
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shimin Lu | -- | 2043 | 2024-01-11 02:17:56 | | | |
| 2 | Peter Tang | Meta information modification | 2043 | 2024-01-11 06:13:37 | | |
Video Upload Options
Biological nitrification is a crucial process in microalgal–bacterial systems. It oxidizes ammonia (NH3) to nitrate (NO3−) via intermediate nitrite (NO2−), which is mainly conducted by ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB). Light is essential for algae photosynthesis; however, nitrifying bacteria are also influenced by light radiation.
1. Introduction
2. Photoinhibition of AOB
|
Nitrifying Microorganism |
Light Level |
Light Source |
Irradiance Time |
Finding |
References |
|---|---|---|---|---|---|
|
AOB and NOB |
63, 74 μmol m−2·s−1 |
White fluorescent tubes |
Continuous illumination for 40 days |
AOB and NOB were not inhibited by 63 or 74 μmol m−2·s−1 light level |
[12] |
|
AOB and NOB |
105 μmol m−2·s−1 |
White fluorescent tubes |
Continuous illumination for 30 days |
NOB, but not AOB, were inhibited |
|
|
Nitrosomonadaceae AOB and Nitrospiraceae NOB |
≥180 μmol m−2·s−1 |
Cool white LED tubes |
With a dark/light cycle of 12 h/12 h |
NOB were significantly inhibited in the batch reactors |
[33] |
|
Nitrosomonadaceae AOB and Nitrospiraceae NOB |
At 225 μmol m−2·s−1 |
Cool white LED tubes |
With a dark/light cycle of 12 h/12 h |
NO2−-N accumulated in batch reactors |
|
|
Nitrosomonadaceae AOB and Nitrospiraceae NOB |
The average visible and UV light intensities were 42 and 3 mW cm−2 |
Sunlight |
Exposed to sunlight for 61 days |
Nitrifying bacteria were substantially inhibited in algal–bacterial symbiosis |
[29] |
|
Nitrosomonadaceae AOB and Nitrospiraceae NOB |
200 μmol m−2·s−1 |
The light panel was 80% similar to solar light |
10−16 h |
The suppression of light on AOB and NOB positively correlated with the light exposure period |
[10] |
|
Nitrosomonadaceae AOB and Nitrospiraceae NOB |
600 μmol m−2·s−1 |
The light panel was 80% similar to solar light |
4−5 h |
The suppression of light on AOB and NOB positively correlated with the light exposure period |
|
|
Nitrosomonadaceae AOB and Nitrospiraceae NOB |
2000 μmol m−2·s−1 |
The light panel was 80% similar to solar light |
2−4 h |
The suppression of light on AOB and NOB positively correlated with the light exposure period |
|
|
Nitrifying granular sludge |
450 μmol m−2·s−1 |
LED light devices |
12 h |
The activity significantly decreased by 50% compared to the dark condition |
[34] |
|
Nitrifying granular sludge |
1600 μmol m−2·s−1 |
LED light devices |
12 h |
The activity significantly decreased by 70% compared to the dark condition, while in the granular sludge reactors, the activity barely changed |
|
|
Nitrosomonadaceae AOB and Nitrospiraceae NOB |
The average light level was 1531 μmol m−2·s−1 |
Sunlight |
63 days |
Sunlight, algae growth, and free nitrous acid decreased the activity of AOB by 25.7% and completely inhibited NOB activity |
[35] |
|
Nitrosomonas-related AOB and Nitrospira-related NOB |
200 μmol m−2·s−1 |
Cool white light-emitting diodes |
In continuous dark/light (12 h/12 h) cycles |
AOB abundance increased from 0.2% to 2.1%, whereas NOB abundance reduced gradually from 0.07% to below 0.01% |
[36] |
|
Nitrifying bacterial |
Below 250 μmol m−2·s−1 |
LED lamps |
Continuous illumination for 15 days |
No significant effect on nitrification activity |
[37] |
|
Nitrifying bacterial |
At 500 μmol m−2·s−1 |
LED lamps |
Continuous illumination for 15 days |
It decreased NH4+-N removal by 20% and NO3−-N production by 26% |
|
|
Nitrifying bacterial |
At 1250 μmol m−2·s−1 |
LED lamps |
Continuous illumination for 15 days |
It decreased NH4+-N removal by 60% and NO3−-N production by 71% |
|
|
Nitrosomonas AOB and Nitrospira NOB |
From 100 to 50 μmol m−2·s−1 |
Continuous illumination for 105 days |
A syntrophic algal/partial nitrification/anammox granular sludge process was developed |
[38] |
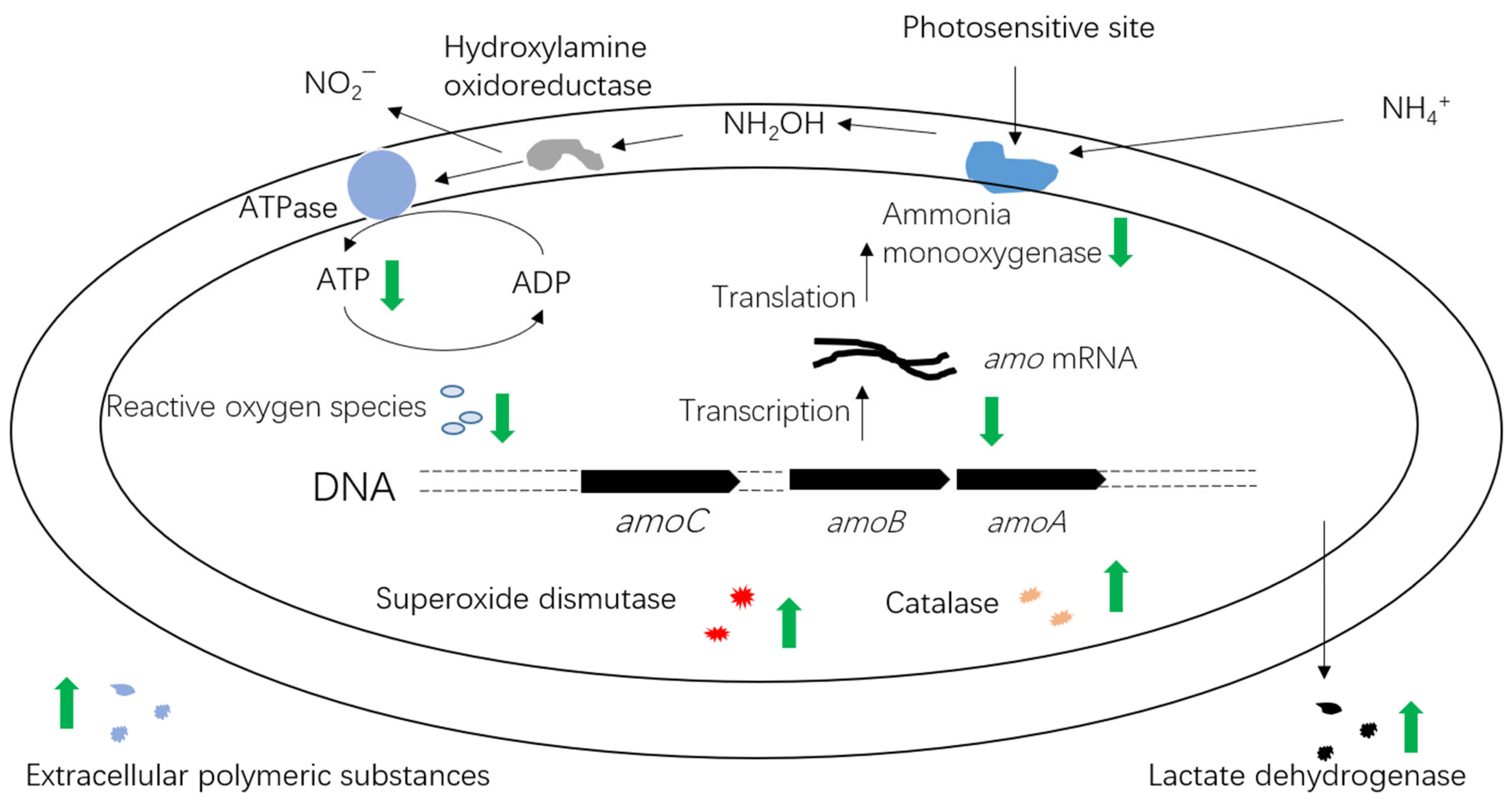
3. Effect of Light on AOB Biodiversity
4. Light Stimulation of AOB Growth
References
- Van Drecht, G.; Bouwman, A.F.; Harrison, J.; Knoop, J.M. Global nitrogen and phosphate in urban wastewater for the period 1970 to 2050. Global Biogeochem. Cycles 2009, 23, GB0A03.
- Rosso, D.; Larson, L.E.; Stenstrom, M.K. Aeration of large-scale municipal wastewater treatment plants: State of the art. Water Sci. Technol. 2008, 57, 973–978.
- Akizuki, S.; Kishi, M.; Cuevas-Rodriguez, G.; Toda, T. Effects of different light conditions on ammonium removal in a consortium of microalgae and partial nitrifying granules. Water Res. 2020, 171, 115445.
- Karya, N.; van der Steen, N.P.; Lens, P.N.L. Photo-oxygenation to support nitrification in an algal-bacterial consortium treating artificial wastewater. Bioresour. Technol. 2013, 134, 244–250.
- Adav, S.S.; Lee, D.J. Extraction of extracellular polymeric substances from aerobic granule with compact interior structure. J. Hazard. Mater. 2008, 154, 1120–1126.
- Wei, X.M.; Sayavedra-Soto, L.A.; Arp, D.J. The transcription of the cbb operon in Nitrosomonas europaea. Microbiology 2004, 150, 1869–1879.
- Soliman, M.; Eldyasti, A. Ammonia-oxidizing bacteria (AOB): Opportunities and applications-a review. Rev. Environ. Sci. Bio-Technol. 2018, 17, 285–321.
- Daims, H.; Lucker, S.; Wagner, M. A New perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol. 2016, 24, 699–712.
- Juliette, L.Y.; Hyman, M.R.; Arp, D.J. Mechanism-based inactivation of ammonia monooxygenase in Nitrosomonas europaea by allylsulfide. Appl. Environ. Microbiol. 1993, 59, 3728–3735.
- Wang, L.F.; Qiu, S.; Guo, J.H.; Ge, S.J. Light irradiation enables rapid start-up of nitritation through suppressing nxrB gene expression and stimulating ammonia oxidizing bacteria. Environ. Sci. Technol. 2021, 55, 13297–13305.
- Nishi, K.; Akizuki, S.; Toda, T.; Matsuyama, T.; Ida, J. Advanced light-tolerant microalgae-nitrifying bacteria consortia for stable ammonia removal under strong light irradiation using light-shielding hydrogel. Chemosphere 2022, 297, 134252.
- Peng, L.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Wang, D.; Song, S.; Wei, W.; Nghiem, L.D.; Ni, B.J. A novel mechanistic model for nitrogen removal in algal-bacterial photo sequencing batch reactors. Bioresour. Technol. 2018, 267, 502–509.
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia-nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358.
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024.
- Lu, S.M.; Liu, X.G.; Liu, C.; Wang, X.D.; Cheng, G.F. Review of ammonia-oxidizing bacteria and archaea in freshwater ponds. Rev. Environ. Sci. Bio-Technol. 2019, 18, 1–10.
- Cho, S.; Fujii, N.; Lee, T.; Okabe, S. Development of a simultaneous partial nitrification and anaerobic ammonia oxidation process in a single reactor. Bioresour. Technol. 2011, 102, 652–659.
- Kim, K.; Park, Y.G. Light as a novel inhibitor of nitrite-oxidizing bacteria (NOB) for the mainstream partial nitrification of wastewater treatment. Processes 2021, 9, 346.
- Schön, G.H.; Engel, H. Der Einfluß des Lichtes auf Nitrosomonas europaea Win. Arch. Mikrobiol. 1962, 42, 415–428.
- Guerrero, M.A. Photoinhibition of marine nitrifying bacteria. I. Wavelength-dependent response. Mar. Ecol. Prog. Ser. 1996, 141, 183–192.
- Liu, Q.; Tolar, B.B.; Ross, M.J.; Cheek, J.B.; Sweeney, C.M.; Wallsgrove, N.J.; Popp, B.N.; Hollibaugh, J.T. Light and temperature control the seasonal distribution of thaumarchaeota in the South Atlantic bight. Isme J. 2018, 12, 1473–1485.
- Peng, X.F.; Fawcett, S.E.; van Oostende, N.; Wolf, M.J.; Marconi, D.; Sigman, D.M.; Ward, B.B. Nitrogen uptake and nitrification in the subarctic North Atlantic Ocean. Limnol. Oceanogr. 2018, 63, 1462–1487.
- Lipschultz, F.; Wofsy, S.C.; Fox, L.E. The effects of light and nutrients on rates of ammonium transformation in a eutrophic river. Mar. Chem. 1985, 16, 329–341.
- Merbt, S.N.; Auguet, J.C.; Casamayor, E.O.; Marti, E. Biofilm recovery in a wastewater treatment plant-influenced stream and spatial segregation of ammonia-oxidizing microbial populations. Limnol. Oceanogr. 2011, 56, 1054–1064.
- Fisher, O.S.; Kenney, G.E.; Ross, M.O.; Ro, S.Y.; Lemma, B.E.; Batelu, S.; Thomas, P.M.; Sosnowski, V.C.; DeHart, C.J.; Kelleher, N.L.; et al. Characterization of a long overlooked copper protein from methane- and ammonia-oxidizing bacteria. Nat. Commun. 2018, 9, 4276.
- Shears, J.H.; Wood, P.M. Spectroscopic evidence for a photosensitive oxygenated state of ammonia mono-oxygenase. Biochem 1985, 226, 499–507.
- Lu, S.M.; Liu, X.G.; Liu, C.; Cheng, G.F.; Shen, H.Y. Influence of photoinhibition on nitrification by ammonia-oxidizing microorganisms in aquatic ecosystems. Rev. Environ. Sci. Bio-Technol. 2020, 19, 531–542.
- Berney, M.; Weilenmann, H.-U.; Egli, T. Gene expression of Escherichia coli in continuous culture during adaptation to artificial sunlight. Environ. Microbiol. 2006, 8, 1635–1647.
- Yang, M.Z.; Qiu, S.; Wang, L.F.; Chen, Z.P.; Hu, Y.B.; Guo, J.H.; Ge, S.J. Effect of short-term light irradiation with varying energy densities on the activities of nitrifiers in wastewater. Water Res. 2022, 216, 118291.
- Huang, W.L.; Li, B.; Zhang, C.; Zhang, Z.Y.; Lei, Z.F.; Lu, B.W.; Zhou, B.B. Effect of algae growth on aerobic granulation and nutrients removal from synthetic wastewater by using sequencing batch reactors. Bioresour. Technol. 2015, 179, 187–192.
- Wu, D.X.; Cheng, M.L.; Zhao, S.M.; Peng, N.; Hu, R.G.; Hu, J.L.; Liang, Y.X. Algal growth enhances light-mediated limitation of bacterial nitrification in an aquaculture system. Water Air Soil Pollut. 2020, 231, 73.
- Smith, J.M.; Chavez, F.P.; Francis, C.A. Ammonium uptake by phytoplankton regulates nitrification in the sunlit ocean. PLoS ONE 2014, 9, e108173.
- Risgaard-Petersen, N.; Nicolaisen, M.H.; Revsbech, N.P.; Lomstein, B.A. Competition between ammonia-oxidizing bacteria and benthic microalgae. Appl. Environ. Microbiol. 2004, 70, 5528–5537.
- Meng, F.S.; Xi, L.M.; Liu, D.F.; Huang, W.W.; Lei, Z.F.; Zhang, Z.Y.; Huang, W.L. Effects of light intensity on oxygen distribution, lipid production and biological community of algal-bacterial granules in photo-sequencing batch reactors. Bioresour. Technol. 2019, 272, 473–481.
- Akizuki, S.; Natori, N.; Cuevas-Rodriguez, G.; Toda, T. Application of nitrifying granular sludge for stable ammonium oxidation under intensive light. Biochem. Eng. J. 2020, 160, 107631.
- Huang, W.L.; Liu, D.F.; Huang, W.W.; Cai, W.; Zhang, Z.Y.; Lei, Z.F. Achieving partial nitrification and high lipid production in an algal-bacterial granule system when treating low COD/NH4-N wastewater. Chemosphere 2020, 248, 126106.
- Si, G.C.; Liu, B.; Liu, Y.R.; Yan, T.; Wei, D. Light-introduced partial nitrification in an algal-bacterial granular sludge bioreactor: Performance evolution and microbial community shift. Bioresour. Technol. 2022, 354, 127226.
- Vergara, C.; Muñoz, R.; Campos, J.L.; Seeger, M.; Jeison, D. Influence of light intensity on bacterial nitrifying activity in algal-bacterial photobioreactors and its implications for microalgae-based wastewater treatment. Int. Biodeterior. Biodegrad. 2016, 114, 116–121.
- Kong, L.R.; Zheng, R.; Feng, Y.M.; Du, W.R.; Xie, C.; Gu, Y.Q.; Liu, S.T. Anammox bacteria adapt to long-term light irradiation in photogranules. Water Res. 2023, 241, 120144.
- Hyman, M.R.; Arp, D. 14C2H2-and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J. Biol. Chem. 1992, 267, 1534–1545.
- Merbt, S.N.; Stahl, D.A.; Casamayor, E.O.; Marti, E.; Nicol, G.W.; Prosser, J.I. Differential photoinhibition of bacterial and archaeal ammonia oxidation. FEMS Microbiol. Lett. 2012, 327, 41–46.
- Harris, S.H.; Smith, R.L. In situ measurements of microbially-catalyzed nitrification and nitrate reduction rates in an ephemeral drainage channel receiving water from coalbed natural gas discharge, Powder River Basin, Wyoming, USA. Chem. Geol. 2009, 267, 77–84.
- Lei, Y.Z. Environmental Chemistry of Aquaculture Water; China Aguriculture Press: Beijing, China, 2003.
- Crab, R.; Defoirdt, T.; Bossier, P.; Verstraete, W. Biofloc technology in aquaculture: Beneficial effects and future challenges. Aquaculture 2012, 356, 351–356.




