field-realistic levels of imidacloprid occurred during days 12–22 [41]. If laboratory insecticide bioassays are to reflect real-world scenarios, then materials used in the field should be tested. Thus, commercially formulated insecticide products [41] rather than active ingredients [27][28][42] should be used. Commercial formulations of insecticides have invariably proven to be more toxic than active ingredients alone [43][44][45]. More work is needed on the impact of nectar-borne neonicotinoids (commercial formulations) on the medium- to long-term survival of adult monarchs. The possibility of ingested neonicotinoids during adult life impacting the survival and development of progeny should also be investigated.
The extent to which neonicotinoids currently contaminate the wider landscape is an important question. The ability of neonicotinoids to move away from sites of application through water, air, and soil [38][46], combined with residual persistence [47], provides the potential for widespread landscape contamination. The contamination of waterways and urban water supplies by neonicotinoids is increasingly being documented [37][48][49] and is concerning not only for pollinators but also for human health [50][51][52]. The ubiquity of neonicotinoids as ingredients in home garden pest control treatments, along with the higher rates used for these applications, has the potential to make urban areas highly contaminated with neonicotinoids. Gardens not directly exposed to neonicotinoid sprays may still be contaminated by run-off from other gardens that use these compounds. Urban areas may be reservoirs of high-level neonicotinoid contamination [53].
Shortened adult longevity has serious consequences for monarch population development, migration, and overwintering. Although egg production appeared to be unaffected by adult exposure to imidacloprid [41], we do not know whether the viability of eggs and resultant larvae is unaffected. Mating behavior could also be affected, as shown in a parasitic wasp species [54]. Monarch migration is powered by feeding on a wide range of nectar in natural, agricultural, and urban landscapes, at least some of which are likely to be contaminated with neonicotinoids. Could the migratory ability of monarchs be affected by these residues? Neonicotinoid-mediated impairment of foraging behavior has been reported for bumblebees [55]. The flight behavior of locusts (Locusta migratoria L.) is impaired by imidacloprid [56], and the migration and metabolism of some birds also appear to be impaired by this chemical [57][58][59].
Correlative declines in butterfly [21][22] and bird faunas [60][61] have been associated with the introduction and widespread adoption of neonicotinoids. The relatively abrupt decline in western Monarch populations between 1997 and 2001 may have been another example of this.
2.4. Sudden Decline 2018–2019
After a 16-year period (2001–2017) of relatively stable overwintering populations fluctuating between 100,000–300,000 butterflies, there was an 86% decline between 2017 (192,624) and 2018 (27,721). A similarly low estimate was made in 2019 (29,436). This sudden drop was considered to mirror a ‘textbook extinction vortex’ [2]. These authors restated a 2016 proposition that 30,000 butterflies represented the quasi-extinction threshold for western monarchs [1][2].
What was the cause of this sudden and rapid decline? Mid-to-late winter (January–March) in 2017, 2018, and 2019 was characterized in coastal California by significant winter storms with substantial rainfall and high winds (e.g., https://www.climate.gov/news-features/event-tracker/soaking-rains-and-massive-snows-pile-california-january-2017, accessed on 2 January 2024) which caused serious damage to some overwintering sites. Monarch populations in late winter are most vulnerable in terms of physical wear and tear to wings and lipid depletion, and it is possible that much mortality and/or early dispersion occurred during these winters, as has been recorded for overwintering monarch populations in Mexico [62]. Substantially earlier dispersal of butterflies was recorded at Lighthouse Field in Santa Cruz, with an 80% decline in January 2017 (J. Dayton, pers. comm). Dispersal at this early time may limit the reproductive potential of females. The first storm event in January–February 2017 was followed by a 35.5% overwintering population decline from 298,464 in 2016 to 192,624 in 2017. The storms during January–March 2018, were followed by an 86% decline to less than 30,000 overwintering butterflies in 2018/19 (Table 1). Although no data are available on the size of reproductive populations in the summers following these severe winter storm events, it is possible that the winter storms caused the decline. An overwintering population of ~200,000 may not be large enough to provide a good buffer against winter storms which may be increasing in frequency and intensity in California [62].
Table 1. Thanksgiving count estimates of monarch butterflies at overwintering sites in California for 2018–2022 (Xerces Society for Invertebrate Conservation).
| Year | Total Monarchs Reported | Number of Sites |
|---|---|---|
| 2018 | 27,721 | 213 |
| 2019 | 29,436 | 242 |
| 2020 | 1899 | 249 |
| 2021 | 247,246 | 284 |
| 2022 | 335,479 | 272 |
2.5. 2020: The Western Monarch Nadir
2020 was the year that the ‘extinction vortex’ seemed to have become a reality for the western monarch butterfly. With just 1899 monarchs counted at 249 overwintering sites, the population declined by 93.6% in a single year (Table 1). Populations at overwintering sites ranged from 1–550 and only 100 sites actually hosted butterflies [https://www.westernmonarchcount.org/ accessed on 2 January 2024]. The western monarch population, at less than 0.5% of its 1980s peak, was considered on the ‘brink of collapse’ with the possibility that they could be lost from the interior west [63].
In October–November 2020, the Washington State University monarch tagging program recorded some unusual data. For the first time, no tagged butterflies were recovered from overwintering sites. During 2017–2019, 67% of recovered monarchs tagged in the Pacific Northwest were found at overwintering sites [64]. Of about 1300 monarchs tagged in late summer-fall 2020 (mostly in southern Oregon), all recoveries (10) were made in northern CA or the San Francisco Bay area, in association with host plant milkweeds. These butterflies, after traveling 300–500 km, appeared to have become reproductive. A precedent for this was seen in 2017 and 2019, when two tagged females from Oregon were observed laying eggs in Santa Barbara [65] and San Francisco after migrating 877 and 537 km, respectively [64]. James [65] suggested that migrants becoming reproductive might contribute to the population decline at overwintering sites.
In December 2020, it was also apparent that citizen scientists were finding large numbers of monarch eggs and larvae in the San Francisco Bay area. An analysis of the number of sightings of monarch larvae and pupae in the San Francisco Bay area in January 2021 showed more than three times as many larvae and pupae reported than in the same month over the previous six years (Figure 2) [66].
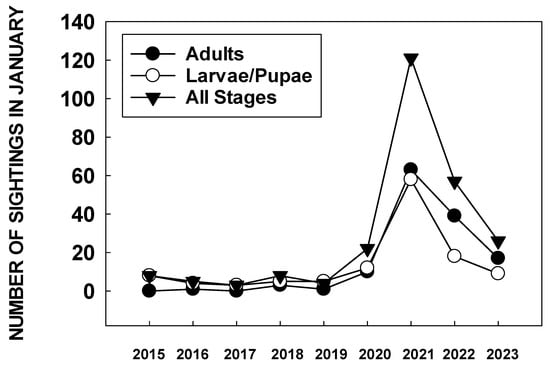
Figure 2. January (2015–2023) sightings of monarch larvae/pupae and adults in the bay area of San Francisco reported to https://www.inaturalist.org./ accessed on August 19 2023.
A study of monarch winter breeding in the San Francisco Bay area during January–April 2021 showed that adults were common during February-March with numbers ranging from 0.23–1.54/min during ~30 min weekly surveys [67]. Eggs and larvae were abundant during the same period. The convergence of data on the absence of butterflies at overwintering sites and the presence of novel breeding populations in the San Francisco Bay area (and anecdotally in other near-coastal urban areas of California, including Los Angeles), indicated a possible shift in overwintering strategy by western monarchs.
Monarch populations in south-eastern Australia in the early 1960s overwintered as non-reproductive populations comparable to California (up to 40,000 butterflies/site) in the Sydney Basin, New South Wales [68]. Research on these populations during 1978–1984 showed the existence of synchronous reproductive and non-reproductive overwintering populations [69][70][71]. Coincident with this apparent shift in overwintering behavior was a fall in the size of overwintering colonies from 40,000 to a maximum of 3500 butterflies per site [70]. This 90%+ decline in overwintering monarch populations was thought to have resulted from the loss of milkweed habitat, but it is possible that the shift towards winter-breeding was at least partly responsible.
If monarch butterflies migrating through the San Francisco Bay area during September 2020 became reproductive and stopped migrating to overwintering sites, as the tag recovery and iNaturalist data indicate, why did this happen? Migrating monarchs in eastern North America are in reproductive diapause [72], which means diapause usually cannot be broken until a period of refraction (when there is no response to temperatures normally stimulatory to reproductive tract development) has passed, normally in mid-winter [73]. While this may apply to most of the population and monarchs arrive at the Mexican overwintering sites with undeveloped reproductive tracts [3], some do break diapause in the southern US and drop out of the migration [74][75]. Australian monarch butterflies possess a flexible reproductive dormancy, oligopause, that has no refractory period [76]. Exposure of reproductively dormant Australian monarchs to temperatures optimal for reproduction at any time results in reproductive tract development. The nature of reproductive dormancy has not been explored in western monarchs but might be expected to be diapause, given that the North American population is genetically homogenous [77]. However, some environmentally induced biological differences have been detected in the western population [77], and reproductive dormancy in western monarchs may be more labile than in the eastern population.
It does seem likely that reproductive dormancy and migratory behavior were terminated prematurely in many western monarchs arriving in California in the autumn of 2020. Temperature is the main driver of reproductive development in adult monarchs [76][78], and the San Francisco Bay area experienced record-breaking temperatures during September–October 2020. The mean daily maximum temperature for San Francisco during September–October 2020 was 24.7 °C, 2.5 °C above the historical mean, and migrants were exposed to temperatures up to 39 °C [66]. If some migrants became reproductive in autumn 2020, forming a substantial winter-breeding population in the San Francisco Bay area, then the official WMTC count of 1899 underestimated the size of the western monarch population. Crone and Schultz [63] estimated a summer breeding population of about 12,000 monarchs in the Bay Area, and it seems likely there were at least that many, if not more, in the Bay Area during winter 2020/21.
2.6. 2021: Monarch Resurrection
In February 2021, the result of a New Year count of the overwintering population was released, showing that the already-small population in late December/early January had fallen further to 1069 butterflies [https://www.westernmonarchcount.org/ accessed on 2 January 2024]. If we assume a 50:50 sex ratio, then there were just 535 females remaining to begin the development of the 2021 summer population. However, as indicated above, a likely substantial population of monarchs also existed as breeding populations in near-coastal urban California, from San Francisco to Los Angeles.
The extent and success of the first generation of eggs and larvae produced in March–April by surviving overwintered females and females in winter-breeding populations in 2021 is unknown. This generation develops within California, and it is notable that the intensity of breeding at South Bay winter-breeding sites increased during March [67]. Adults from this first spring generation migrate during May and June into far northern California, Oregon, and Washington. The number of monarchs seen in Oregon, Idaho, and Washington during migration in April–June 2021 reported to websites like iNaturalist, was the same (25) as during the same period in 2020 [James, unpubl. obs]. This was surprising given the difference in the size of overwintering populations (WMTC January counts) that spring migrants were derived from in 2019/2020 (11,971) and 2020/2021 (1069). This is likely further evidence that the ‘true’ size of overwintering populations in 2019/2020 and 2020/2021 may have been comparable if winter-breeding populations were included. During July and August 2021, 43 monarchs were reported from the Pacific Northwest, almost double the number reported during the same period in 2020 (22).
The summer 2021 population of monarchs in the west appeared robust enough to improve populations at the traditional overwintering sites in winter 2021/2022 as long as migration was strong and temperatures during September–October in California were seasonal. The mean daily maximum temperature during September–October 2021 in San Francisco was 21.3 °C, below the long-term mean of 22.2 °C for this period (Figure 3).
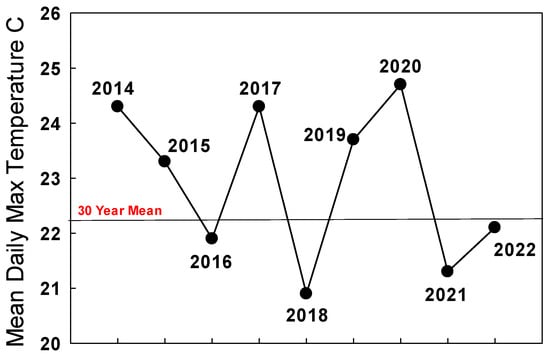
Figure 3. Mean daily maximum temperatures (°C) for San Francisco for September–October 2014–2022. Thirty-year mean: 1985–2015.
Consequently, monarchs migrated through the Bay Area and other parts of California, likely with minimal loss of individuals to reproductive populations. The number of larvae and pupae reported in the San Francisco area during January 2022 to iNaturalist was substantially lower than in 2021 (Figure 2), indicating a lower incidence of winter breeding.
The overwintering monarch population in California increased from 1899 in 2020 to 247,246 in 2021 [https://www.westernmonarchcount.org/ accessed on 2 January 2024], a 130-fold increase (Table 1). Simple mathematics shows that a population of 535 females leaving overwintering sites in late winter 2021 could not result in a population increase of this size. Even with overly-optimistic assessments of development and survival, a population of between 10,000 and 20,000 post-overwintering females would be needed to produce a population of 300,000+ in one season [Chip Taylor, pers. comm.]. This is further evidence that there were other females (e.g., those in winter-breeding populations) in early 2021 to help produce the scale of increase that occurred during the summer. It seems likely that winter-breeding populations were part of this, but it is also possible that some migrants reacted to the above-average warm-hot conditions of September–October 2020 by forming small, inconspicuous, non-reproductive overwintering populations at undiscovered sites, perhaps in the higher-elevation coastal range of California. There is precedent for this, with monarchs sometimes forming temporary roosts in hot autumn conditions in Texas [Chip Taylor, pers. comm.] and in Australia [79]. Another possibility for increasing the western population during a single season could come from spring migrants leaving overwintering populations in Mexico and arriving and laying eggs in Arizona [80]. Most likely, winter-breeding populations, undiscovered overwintering butterflies, and a spring incursion from Mexico all contributed in some way to the recovery of western monarchs in 2021.
Pacific Northwest summer populations in 2022 as judged from iNaturalist reports and personal communications appeared to be almost 10-times greater than in 2021 [James, unpubl. obs.] The mean daily maximum temperature for San Francisco during September–October 2022 (22.1 °C) was close to the long-term average (22.2 °C) (Figure 3), and the overwintering population grew by about 35% to 335,479 [https://www.westernmonarchcount.org/ accessed on 2 January 2024] (Table 1).
2.7. Western Monarchs: The Future
It is likely that there will be wide swings in abundance as the monarch responds to a changing environment, primarily the climate. From their arrival in Australia in 1872 [81][82], monarchs adapted to a new environment by substantially changing their physiology and behavior [83]. Migratory butterflies like the monarch have great genetic diversity and a better ability to adapt and produce larger populations [84].
California’s climate has demonstrably warmed during recent decades [85][86] and may have reached a tipping point in terms of facilitating ‘normal’ migratory and overwintering behavior for monarch butterflies. Autumn temperatures in California may now determine whether the majority of migrants overwinter at traditional sites in reproductive dormancy or develop winter-breeding populations in near-coastal urban areas. Warmer conditions may enable monarchs to breed during the winter in coastal areas north of San Francisco. Since the 1960s, a northward spread of monarch winter-breeding from San Diego to San Francisco [67][87]. A warming climate is also predicted to result in more frequent and intense winter storms in California [88], which will increase monarch mortality at traditional overwintering sites (but not necessarily at winter-breeding sites). Traditional overwintering populations in the future will be the product of interaction between higher fall temperatures, an increased frequency of winter storms, and the ability of monarchs to cope with and adapt to these environmental changes.
Future Thanksgiving counts at traditional overwintering sites may not always provide a realistic assessment of western monarch population size and trends, depending on the proportion that develops winter-breeding populations. There may also be a shift in occupied overwintering sites towards the north or to higher elevations as winter temperatures rise [5]. The future of Monarch populations in western North America will depend on how successful the butterfly is in responding and adapting to a changing environment, primarily a warming climate.
References
- Schultz, C.B.; Brown, L.M.; Pelton, E.; Crone, E.E. Citizen science monitoring demonstrates dramatic declines of monarch butterflies in western North America. Biol. Conserv. 2017, 214, 343–346.
- Pelton, E.M.; Schultz, C.B.; Jepsen, S.J.; Hoffman Black, S.; Crone, E.E. Western monarch population plummets: Status, probable causes and recommended conservation actions. Front. Ecol. Evol. 2019, 7, 258.
- Brower, L.P. Understanding and misunderstanding the migration of the monarch butterfly (Nymphalidae) in North America. J. Lepid. Soc. 1995, 49, 304–385.
- Leong, K.L.H.; Sakai, W.H.; Bremer, W.; Feurstein, D.; Yoshimura, G. Analysis of the pattern of distribution and abundance of overwintering sites along the California coastline. In The Monarch Butterfly: Biology and Conservation; Oberhauser, K.S., Solensky, M.J., Eds.; Cornell University Press: Ithaca, NY, USA, 2004; pp. 177–185.
- Fisher, A.; Saniee, K.; van der Heide, C.; Griffiths, J.; Meade, D.; Villablanca, F. Climatic niche model for overwintering monarch butterflies in a topographically complex region of California. Insects 2018, 9, 167.
- Tuskes, P.M.; Brower, L.P. Overwintering ecology of the monarch butterfly, Danaus plexippus L., in California. Ecol. Entomol. 1978, 3, 141–153.
- Chaplin, S.B.; Wells, P.H. Energy reserves and metabolic expenditures of monarch butterflies overwintering in southern California. Ecol. Entomol. 1982, 7, 249–256.
- Oberhauser, K.; Ries, L.; Altizer, S.; Batalden, R.V.; Kudell-Ekstrum, J.; Garland, M.; Howard, E.; Jepsen, S.; Lovett, J.; Monroe, M.; et al. Contributions to monarch biology and conservation through citizen science: Seventy years and counting. In Monarchs in a Changing World: Biology and Conservation of an Iconic Butterfly; Oberhauser, K.S., Nail, K.R., Altizer, S., Eds.; Cornell University Press: Ithaca, NY, USA, 2015; pp. 13–29.
- Williams, E.H.; Brower, L.P. A conservation concern: How many monarchs are there? News Lepid. Soc. 2016, 58, 90–93.
- Brower, L.P.; Taylor, O.R.; Williams, E.H.; Slayback, D.A.; Zubieta, R.R.; Ramirez, I. Decline of monarch butterflies overwintering in Mexico: Is the migratory phenomenon at risk? Insect Cons. Divers. 2012, 5, 95–100.
- Pleasants, J.M.; Oberhauser, K. Milkweed loss in agricultural fields because of herbicide use: Effect on the monarch butterfly population. Insect Cons. Divers. 2013, 6, 135–144.
- Yu, Z.; Lu, C. Historical cropland expansion and abandonment in the continental US during 1850 to 2016. Glob. Ecol. Biogeogr. 2017, 27, 322–333.
- Thogmartin, W.E.; Widerholt, R.; Oberhauser, K.; Drum, R.G.; Diffendorfer, J.E.; Altizer, S.; Taylor, O.R.; Pleasants, J.; Semmens, D.; Semmens, B.; et al. Monarch butterfly decline: Identifying the threatening processes. Roy. Soc. Open Sci. 2017, 4, 170760.
- Flockhart, D.T.T.; Pichancourt, J.; Norris, D.R.; Martin, T.G. Unravelling the annual cycle in a migratory animal: Breeding season habitat loss drives population declines of monarch butterflies. J. Anim. Ecol. 2015, 84, 155–165.
- Jeschke, P.; Nauen, R. Neonicotinoids-from zero to hero in insecticide chemistry. Pest Sci. Man. 2008, 64, 1084–1098.
- Douglas, M.R.; Tooker, J.F. Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in US field crops. Environ. Sci. Technol. 2015, 49, 5088–5097.
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Codova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D.; et al. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification-a tool for resistance management. Pest. Biochem. Phys. 2020, 167, 104587.
- Godfray, H.C.J.; Blacquiere, T.; Field, L.M.; Hails, R.S.; Petrokofsky, G.; Potts, S.G.; Raine, N.E.; Vanbergen, A.J.; McLean, A.R. A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140558.
- Lu, C.; Hung, Y.T.; Cheng, Q. A review of sub-lethal neonicotinoid insecticides exposure and effects on pollinators. Curr. Pollut. Rep. 2020, 6, 137–151.
- Singla, A.; Barmota, H.; Sahoo, K.J.; Kang, B.K. Influence of neonicotinoids on pollinators: A review. J. Apic. Res. 2021, 60, 19–32.
- Gilburn, A.S.; Bunnefield, N.; Wilson, J.M.; Botham, M.S.; Brereton, T.M.; Fox, R.; Goulson, D. Are neonicotinoid insecticides driving declines of widespread butterflies? Peer J. 2015, 3, e1402.
- Forister, M.L.; Cousens, B.; Harrison, J.G.; Anderson, K.; Thorne, J.H.; Waetjen, D.; Nice, C.C.; Parsia, M.D.; Hladik, M.L.; Meese, R.; et al. Increasing neonicotinoid use and the declining butterfly fauna of lowland California. Biol. Lett. 2016, 12, 20160475.
- Alkassab, A.T.; Kirchner, W.H. Sublethal exposure to neonicotinoids and related side effects on insect pollinators: Honeybees, bumblebees and solitary bees. J. Plant Dis. Prot. 2017, 124, 1–30.
- Siviter, H.; Richman, S.K.; Muth, F. Field-realistic neonicotinoid exposure has sub-lethal effects on non-Apis bees: A meta-analysis. Ecol. Lett. 2021, 24, 2586–2597.
- Braak, N.; Neve, R.; Jones, A.K.; Gibbs, M.; Breuker, C.J. The effects of insecticides on butterflies: A review. Environ. Pollut. 2018, 242, 507–518.
- Pecenka, J.R.; Lundgren, J.G. Non-target effects of clothianidin on monarch butterflies. Sci. Nat. 2015, 102, 19.
- Krischik, V.; Rogers, M.; Gupta, G.; Varshney, A. Soil-applied imidacloprid translocates to ornamental flowers and reduces survival of adult Coleomegilla maculata, Harmonia axyridis and Hippodamia convergens Lady beetles and larval Danaus plexippus and Vanessa cardui butterflies. PLoS ONE 2015, 10, 1371.
- Krishnan, N.; Zhang, Y.; Aust, M.E.; Hellmich, R.L.; Coats, J.R.; Bradbury, S.P. Monarch butterfly (Danaus plexippus) life-stage risks from foliar and seed treatment insecticides. Environ. Tox. Chem. 2021, 40, 1761–1777.
- Krishnan, N.; Jurenka, R.A.; Bradbury, S.P. Neonicotinoids can cause arrested pupal ecdysis in lepidoptera. Sci. Rep. 2021, 11, 15787.
- Jeschke, P.; Nauen, R.; Schindler, M.; Elbert, A. Overview of the status and global strategy for neonicotinoids. J. Agric. Food Chem. 2011, 59, 2897–2908.
- David, A.; Botias, C.; Abdul-Sada, A.; Nicholls, E.; Rotheray, E.L.; Hill, E.M.; Goulson, D. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 2016, 88, 169–178.
- Douglas, M.; Rohr, J.R.; Tooker, J.F. Neonicotinoid insecticide travels through a soil food chain, disrupting biological control of non-target pests and decreasing soya bean yield. J. Appl. Ecol. 2014, 52, 250–260.
- Sanchez-Bayo, F.; Hyne, R.V. Detection and analysis of neonicotinoids in river waters-development of a passive sampler for three commonly used insecticides. Chemosphere 2014, 99, 143–151.
- Bottias, C.; David, A.; Hill, E.M.; Goulson, D. Contamination of wild plants near neonicotinoid seed-treated crops and implications for non-target insects. Sci. Total Environ. 2016, 566, 269–278.
- Hladik, M.L.; Kolpin, D.W.; Kuivila, K.M. Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA. Environ. Pollut. 2014, 193, 189–196.
- Morrissey, C.A.; Mineau, P.; Devries, J.H.; Sanchez-Bayo, F.; Liess, M.; Cavallaro, M.C.; Liber, K. Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: A review. Environ. Int. 2015, 74, 291–303.
- Schmidt, T.S.; Miller, J.L.; Mahler, B.J.; Van Metre, P.C.; Nowell, L.H.; Sandstrom, M.W.; Carlisle, D.M.; Moran, P.W.; Bradley, P.M. Ecological consequences of neonicotinoid mixtures in streams. Sci. Adv. 2022, 8, eabj8182.
- Main, A.R.; Michel, N.L.; Cavallaro, M.C.; Headley, J.V.; Peru, K.M.; Morrissey, C.A. Snowmelt transport of neonicotinoid insecticides to Canadian prairie wetlands. Agric. Ecosys. Environ. 2016, 215, 76–84.
- Keim, B. Backyard Pesticide May Fuel Bee Die-Offs. 2012. Available online: www.wired.com/2012/04/neonicotinoids-gardens/ (accessed on 10 June 2023).
- Morrison, B.A.; Xia, K.; Stewart, R.D. Evaluating neonicotinoid insecticide uptake by plants used as buffers and cover crops. Chemosphere 2023, 322, 138154.
- James, D.G. A neonicotinoid insecticide at a rate found in nectar reduces longevity but not oogenesis in monarch butterflies, Danaus plexippus (L.) (Lepidoptera: Nymphalidae). Insects 2019, 10, 276.
- Prouty, C.; Bartlett, L.J.; Krischik, V.; Altizer, S. Adult monarch butterflies show high tolerance to neonicotinoid insecticides. Ecol. Entomol. 2023, 48, 531–543.
- Mullin, C.A. Effects of ‘inactive’ ingredients on bees. Curr. Opin. Insect Sci. 2015, 10, 194–200.
- Mesnage, R.; Antoniou, M.N. Ignoring adjuvant toxicity falsifies the safety profile of commercial pesticides. Front. Public Health 2018, 5, 361.
- Nagy, K.; Duca, R.C.; Lovas, S.; Creta, M.; Scheepers, P.; Godderis, L.; Adam, B. Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ. Res. 2020, 181, 108926.
- Krupke, C.H.; Holland, J.D.; Long, E.Y.; Eltzer, B.D. Planting of neonicotinoid-treated maize poses risks for honey bees and other non-target organisms over a wide area without consistent crop yield benefit. J. Appl. Ecol. 2017, 54, 1449–1458.
- Bonmatin, J.M.; Giorio, C.; Girolami, V.; Goulson, D.; Kreutzwizer, D.P.; Krupke, C.; Liess, M.; Long, E.; Marzaro, M.; Mitchell, E.A.D.; et al. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 2014, 22, 35–67.
- Thompson, D.A.; Lehmler, H.J.; Kolpin, D.W.; Hladik, M.L.; Vargo, J.D.; Schilling, K.E.; LeFevre, G.H.; Peeples, T.L.; Poch, M.C.; LaDuca, L.E.; et al. A critical review on the potential impacts of neonicotinoid insecticide use: Current knowledge of environmental fate, toxicity and implications for human health. Environ. Sci. Process. Impacts 2020, 22, 1315–1346.
- Thompson, D.A.; Kolpin, D.W.; Hladik, M.L.; Lehmler, H.J.; Meppelink, S.M.; Poch, M.C.; Vargo, J.D.; Soupene, V.A.; Irfan, N.M.; Robinson, M.; et al. Prevalence of neonicotinoid insecticides in paired private-well tap water and human urine samples in a region of intense agriculture overlying vulnerable aquifers in eastern Iowa. Chemosphere 2023, 319, 137904.
- Zhou, Y.; Guo, J.; Wang, Z.; Sun, Z.; Yun, X.; Zhang, J. Levels and inhalation risk of neonicotinoid insecticides in fine particulate matter in urban and rural areas of China. Environ. Int. 2020, 142, 105822.
- Zhang, D.; Lu, S. Human exposure to neonicotinoids and the associated health risks. Environ. Int. 2022, 163, 107201.
- Chen, Y.; Yu, W.; Zhang, L.; Cao, L.; Ling, J.; Liao, K.; Shen, G.; Du, W.; Chen, K.; Zhao, M.; et al. First evidence of neonicotinoid insecticides in human bile and associated hepatotoxicity risk. J. Hazard. Mater. 2023, 446, 130715.
- Frank, S.D.; Tooker, J.F. Neonicotinoids pose undocumented threats to food webs. Proc. Natl. Acad. Sci. USA 2020, 117, 22609–22613.
- Kremer, A.N.; King, B.H. A neonicotinoid affects the mating behavior of Spalangia endius (Hymenoptera: Pteromalidae), a biological control agent of filth flies. Environ. Entomol. 2019, 48, 489–495.
- Muth, F.; Leonard, A.S. A neonicotinoid pesticide impairs foraging, but not learning, in free-flying bumblebees. Sci. Rep. 2019, 9, 4764.
- Gill, R.J.; Raine, N.E. Chronic impairment of bumblebee natural foraging behavior induced by sublethal pesticide exposure. Funct. Ecol. 2014, 28, 1459–1471.
- Eng, M.L.; Stutchbury, B.J.M.; Morrissey, C.A. Imidacloprid and chlorpyrifos insecticides impair migratory ability in a seed-eating songbird. Sci. Rep. 2017, 7, 15176.
- Eng, M.L.; Stutchbury, B.J.M.; Morrissey, C.A. A neonicotinoid reduces fueling and delays migration in songbirds. Science 2019, 365, 1177–1180.
- English, S.G.; Sandoval-Herrera, N.I.; Bishop, C.A.; Bishop, M.; Maisonneuve, F.; Elliot, J.E.; Welch, K.C., Jr. Neonicotinoid pesticides exert metabolic effects on avian pollinators. Sci. Rep. 2021, 11, 2914.
- Hallman, C.A.; Foppen, R.B.D.; Van Turnhout, C.A.M.; De Kroon, H.; Jongejans, E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 2014, 511, 341–343.
- Erti, H.M.H.; Mora, M.A.; Brightsmith, D.J.; Navarro-Alberto, J.A. Potential impact of neonicotinoid use on Northern Bobwhite (Colinus virginianus) in Texas: A historical analysis. PLoS ONE. 2018, 13, e0191100.
- Brower, L.P.; Williams, E.H.; Jaramillo-Lopez, P.; Kust, D.R.; Slayback, D.A.; Ramirez, M.I. Butterfly mortality and salvage logging from the March 2016 storm in the monarch butterfly biosphere reserve in Mexico. Am. Entomol. 2017, 63, 151–164.
- Crone, E.E.; Schultz, C.B. Resilience or catastrophe? A possible state change for monarch butterflies in western North America. Ecol. Lett. 2021, 24, 1533–1538.
- James, D.G.; Kappen, L. Further insights on the migration biology of monarch butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) from the Pacific Northwest. Insects 2021, 12, 161.
- James, D.G. Do some fall migrants from the Pacific Northwest augment winter breeding populations of monarch butterflies in southern California? J. Lepid. Soc. 2018, 72, 244–246.
- James, D.G. Western North American monarchs: Spiraling into oblivion or adapting to a changing environment? Anim. Migr. 2021, 8, 19–26.
- James, D.G.; Schaefer, M.C.; Easton, K.K.; Carl, A. First population study on winter breeding monarch butterflies, Danaus plexippus (Lepidoptera: Nymphalidae) in the urban south bay of San Francisco. Insects 2021, 12, 946.
- Smither, C.N. A note on overwintering in Danaus plexippus (Linnaeus) (Lepidoptera: Nymphalidae in Australia. Aust. Zool. 1965, 13, 135–136.
- James, D.G. Studies on a winter breeding population of Danaus plexippus (L.) (Lepidoptera: Nymphalidae) at Spencer, New South Wales. Gen. Appl. Ent. 1981, 13, 47–53.
- James, D.G. Population and general biology of non-reproductive colonies of the monarch butterfly, Danaus plexippus (L.) (Lepidoptera: Nymphalidae) in New South Wales. Aust. J. Zool. 1984, 32, 663–670.
- James, D.G. Migration biology of the monarch butterfly in Australia. In Biology and Conservation of the Monarch Butterfly; Malcolm, S.B., Zalucki, M.P., Eds.; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 1993; pp. 189–200.
- Goehring, L.; Oberhauser, K.S. Effects of photoperiod, temperature and host plant age on induction of reproductive diapause and development time in Danaus plexippus. Ecol. Entomol. 2002, 27, 674–685.
- Mansingh, A. Physiological classification of dormancies in insects. Can. Entomol. 1971, 103, 983–1009.
- Howard, E.; Aschen, H.; Davis, A.K. Citizen science observations of monarch butterfly overwintering in the southern United States. Psyche 2010, 2010, 689301.
- Satterfield, D.A.; Maerz, J.C.; Hunter, M.D.; Flockhart, D.T.T.; Hobson, K.A.; Norris, D.R.; Streit, A.; De Roode, J.C.; Altizer, S. Migartory monarchs that encounter resident monarchs show life history differences and higher rates of parasite infection. Ecol. Lett. 2018, 21, 1670–1680.
- James, D.G. Ovarian dormancy in Danaus plexippus (L.) (Lepidoptera: Nymphalidae)-Oligopause not diapause. J. Aust. Ent. Soc. 1982, 21, 31–35.
- Talla, V.; Pierce, A.A.; Adams, K.L.; De Man, T.J.B.; Nallu, S.; Villablanca, F.X.; Kronforst, M.R.; De Roode, J.C. Genomic evidence for gene flow between monarchs with divergent migratory phenotypes and flight performance. Mol. Ecol. 2020, 29, 2567–2582.
- Barker, J.F.; Herman, W.S. Effect of photoperiod and temperature on reproduction of the monarch butterfly. J. Insect Physiol. 1976, 12, 1565–1568.
- James, D.G. Effect of temperature upon energy reserves of the monarch butterfly, Danaus plexippus (L.) (Lepidoptera: Danaidae). Aust. J. Zool. 1986, 34, 373–379.
- Brower, L.P.; Pyle, R.M. The interchange of migratory monarchs between Mexico and the western United States and the importance of floral corridors to the fall and spring migrations. In Conserving Migratory Pollinators and Nectar Corridors in the Western United States; Nabhan, G., Ed.; University of Arizona Press: Tucson, AZ, USA, 2004; pp. 144–166.
- Vane-Wright, R.I. The Columbus hypothesis: An explanation for the dramatic 19th Century range expansion of the monarch butterfly. In Biology and Conservation of the Monarch Butterfly; Malcolm, S.B., Zalucki, M.P., Eds.; Natural History Museum of Los Ange-les County: Los Angeles, CA, USA, 1993; pp. 179–187.
- Zalucki, M.P.; Clarke, A.R. Monarchs across the Pacific: The Columbus hypothesis revisited. Biol. J. Linn. Soc. 2004, 82, 111–121.
- James, D.G. Overwintering Biology of the Monarch Butterfly, Danaus plexippus, in New South Wales. Ph.D. Thesis, Macquarie University, Sydney, NSW, Australia, 1984; p. 199.
- Garcia-Berro, A.; Talla, V.; Vila, R.; Kar Wai, H.; Shipilina, D.; Chan, K.G.; Pierce, N.E.; Backstrom, N.; Talavera, G. Migratory behavior is positively associated with genetic diversity in butterflies. Mol. Ecol. 2023, 32, 560–574.
- Mann, M.E.; Gleick, P.H. Climate change and California drought in the 21st century. Proc. Natl. Acad. Sci. USA 2015, 112, 3858–3859.
- Diffenbaugh, N.S.; Swain, D.L.; Touma, D. Anthropogenic warming has increased drought risk in California. Proc. Natl. Acad. Sci. USA 2015, 112, 3931–3936.
- Urquhart, F.A.; Urquhart, N.R.; Munger, F. A study of a continuously breeding population of Danaus plexippus in southern California compared to a migratory population and its significance in the study of insect movement. J. Res. Lep. 1970, 7, 169–181.
- Neelin, J.D.; Langenbrunner, B.; Meyerson, J.; Hall, A.; Berg, N. California winter precipitation change under global warming in the coupled model intercomparison project phase 5 ensemble. J. Clim. 2013, 26, 6238–6256.
More
Information
Subjects:
Entomology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
604
Revisions:
2 times
(View History)
Update Date:
11 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




