The global discharge of nitrogen into wastewater is expected to increase from 6.4 Tg-N yr
−1 in 2000 to 12.0–15.5 Tg-N yr
−1 in 2050 [
1], and nitrogen levels in water have become one of the most important pollution problems facing humanity today. Currently, biological nitrification and denitrification processes mainly remove nitrogen from wastewater. Biological nitrification is an aerobic process. However, aeration, which is required for this process, is an energy-intensive and costly process, potentially accounting for 45–75% of energy consumption in mechanized wastewater treatment plants [
2]. The use of microalgal–bacterial systems to remove nitrogen from wastewater has gained attention over the past decade as an alternative to conventional nitrification, owing to their potential to reduce the energy consumption associated with mechanical oxygenation [
3,
4]. In addition to reducing the energy requirements associated with mechanical oxygenation, the system also offers several advantages, such as excellent settleability, high biomass production, and the ability to withstand toxicity and organic loading [
5].
Biological nitrification is a crucial process in microalgal–bacterial systems. It oxidizes ammonia (NH
3) to nitrate (NO
3−) via intermediate nitrite (NO
2−), which is mainly conducted by ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB). Ammonia-oxidizing bacteria participate in the carbon (C) and nitrogen (N) cycles and are thus involved in environmental processes. AOB assimilate carbon dioxide (CO
2) via the Calvin–Benson–Bassham (CBB) cycle. The enzyme that catalyzes CO
2 fixation in AOB is encoded by the
cbb genes [
6]. The oxidation of NH
3 to NO
2− requires two steps [
7]. First, NH
3 is oxidized to hydroxylamine (NH
2OH) by ammonia monooxygenase (AMO), and then NH
2OH is further oxidized to NO
2− by hydroxylamine reductase [
7]. The key NOB enzyme, nitrite oxidoreductase (NXR), catalyzes the oxidation of nitrite to nitrate and can also facilitate the reduction of nitrate to nitrite. The
nxrA and the
nxrB genes are powerful functional and phylogenetic markers that can detect and identify uncultured NOB [
8].
Light is an essential ecological factor for algae photosynthesis, yet the impact of light on nitrifying bacteria is also significant. Previous studies have shown that strong light radiation causes photo-oxidation damage to the AMO in
Nitrosomonas europaea AOB, decreasing their ammonia oxidation activity [
9]. In addition, light has also been shown to have a significantly negative effect on NOB in microalgal–bacterial systems [
10]. In contrast, a recent study showed that appropriate light irradiation stimulated AOB growth [
10]. Furthermore, previous research indicates that nitrification is closely related to light levels in algal–bacterial systems [
10,
11,
12]. To save mechanical oxygenation and control the ammonia concentration of aquaculture water, the algal–bacterial symbiosis approach has been widely used in pond aquaculture [
13,
14,
15]. With the development of anammox in recent years, the combined process of partial nitrification and anammox, utilizing light to suppress NOB, has garnered increasing interest [
16,
17].
2. Photoinhibition of AOB
The photoinhibition of AOB was discovered under laboratory cultivation conditions in 1962 [
18]. Subsequently, the photoinhibition of nitrification has been found in many natural aquatic environments, such as oceans [
19,
20,
21] and rivers [
22,
23]. Furthermore, in the past decade, it has been shown that the photoinhibition of AOB also widely occurs in microalgal–bacterial systems for treating artificial wastewater (
Table 1).
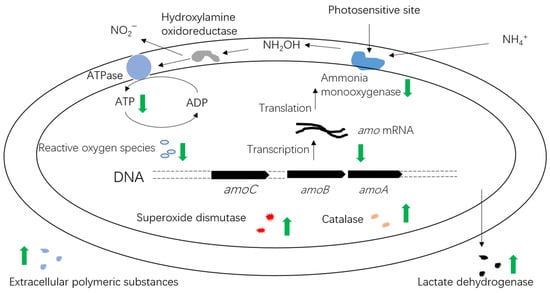
As shown in
Figure 1, previous research indicated that the photoinhibition of AOB by visible light was mainly caused by irreversible damage to copper-containing AMO [
27,
28]. Subsequently, Lu et al. deduced that the conformational change in AMO was likely involved in converting NH
3 to NH
2OH. During this conformational change, if the photosensitive sites of AMO are exposed to visible light, AMO becomes inactivated. Additionally, the synthesis of new AMO is necessary for recovery post-photoinactivation [
29]. The extent of photoinhibition increases as the wavelength decreases in the 300–623 nm range, while recovery from photoinactivity accelerates with increasing wavelength [
29]. Recent studies have shown that
amoA gene expression is downregulated when AOB are exposed to white light radiation in a microalgal–bacterial system, shedding light on the mechanism of AOB photoinhibition at the genetic level [
10]. In addition, light shock could lead to cellular metabolic disorders and membrane oxidation (
Figure 1). After light shock, the activities of two antioxidant enzymes, superoxide dismutase (SOD) and catalase (CAT), increase, while the levels of reactive oxygen species (ROS) decrease. Near-UV radiation (300–400 nm) can damage nucleic acids, cell membranes, and more, affecting not just AMO [
29,
30]. It is possible that lactate dehydrogenase (LDH) is released following light shock due to the cell membrane damage caused by near-UV radiation [
31]. AOB may secrete excessive extracellular polymeric substances (EPSs) under light stress to improve their resistance to external light stress.
Besides directly damaging AOB cells, light can also indirectly inhibit AOB through microalgae [
32,
33,
34]. This is because microalgae are more competitive for nitrogen than AOB in microalgal–bacterial systems with low ammonia concentrations [
32,
33,
34,
35]. In microalgal–bacterial systems for treating wastewater with a high ammonia content, the competitive advantage of microalgae in carbon sources offers the most plausible explanation for their inhibitory effect on AOB or NOB. It was reported that
Nitrosomonas europaea grew poorly in Na
2CO
3-deficient media, where C was available only from the atmosphere, and when the cultures exhibited a long lag phase (~5 days, compared to 1 day in a carbonate-containing medium) [
6]. Microalgae utilize CO
2 for photosynthesis, thereby lowering the concentration of carbonate ions and increasing the oxygen concentration in water. Evidence has suggested that the transcription of
cbb genes is upregulated when the carbon source is limited, while the
amo,
hao, and other energy-harvesting-related genes are downregulated. Consequently, the ammonia oxidation ability of AOB becomes weak, leading to light suppression [
6].
Table 1. Effect of light level on nitrification activities reported by different studies.
Figure 1. Possible mechanisms of photoinhibition of ammonia-oxidizing bacteria (AOB) include the location of photosensitive sites on ammonia monooxygenase (AMO) that are susceptible to visible light [
29]. When exposed to intense light, AMO becomes irreversibly deactivated [
40]. Light radiation can lead to cellular metabolic disorders and membrane oxidation. In this context, the activities of superoxide dismutase (SOD) and catalase (CAT) increase, while the levels of reactive oxygen species (ROS) decrease. Additionally, the release rate of lactate dehydrogenase (LDH) and the production of extracellular polymeric substances (EPSs) increase [
31]. The expression of the
amoA gene is downregulated [
10], leading to a weakened ammonia oxidation ability in AOB. The green arrow in the blue box means the activities of two antioxidant enzymes, superoxide dismutase (SOD) and catalase (CAT) increase, after light shock.
3. Effect of Light on AOB Biodiversity
Previous studies have shown that photoinhibition is associated with specific AOB strains under pure culture conditions. For example, Merbt et al. reported that
Nitrosomonas europaea ATCC19718 was more sensitive than
Nitrosospira multiformis ATCC25196, with decreases in specific growth rates of 91% and 41%, respectively, at a light level of 60 μmol m
−2·s
−1 [
26]. It has also been suggested that light plays a selective role in AOB biodiversity in a microalgal–bacterial system. For example, after prolonged exposure to light in light-treated reactors, the population of the genus
Nitrosomonadaceae Ellin6067 doubled, the number of AOBs in the other four genera reduced, and
Nitrosomonas (a typical AOB) disappeared [
10]. The percentage of
Nitrosomonadaceae gradually increased from 2% to 4% under intense light illumination in photo-sequencing batch reactors, and no other AOB were detected [
36]. Similarly, a study by Kim and Park [
17] showed that the proportion of the family
Nitrosomonadaceae increased from 0.126% to 0.379% after blue light illumination. Recently, it was reported that
Nitrosomonas spp., when coupled with anammox bacteria, could adapt to long-term light irradiation in photogranules, successfully establishing a synthetic algal/partial discrimination/anammox gross sludge process [
39]. As described in numerous studies,
Nitrosomonadaceae AOB exhibit a flexible response to light irradiation, making it advantageous over other AOB types in the microalgal–bacterial system.
4. Light Stimulation of AOB Growth
Recent studies have shown that an optimum light level could stimulate the growth of AOB in microalgal–bacterial granular reactors. Wang et al. [
10] first reported the stimulatory effects of energy densities of 0.03−0.08 kJ mg
−1 VSSs (volatile suspended solids), corresponding to 80–160 W and 400–1000 μmol m
−2·s
−1 for 2.0–5.0 h at concentrations of 2750–4250 mg L
−1, on AOB in sequencing batch reactors when treating real or synthetic municipal wastewater. Subsequently, Yang et al. [
31] confirmed that light could increase AOB activity by 120% at a specific light energy density (Es) ranging from 0.0203 to 0.1571 kJ·mg
−1 VSS. These stimulatory effects were supported by increased electron transport system activity, key enzyme activity (AMO), gene expression (
amoA), and energy generation (ATP consumption) during light treatment. To date, few reports exist on the stimulation of AOB growth by light irradiation in pure cultures. It is possible that light itself does not stimulate AOB growth. It is likely that the appropriate light needed to promote AOB growth is closely related to algae metabolic activity. The nitrification process, as shown in stoichiometric Equation (1) [
41], produces 4 mol of H
+ for 2 mol of ammonia consumed, which can result in a decrease in solution pH. The photosynthesis process in algae can be represented by stoichiometric Equation (2) [
42], where, for every 106 mol of CO
2 assimilated, 18 mol of H
+ is consumed, generating 138 mol of O
2. Photosynthesis not only consumes the H
+ generated during nitrification but also provides substrate O
2 for nitrification. Furthermore, nitrification bacteria and algae generally coexist in the system as bioflocs [
43], meaning that nitrification and photosynthesis simultaneously occur in a very small space, and the metabolites produced by the two reaction processes can be rapidly transported away. Therefore, appropriate light can stimulate the growth of AOB in microalgal–bacterial granular reactors more effectively than dark conditions.