
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | William Parker | -- | 2978 | 2024-01-10 15:18:42 | | | |
| 2 | Rita Xu | -10 word(s) | 2968 | 2024-01-11 03:22:19 | | | | |
| 3 | William Parker | + 204 word(s) | 3172 | 2025-07-18 03:22:48 | | |
Video Upload Options
Based on available data that include approximately 20 lines of evidence from studies in laboratory animal models, observations in humans, correlations in time, and pharmacological/toxicological considerations, it has been concluded without reasonable doubt and with no evidence to the contrary that exposure of susceptible babies and children to acetaminophen (paracetamol) induces many, if not most, cases of autism spectrum disorder (ASD).
1. Introduction
Acetaminophen became widely available in the 1950s and eventually became one of the mostly frequently used medications for the treatment of pains and fevers. By the 1970s, a consensus was reached in the medical community that acetaminophen is safe and effective when used as directed in the pediatric population [1]. Following this consensus, the discovery of an association between aspirin exposure and Reye Syndrome in the early 1980s led to increased dependence on acetaminophen for treatment of pediatric populations, with both prescription and over-the-counter formulations available.
As early as 1999 it was noted that many children with ASD have difficulty metabolizing acetaminophen [2]. Although it had long been understood that difficulty with acetaminophen metabolism increases the toxicity of the drug [3], the observation that individuals with ASD had difficulty with acetaminophen metabolism did not, prior to 2008, elicit the idea that acetaminophen might be a source of injury in individuals with ASD. After decades of acetaminophen use in the pediatric population, strongly encouraged by direct-to-consumer advertising by pharmaceutical manufacturers, a case-controlled study published by Stephen Schultz in 2008 involving 81 children with autism spectrum disorder (ASD) found a 20-fold greater risk of regressive ASD when acetaminophen was used between 12 and 18 months of age compared to controls [4]. Two years later, studies in adult laboratory rats show neuronal cell death with non-lethal doses of acetaminophen [5]. Five years after the study by Schultz, the first study in neonatal laboratory animals using acetaminophen showed profound neurodevelopmental injury at doses only 2-fold higher than doses administered to human children [6]. Subsequent evaluation of safety claims found that the consensus of safety was based on the false assumption that acetaminophen is metabolized the same in babies and in children as in adults [1]. By 2022, mounting evidence that acetaminophen (paracetamol) use in susceptible babies and children is associated with the development of ASD and other neurodevelopmental disorders was overwhelming [7][8][9]. Based on approximately 20 independent lines of evidence, it was concluded without reasonable doubt and with no evidence to the contrary that acetaminophen administration in susceptible babies and children is a causative agent for the induction of many, if not most, cases of ASD [7][8][9]. The conclusion was based on (a) studies in laboratory animal models [10][11][12][13][14][15], (b) understanding of the pharmacological mechanisms associated with acetaminophen toxicity [16], (c) connections between ASD, acetaminophen exposure, and human activities such as vaccination [4] and circumcision [17], (d) associations between acetaminophen administration and ASD during the later stages of pregnancy [18] and in early childhood [4][19], and (e) associations between acetaminophen use and ASD through time [16][20].
2. Summary of Evidence
Fourteen associations between acetaminophen use during early neurodevelopment and ASD were evident as of late 2023 [7]. A summary of those associations, along with additional lines of evidence available at that time [7] and in a follow-up study [21] are as follows:
- Association #1: Circumcision of males, often performed using acetaminophen as an analgesic, is associated with a 2-fold increase in the risk for early-onset (infantile) ASD [17].
- Association #2: Acetaminophen-containing products used by South Korean children were repeatedly found to contain amounts of drug exceeding the package label [22], and an exceptionally high prevalence of ASD was identified in South Korea [23][24].
- Association #3: The popularity of acetaminophen use and the prevalence of ASD was substantially higher in Denmark than in Finland in the mid-2000s (Figure 1).
- Association #4: Ultra-Orthodox Jews [25] and Arabs [25][26] in Israel have a reported prevalence of ASD less than half of that of other Israelis. Traditional circumcision practices employed by Ultra-Orthodox Jews do not utilize acetaminophen, and circumcision practices in Arab communities take place outside of the neurodevelopmental window sensitive to ASD induction (Figure 3).
- Association #5: Analysis of 61,430 babies in the Danish National Birth Cohort found an odds ratio (OR) of 1.3 (CI 1.02-1.66) for ASD associated with postnatal acetaminophen exposure [19]. The approach used in the analysis is expected to dramatically underestimate the real odds ratio [8]. Other studies have supported this association [27][28].
- Association #6: The ratio of regressive to infantile ASD rose at the same time as pediatric acetaminophen use rose [20] after aspirin was associated with Reye’s syndrome [16].
- Association #7: The prevalence of ASD began to increase in the early 1980s, coinciding with the increase in acetaminophen use after aspirin was associated with Reye’s syndrome [16].
- Association #8: The prevalence of ASD has steadily increased [16] as direct-to-consumer advertising [29] and perhaps other factors have driven up use of pharmaceutical products. In 1998, Forbes Magazine reported that the primary manufacturer of acetaminophen in the US spent $250 million on advertising for acetaminophen containing products in the US in one year [30].
- Association #9: Maternal use of acetaminophen during pregnancy is associated with long-term effects that include lower IQ, increased ASD, and increased ADHD in their children [18][19][31][32][33][34][35][36][37][38][39][40][41][42][43].
- Association #10: Levels of acetaminophen in cord blood are associated with ASD [35].
- Association #11: Acetaminophen given alongside the MMR vaccine but not the MMR vaccination alone was associated with ASD [4].
- Association #12: Acetaminophen use during early childhood is associated with a dramatic increase in regressive ASD [4].
- Association #13: Many parents believe that their children’s ASD was induced by a vaccine [44][45]. acetaminophen is frequently used with vaccinations, although vaccinations alone do not cause ASD [46][47].
- Association #14: Cystic fibrosis is associated with unusually efficient (effective) metabolism of acetaminophen [48][49], and evidence suggests that the prevalence of ASD is very low in patients with cystic fibrosis [16].
- Plausible mechanism #1: Acetaminophen use in adults temporarily blunts social trust [50] and awareness [51], emotional responses to external stimuli [52], and the ability to identify errors [53], indicating that the drug targets regions of the brain affected in patients with ASD.
- Plausible mechanism #2: Genetic and immune factors associated with an increased risk of ASD have a detrimental effect on the body’s ability to metabolize acetaminophen [2][16][54]. Difficulties in metabolizing acetaminophen have long been known increase the toxicity of the drug [3].
- Plausible mechanism #3: Acetaminophen is known to be highly toxic in the presence of oxidative stress [55] via a mechanism that involves the formation of the toxic metabolite, NAPQI [56][57][58] and concomitant mitochondrial damage [59]. Oxidative stress [16] and possibly mitochondrial dysfunction [60] also play a role in ASD.
- Laboratory animal studies #1: Early life exposure to acetaminophen at doses similar to or even less than doses received by human babies and children results in long term, profound modification of brain function in both laboratory mice and rats [12][13][14][15][61], by definition a severe adverse event that would have precluded any clinical testing of acetaminophen in babies and small children.
- Laboratory animal studies #2: In laboratory rats, acetaminophen affects the developing male brain more than the female brain [15]. In laboratory mice, males are more susceptible to acetaminophen-mediated liver injury than are females [62]. ASD is more prevalent in males than in females [63].
- Laboratory animal studies #3: Acetaminophen causes apoptosis-mediated death of cortical neurons in adult laboratory rats at concentrations lower than it causes liver failure [5]. Affected cortical neurons are implicated in ASD [64][65], and individuals with ASD have increased levels of biomarkers for neuronal apoptosis [66][67][68].
- Observation in veterinary medicine: Adult cats are susceptible to acetaminophen-mediated injury due to the lack of a robust glucuronidation-dependent capacity for metabolism [69][70][71][72] Human neonates similarly lack a robust glucuronidation-dependent pathway [73][74].
- Underlying assumptions of safety proven to be misguided: Acetaminophen use in babies and children was assumed to be safe during the 1970s because it did not cause liver damage, despite the fact that it targets brain function and was never shown to be safe for neurodevelopment [1].
- ASD and fetal alcohol spectrum disorder (FASD) are similar in many regards [21], demonstrating that a complex spectrum disorder can be induced by a single chemical.
- Alternative explanations for the current prevalence of ASD are not consistent with available evidence [21].
Any one geographic or temporal association might be spurious, possibly unrelated to causality. For example, two independent studies, when taken together, show that the popularity of acetaminophen in two Scandinavian countries, Denmark and Finland, correlated with the prevalence of ASD in those countries (Figure 1). First, the sales of acetaminophen per unit population from 2006 through 2010 in Denmark were more than 2-fold greater than the sales of acetaminophen in Finland during the same time period [75]. Second, for children born in 2006, whose brain development might have been influenced by exposure to acetaminophen between 2006 and 2010, the prevalence of ASD in children born in Denmark was about 70% greater than the prevalence of ASD in children born in Finland [76]. Limitations to the conclusions that can be drawn from this previously unreported geographic association are evident. For example, total sales of acetaminophen do not necessarily reflect use of the drug during early development, when induction of ASD is possible, and therefore this association does not necessarily reflect a direct association between pediatric use of acetaminophen and the prevalence of ASD. Nevertheless, when considered in light of other lines of evidence [7][8][9], this association adds to the burden of evidence demonstrating without reasonable doubt that acetaminophen use in susceptible babies and children causes many if not most cases of ASD.
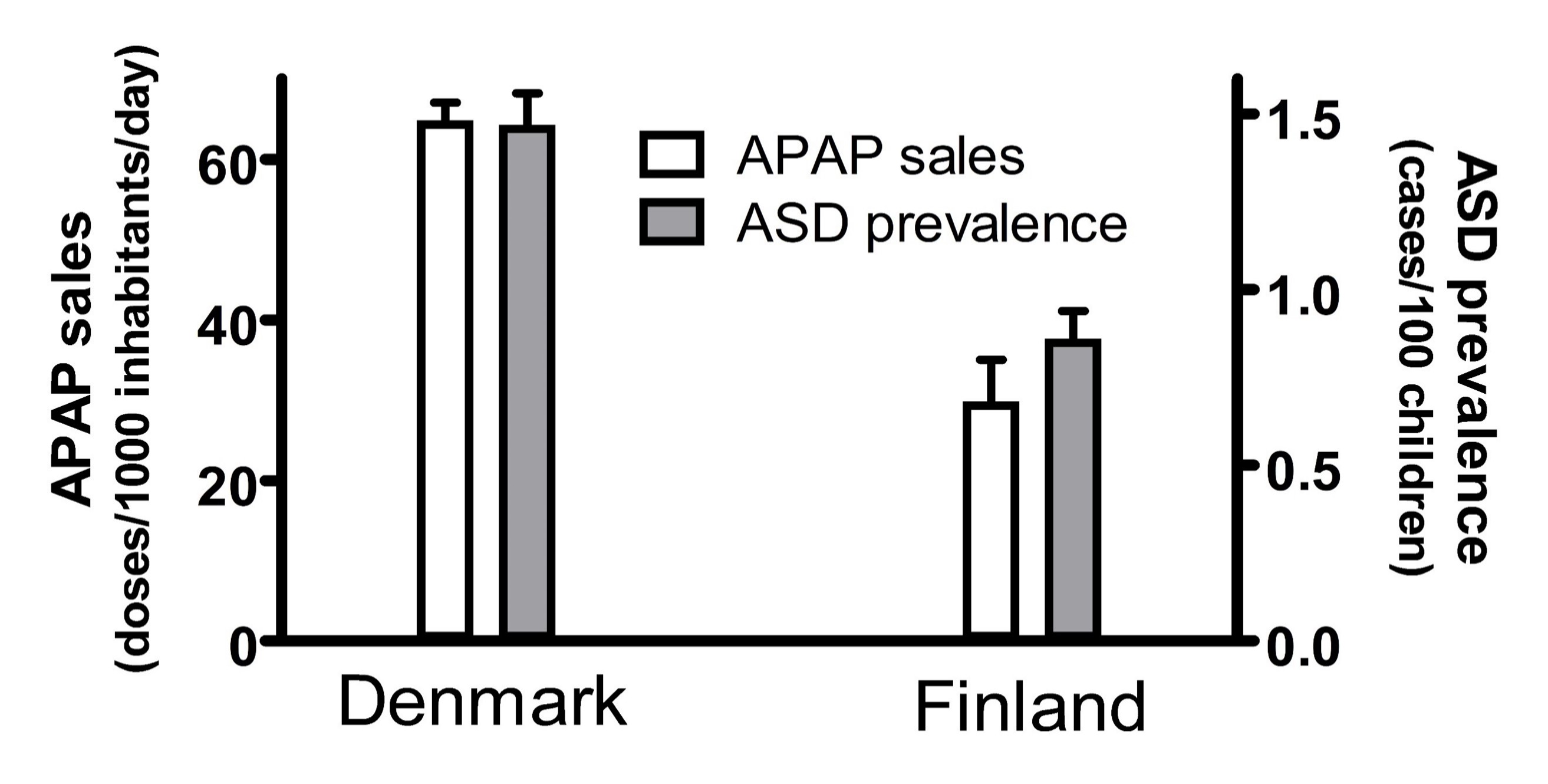
Figure 1. The sales of acetaminophen (APAP) in Denmark and Finland correlated with the prevalence of autism spectrum disorder (ASD) in those countries. The sale of acetaminophen is limited to the sale of drugs containing acetaminophen as the only active ingredient, and is measured in units of a “defined daily doses” (equal to 3 grams of active ingredient) per 1000 inhabitants per day. The sales for each year from 2006 to 2010 were averaged, and the mean plotted. Trends in sales for both Denmark and Finland showed an upward trend every year, and the upper limit of the range (data from 2010) is shown by the error bars. The prevalence of ASD is shown for all children, including males and females, born in 2006 with prevalence measured at 9 years of age. The 95% confidence interval reported by Delobel-Ayoub and colleagues is indicated by the error bars.
A summary of evidence that acetaminophen causes many if not most cases of ASD was published in mid-2022 [8]. In that summary of evidence, it was noted that
“…if paracetamol exposure is not toxic to neurodevelopment, then a number of observations remain unexplained. The tally shown in [the published manuscript] describes six unknown factors that must be invoked to account for all observations, and eight largely independent observations that must be attributed to coincidence.”
McFadden [77] convincingly argues that Occam’s Razor, when applied properly, is a guiding principle in modern science, distinguishing between conspiracy theory and science. Occam’s Razor can be described as the principle that the simplest explanation that accounts for available observations is probably the correct explanation. When applying this Razor, the addition of undefined factors is of considerable concern when used to support an explanation, particularly when such factors need not be employed when another explanation is available. As pointed out in McFadden’s closing statement [77]:
“…science is, ultimately, the method by which we use the tools of experimentation, mathematics, and logic to find the simplest explanations of the complex phenomena of our world…”
With this guiding principle in mind, the idea that acetaminophen is not responsible for extensive neurodevelopmental injury in the world today has the hallmarks of pseudoscience. Indeed, consensus without supporting data is consensus, not science.
3. Factors Affecting Susceptibility to Acetaminophen-Mediated Injury during Neurodevelopment
Most babies and children who are exposed to acetaminophen do not sustain injuries leading to ASD, indicating that some factor or factors render some individuals particularly susceptible to acetaminophen-induced injury, while others are resistant. Fortunately, the metabolism of acetaminophen has been widely studied for over half a century, and is exceptionally well characterized. A synopsis of some of the major biochemical pathways of acetaminophen metabolism are shown in Figure 2. Unfortunately, a wide range of factors, including various genetic factors, antibiotic use, infection, temporary inability to eat, exposure to environmental toxins, and problems with folate metabolism or transport can all induce oxidative stress, which leads to susceptibility to acetaminophen-induced injury. This issue was reviewed in detail in 2017 [16]. The result of this situation is that a variety of factors that induce oxidative stress and render acetaminophen more toxic are associated with ASD, but no one factor alone accounts for susceptibility to acetaminophen or for the occurrence of acetaminophen-induced ASD [16].
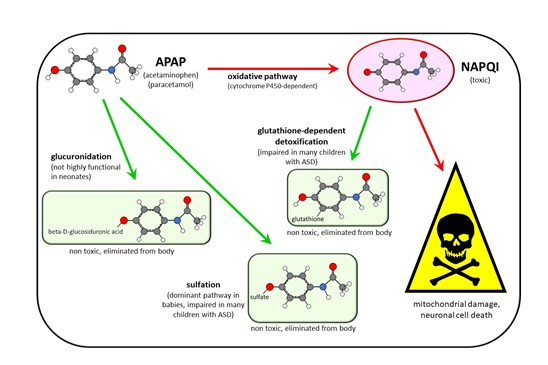
Figure 2. Schematic diagram showing some of the major metabolic pathways of acetaminophen (APAP) metabolism in humans. Three pathways—glucuronidation, sulfation, and oxidation—followed by a reaction with glutathione are shown. The major pathway in babies and in children, sulfation, tends to be impaired in children with autism spectrum disorder (ASD). This is expected to shunt more of the drug through the oxidative pathway, resulting in the production of excess N-acetyl-p-benzoquinone imine (NAPQI), the toxic compound shown in the diagram. Unfortunately, children with ASD also tend to have a reduced ability to detoxify NAPQI, resulting in increased toxicity of APAP due to excess NAPQI. The therapeutically important deacetylation reaction resulting in the production of p-aminophenol, and subsequent downstream reactions in that pathway, are not shown.
Some studies using multivariate analyses of cohort data adjusting for inflammation-associated factors have tended to show little to no risk of acetaminophen use for neurodevelopment [78][79][80][81]. To resolve the discrepancy between these studies and overwhelming evidence pointing toward a critical role of acetaminophen in the etiology of ASD, Parker and colleagues used in-silico methods to show that, even when acetaminophen combined with oxidative stress cause half of all cases of ASD, standard and commonly used statistical analysis of healthcare data shows little to no risk of acetaminophen use [21]. The problem identified was that cofactors which create aberrant metabolism of acetaminophen were adjusted for in the analysis. This issue is explained in more detail here (https://encyclopedia.pub/video/1656). It was concluded that risks of acetaminophen use for neurodevelopment obtained from multivariate analysis of cohort data depend on underlying assumptions in the analyses, and that other evidence, both abundant and robust, demonstrate the critical role of acetaminophen in the etiology of ASD [21].
4. Periods of Sensitivity to Acetaminophen during Neurodevelopment
The timing of acetaminophen-mediated induction of ASD during brain development is of considerable interest. Based on an analysis of cohort data by Liew and colleagues [18], the neurodevelopmental window sensitive to acetaminophen may begin very early during pregnancy, perhaps in the first trimester (Figure 3). However, the time of birth is evidently the most critical period in terms of sensitivity to acetaminophen-induced neurodevelopmental injury (Figure 2). The view that newborns are exquisitely sensitive to acetaminophen is consistent with the 2-fold greater prevalence of infantile ASD associated with circumcision [82] and the 3.6-fold greater prevalence of ASD in the third of children with the highest levels of acetaminophen in their cord blood compared to the third with the lowest levels [35]. As discussed previously [9], the well-established age-dependent pharmacology of acetaminophen [83][84] dictates that, by unit weight, the mother/fetus dyad is far more capable of metabolizing and detoxifying acetaminophen than the newborn alone. The dramatic differences in weight-adjusted capacity to metabolize and detoxify acetaminophen between pregnant women and newborns [9] are expected to adversely affect the developing brain of susceptible newborns when the umbilical cord is cut in the presence of acetaminophen. Based on pharmacokinetic considerations, the first 10 days of life in particular should be the most sensitive to acetaminophen, when the glucuronidation process, important for the detoxification of acetaminophen [9], is not yet functional [85]. Following the perinatal period, the work by Schultz [4] discussed above and the observations from parents discussed previously [16] indicate that acetaminophen induces many, if not most, cases of regressive ASD. Based on a meta-analysis by Tan and colleagues [86], regression can occur in children as old as 4 or 5 years, but the time distribution of regression is skewed toward earlier ages, with half of all cases of regression probably occurring before about 1.5 years of age. Thus, as shown in Figure 3, the neurodevelopmental window for the induction of ASD by acetaminophen is broad, with the prevalence of induction apparently dictated by levels of drug exposure and by susceptibility.
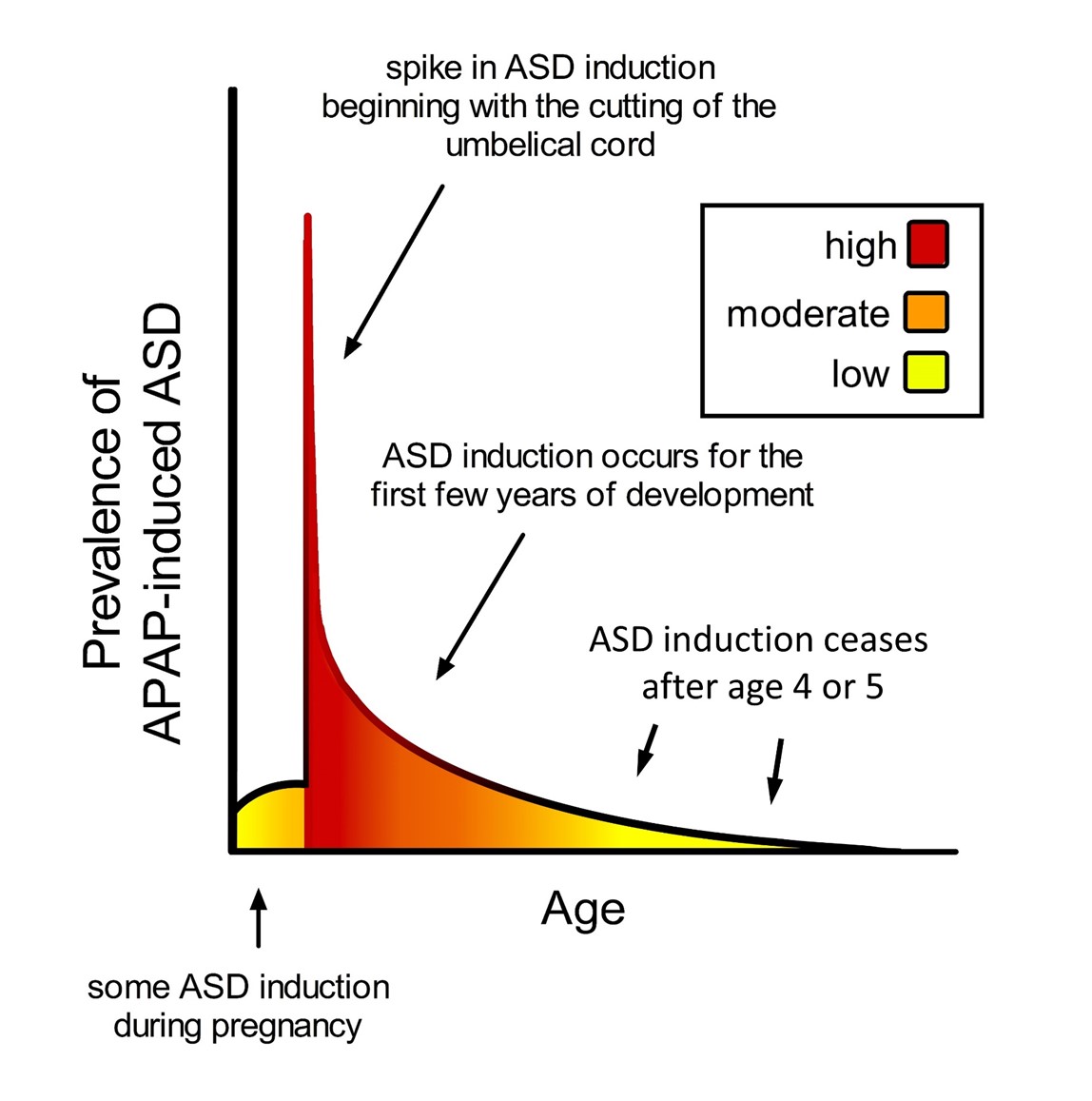
Figure 3. Schematic diagram showing prevalence of acetaminophen (APAP)-induced ASD as a function of age. The neurodevelopmental window of sensitivity is broad, possibly bounded by the first trimester of pregnancy and the end of the 4th or 5th year of life. The shape of the curve is apparently determined by (a) levels of exposure to acetaminophen, and (b) sensitivity to acetaminophen, a function of oxidative stress and ability to metabolize acetaminophen. ASD, autism spectrum disorder; APAP, acetaminophen (paracetamol).
5. Regulatory Action and Public Awareness
The initial report of a strong association between acetaminophen and ASD published in 2008 [4] was immediately questioned and largely forgotten, although subsequent analysis demonstrated that published study criticisms were not valid [9]. Likewise, numerous studies in animal models were ignored despite compelling results using a variety of study designs [10][11][12][13][14][15]. Rather, the consensus, based on false assumptions [1], that acetaminophen is safe for pediatric use prevails in both the medical community and the public consciousness as of early 2024. Progress toward public awareness was likely hampered because classic multivariable regression analysis used to assess the connection between acetaminophen and ASD is widely trusted, but was demonstrated to be invalid [8] as a result of (a) extremely widespread use of acetaminophen, and (b) a variety of factors that make acetaminophen more toxic and act as cofactors for injury induction, but which were treated as confounding factors in the analysis.
Given that the therapeutic target of acetaminophen involves brain function, pediatric use of acetaminophen should have been preceded by extensive tests using animal models for neurodevelopment [8]. Preclinical toxicity screens using laboratory animals often fail to detect drug toxicity in humans [87]. However, neurodevelopment is a conserved process across mammalian species, and laboratory animals provide a very good model for examining brain sensitivity in the perinatal period [88]. Indeed, the toxicity of acetaminophen for the developing brain is readily detected using perinatal laboratory animal models [8][10][11][12][13][14][15]. Given the severe adverse effects of acetaminophen on neurodevelopment observed in laboratory animals by several independent laboratories using a variety of experimental designs, Parker and colleagues conclude that the standard safeguards involving preclinical testing that protect the population from exposure to drugs with a poor benefit-to-risk ratio were not employed when acetaminophen was introduced into widespread pediatric use in the 1980s [7].
Parker and colleagues have encouraged regulatory and policy-making bodies to restrict the pediatric use of acetaminophen, and they stress the importance of consumer education to inform individuals of current knowledge regarding the impact of acetaminophen on the developing brain, particularly in the perinatal period [7]. The fact that consumers lack education regarding the dangers of acetaminophen for neurodevelopment is evident from numerous studies showing widespread frivolous use of the drug for lowering body temperatures that are not actually high enough to be classified as a fever [89][90][91][92][93], and misuse of the drug either by giving too high of a dose or administering the drug too frequently [91][92][93][94][95][96][97][98][99][100]. Of particular concern is the use of acetaminophen immediately before and after birth, both of which affect the neonate, when susceptibility to injury is greatest (Figure 3). It is within this time frame that the benefits of acetaminophen have not been proven [7], and when 50% or even more of all cases of ASD may be induced based on the estimate by Parker and colleagues [7].
References
- Cendejas-Hernandez, J.; Sarafian J.; Lawton V.; Palkar A.; Anderson L.; Lariviere V.; Parker W. Paracetamol (Acetaminophen) Use in Infants and Children was Never Shown to be Safe for Neurodevelopment: A Systematic Review with Citation Tracking. . Eur J Pediatr. 2022;181:1835-57. doi: 10.1007/s00431-022-04407-w.
- Alberti, A.; Pirrone P.; Elia M.; Waring R.H.; Romano C. Sulphation deficit in "low-functioning" autistic children: a pilot study. Biol Psychiatry. 1999;46(3):420-4. Epub 1999/08/06. doi: 10.1016/s0006-3223(98)00337-0. PubMed PMID: 10435209.
- Spielberg, S.P.; Gordon G.B. Glutathione synthetase-deficient lymphocytes and acetaminophen toxicity. Clinical pharmacology and therapeutics. 1981;29(1):51-5. doi: 10.1038/clpt.1981.9. PubMed PMID: 7460474.
- Schultz, S.T.; Klonoff-Cohen H.S.; Wingard D.L.; Akshoomoff N.A.; Macera C.A.; Ji M. Acetaminophen (paracetamol) use, measles-mumps-rubella vaccination, and autistic disorder. The results of a parent survey. Autism. 2008;12(3):293-307.
- Posadas, I.; Santos P.; Blanco A.; Muñoz-Fernández M.; Ceña V. Acetaminophen induces apoptosis in rat cortical neurons. PLoS One. 2010;5(12):e15360. Epub 2010/12/21. doi: 10.1371/journal.pone.0015360. PubMed PMID: 21170329; PubMed Central PMCID: PMCPMC3000821.
- Viberg, H.; Eriksson P.; Gordh T.; Fredriksson A. Paracetamol (Acetaminophen) Administration During Neonatal Brain Development Affects Cognitive Function and Alters Its Analgesic and Anxiolytic Response in Adult Male Mice. Toxicol Sci. 2013;138(1):139-47. doi: 10.1093/toxsci/kft329.
- Parker, W.; Anderson L.G.; Jones J.P.; Anderson R.; Williamson L.; Bono-Lunn D.; Konsoula Z. The Dangers of Acetaminophen for Neurodevelopment Outweigh Scant Evidence for Long-Term Benefits. Children. 2024;11(1):44. PubMed PMID: doi:10.3390/children11010044.
- Patel, E.; Jones Iii J.P., 3rd; Bono-Lunn D.; Kuchibhatla M.; Palkar A.; Cendejas Hernandez J.; Sarafian J.T.; Lawton V.G.; Anderson L.G.; Konsoula Z., et al. The safety of pediatric use of paracetamol (acetaminophen): a narrative review of direct and indirect evidence. Minerva pediatrics. 2022;74(6):774-88. Epub 2022/07/14. doi: 10.23736/s2724-5276.22.06932-4. PubMed PMID: 35822581.
- Zhao, L.; Jones J.; Anderson L.; Konsoula Z.; Nevison C.; Reissner K.; Parker W. Acetaminophen causes neurodevelopmental injury in susceptible babies and children: no valid rationale for controversy. Clinical and experimental pediatrics. 2023. Epub 2023/06/16. doi: 10.3345/cep.2022.01319. PubMed PMID: 37321575.
- Herrington, J.A.; Guss Darwich J.; Harshaw C.; Brigande A.M.; Leif E.B.; Currie P.J. Elevated ghrelin alters the behavioral effects of perinatal acetaminophen exposure in rats. Dev Psychobiol. 2022;64(3):e22252. Epub 2022/03/22. doi: 10.1002/dev.22252. PubMed PMID: 35312061.
- Harshaw, C.; Warner A.G. Interleukin-1β-induced inflammation and acetaminophen during infancy: Distinct and interactive effects on social-emotional and repetitive behavior in C57BL/6J mice. Pharmacol Biochem Behav. 2022;220:173463. doi: https://doi.org/10.1016/j.pbb.2022.173463.
- Viberg, H.; Eriksson P.; Gordh T.; Fredriksson A. Paracetamol (acetaminophen) administration during neonatal brain development affects cognitive function and alters its analgesic and anxiolytic response in adult male mice. Toxicol Sci. 2014;138(1):139-47. Epub 2013/12/24. doi: 10.1093/toxsci/kft329. PubMed PMID: 24361869.
- Suda, N.; Cendejas Hernandez J.; Poulton J.; Jones J.P.; Konsoula Z.; Smith C.; Parker W. Therapeutic doses of acetaminophen with co-administration of cysteine and mannitol during early development result in long term behavioral changes in laboratory rats. PLoS One. 2021;16(6):e0253543. Epub 2021/06/26. doi: 10.1371/journal.pone.0253543. PubMed PMID: 34170958; PubMed Central PMCID: PMCPMC8232535.
- Philippot, G.; Gordh T.; Fredriksson A.; Viberg H. Adult neurobehavioral alterations in male and female mice following developmental exposure to paracetamol (acetaminophen): characterization of a critical period. J Appl Toxicol. 2017;37(10):1174-81. Epub 2017/04/28. doi: 10.1002/jat.3473. PubMed PMID: 28448685.
- Dean, S.L.; Knutson J.F.; Krebs-Kraft D.L.; McCarthy M.M. Prostaglandin E2 is an endogenous modulator of cerebellar development and complex behavior during a sensitive postnatal period. Eur J Neurosci. 2012;35(8):1218-29. Epub 2012/04/20. doi: 10.1111/j.1460-9568.2012.08032.x. PubMed PMID: 22512254; PubMed Central PMCID: PMCPmc3534986.
- Parker, W.; Hornik C.D.; Bilbo S.; Holzknecht Z.E.; Gentry L.; Rao R.; Lin S.S.; Herbert M.R.; Nevison C.D. The role of oxidative stress, inflammation and acetaminophen exposure from birth to early childhood in the induction of autism. J Int Med Res. 2017;45(2):407-38.
- Frisch, M.; Simonsen J. Ritual circumcision and risk of autism spectrum disorder in 0- to 9-year-old boys: national cohort study in Denmark. J R Soc Med. 2015;108(7):266-79. Epub 2015/01/13. doi: 10.1177/0141076814565942. PubMed PMID: 25573114; PubMed Central PMCID: PMCPMC4530408.
- Liew, Z.; Ritz B.; Virk J.; Olsen J. Maternal use of acetaminophen during pregnancy and risk of autism spectrum disorders in childhood: A Danish national birth cohort study. Autism research : official journal of the International Society for Autism Research. 2016;9(9):951-8. Epub 2015/12/22. doi: 10.1002/aur.1591. PubMed PMID: 26688372.
- Alemany, S.; Avella-García C.; Liew Z.; García-Esteban R.; Inoue K.; Cadman T.; López-Vicente M.; González L.; Riaño Galán I.; Andiarena A., et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. Eur J Epidemiol. 2021;36(10):993-1004. Epub 2021/05/29. doi: 10.1007/s10654-021-00754-4. PubMed PMID: 34046850.
- Rimland, B. The autism increase: research needed on the vaccine connection. Autism Research Review International. 2000.
- John P. Jones; Lauren Williamson; Zacharoula Konsoula; Rachel Anderson; Kathryn J. Reissner; William Parker; Evaluating the Role of Susceptibility Inducing Cofactors and of Acetaminophen in the Etiology of Autism Spectrum Disorder. Life. 2024, 14, 918.
- Hall, C.; Smith M. Increased cGMP enforcement has gone international: South Korean action against Johnson & Johnson serves as warning. White Collar Watch. 2013;June.
- Kim, Y.S.; Leventhal B.L.; Koh Y.J.; Fombonne E.; Laska E.; Lim E.C.; Cheon K.A.; Kim S.J.; Kim Y.K.; Lee H., et al. Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168(9):904-12. Epub 2011/05/12. doi: 10.1176/appi.ajp.2011.10101532. PubMed PMID: 21558103.
- Baird, G. 2.64% of South Korean children aged 7 to 12 have autism spectrum disorders. Evidence Based Mental Health. 2012;15(1):11. doi: 10.1136/ebmental-2011-100289.
- Raz, R.; Weisskopf M.G.; Davidovitch M.; Pinto O.; Levine H. Differences in autism spectrum disorders incidence by sub-populations in Israel 1992-2009: a total population study. J Autism Dev Disord. 2015;45(4):1062-9. doi: 10.1007/s10803-014-2262-z. PubMed PMID: 25287899.
- Levaot, Y.; Meiri G.; Dinstein I.; Menashe I.; Shoham-Vardi I. Autism Prevalence and Severity in Bedouin-Arab and Jewish Communities in Southern Israel. Community mental health journal. 2019;55(1):156-60. Epub 2018/02/02. doi: 10.1007/s10597-018-0236-x. PubMed PMID: 29388003.
- Bittker, S.S.; Bell K.R. Acetaminophen, antibiotics, ear infection, breastfeeding, vitamin D drops, and autism: an epidemiological study. Neuropsychiatric disease and treatment. 2018;14:1399-414. Epub 2018/06/19. doi: 10.2147/ndt.s158811. PubMed PMID: 29910617; PubMed Central PMCID: PMCPMC5987866.
- Bittker, S.S.; Bell K.R. Postnatal Acetaminophen and Potential Risk of Autism Spectrum Disorder among Males. Behavioral sciences (Basel, Switzerland). 2020;10(1). Epub 2020/01/08. doi: 10.3390/bs10010026. PubMed PMID: 31906400; PubMed Central PMCID: PMCPMC7017213.
- Donohue, J. A history of drug advertising: the evolving roles of consumers and consumer protection. Milbank Q. 2006;84(4):659-99. doi: 10.1111/j.1468-0009.2006.00464.x. PubMed PMID: 17096638.
- Easton, T.; Herrera S. J&J's dirty little secret. Forbes. 1998:42-4.
- Tovo-Rodrigues, L.; Schneider B.C.; Martins-Silva T.; Del-Ponte B.; Loret de Mola C.; Schuler-Faccini L.; Vianna F.S.L.; Munhoz T.N.; Entiauspe L.; Silveira M.F., et al. Is intrauterine exposure to acetaminophen associated with emotional and hyperactivity problems during childhood? Findings from the 2004 Pelotas birth cohort. BMC Psychiatry. 2018;18(1):368. doi: 10.1186/s12888-018-1942-1.
- Vlenterie, R.; Wood M.E.; Brandlistuen R.E.; Roeleveld N.; van Gelder M.M.; Nordeng H. Neurodevelopmental problems at 18 months among children exposed to paracetamol in utero: a propensity score matched cohort study. Int J Epidemiol. 2016;45(6):1998-2008. Epub 2016/09/03. doi: 10.1093/ije/dyw192. PubMed PMID: 27585674; PubMed Central PMCID: PMCPMC5841617.
- Liew, Z.; Ritz B.; Virk J.; Arah O.A.; Olsen J. Prenatal Use of Acetaminophen and Child IQ: A Danish Cohort Study. Epidemiology. 2016;27(6):912-8. Epub 2016/08/02. doi: 10.1097/ede.0000000000000540. PubMed PMID: 27479646.
- Liew, Z.; Bach C.C.; Asarnow R.F.; Ritz B.; Olsen J. Paracetamol use during pregnancy and attention and executive function in offspring at age 5 years. Int J Epidemiol. 2016;45(6):2009-17. Epub 2016/12/30. doi: 10.1093/ije/dyw296. PubMed PMID: 28031314.
- Ji, Y.; Azuine R.E.; Zhang Y.; Hou W.; Hong X.; Wang G.; Riley A.; Pearson C.; Zuckerman B.; Wang X. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure With Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry. 2020;77(2):180-9. Epub 2019/10/31. doi: 10.1001/jamapsychiatry.2019.3259. PubMed PMID: 31664451; PubMed Central PMCID: PMCPMC6822099.
- Avella-Garcia, C.B.; Julvez J.; Fortuny J.; Rebordosa C.; Garcia-Esteban R.; Galan I.R.; Tardon A.; Rodriguez-Bernal C.L.; Iniguez C.; Andiarena A., et al. Acetaminophen use in pregnancy and neurodevelopment: attention function and autism spectrum symptoms. Int J Epidemiol. 2016;45(6):1987-96. Epub 2016/06/30. doi: 10.1093/ije/dyw115. PubMed PMID: 27353198.
- Skovlund, E.; Handal M.; Selmer R.; Brandlistuen R.E.; Skurtveit S. Language competence and communication skills in 3-year-old children after prenatal exposure to analgesic opioids. Pharmacoepidemiol Drug Saf. 2017;26(6):625-34. Epub 2017/02/09. doi: 10.1002/pds.4170. PubMed PMID: 28168770.
- Liew, Z.; Ritz B.; Rebordosa C.; Lee P.C.; Olsen J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA pediatrics. 2014;168(4):313-20. Epub 2014/02/26. doi: 10.1001/jamapediatrics.2013.4914. PubMed PMID: 24566677.
- Ystrom, E.; Gustavson K.; Brandlistuen R.E.; Knudsen G.P.; Magnus P.; Susser E.; Davey Smith G.; Stoltenberg C.; Suren P.; Haberg S.E., et al. Prenatal Exposure to Acetaminophen and Risk of ADHD. Pediatrics. 2017;140(5). Epub 2017/11/01. doi: 10.1542/peds.2016-3840. PubMed PMID: 29084830; PubMed Central PMCID: PMCPMC5654387 conflicts of interest to disclose.
- Thompson, J.M.; Waldie K.E.; Wall C.R.; Murphy R.; Mitchell E.A. Associations between acetaminophen use during pregnancy and ADHD symptoms measured at ages 7 and 11 years. PLoS One. 2014;9(9):e108210. Epub 2014/09/25. doi: 10.1371/journal.pone.0108210. PubMed PMID: 25251831; PubMed Central PMCID: PMCPMC4177119.
- Stergiakouli, E.; Thapar A.; Davey Smith G. Association of Acetaminophen Use During Pregnancy With Behavioral Problems in Childhood: Evidence Against Confounding. JAMA pediatrics. 2016;170(10):964-70. Epub 2016/08/18. doi: 10.1001/jamapediatrics.2016.1775. PubMed PMID: 27533796; PubMed Central PMCID: PMCPMC5300094.
- Brandlistuen, R.E.; Ystrom E.; Nulman I.; Koren G.; Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702-13. Epub 2013/10/29. doi: 10.1093/ije/dyt183. PubMed PMID: 24163279; PubMed Central PMCID: PMCPMC3887567.
- Woodbury, M.L.; Cintora P.; Ng S.; Hadley P.A.; Schantz S.L. Examining the relationship of acetaminophen use during pregnancy with early language development in children. Pediatr Res. 2023. doi: 10.1038/s41390-023-02924-4.
- Freed, G.L.; Clark S.J.; Butchart A.T.; Singer D.C.; Davis M.M. Parental vaccine safety concerns in 2009. Pediatrics. 2010;125(4):654-9. Epub 2010/03/03. doi: 10.1542/peds.2009-1962. PubMed PMID: 20194286.
- Bazzano, A.; Zeldin A.; Schuster E.; Barrett C.; Lehrer D. Vaccine-related beliefs and practices of parents of children with autism spectrum disorders. American journal on intellectual and developmental disabilities. 2012;117(3):233-42. Epub 2012/06/22. doi: 10.1352/1944-7558-117.3.233. PubMed PMID: 22716265.
- Abu Kuwaik, G.; Roberts W.; Zwaigenbaum L.; Bryson S.; Smith I.M.; Szatmari P.; Modi B.M.; Tanel N.; Brian J. Immunization uptake in younger siblings of children with autism spectrum disorder. Autism. 2014;18(2):148-55. Epub 2012/10/10. doi: 10.1177/1362361312459111. PubMed PMID: 23045216.
- Jain, A.; Marshall J.; Buikema A.; Bancroft T.; Kelly J.P.; Newschaffer C.J. Autism occurrence by MMR vaccine status among US children with older siblings with and without autism. Jama. 2015;313(15):1534-40. Epub 2015/04/22. doi: 10.1001/jama.2015.3077. PubMed PMID: 25898051.
- Hutabarat, R.M.; Unadkat J.D.; Kushmerick P.; Aitken M.L.; Slattery J.T.; Smith A.L. Disposition of drugs in cystic fibrosis. III. Acetaminophen. Clin Pharmacol Ther. 1991;50(6):695-701. Epub 1991/12/01. PubMed PMID: 1752114.
- Kearns, G.L. Hepatic drug metabolism in cystic fibrosis: recent developments and future directions. Ann Pharmacother. 1993;27(1):74-9. Epub 1993/01/01. PubMed PMID: 8431626.
- Roberts, I.D.; Krajbich I.; Way B.M. Acetaminophen influences social and economic trust. Scientific Reports. 2019;9(1):4060. doi: 10.1038/s41598-019-40093-9.
- Dewall, C.N.; Macdonald G.; Webster G.D.; Masten C.L.; Baumeister R.F.; Powell C.; Combs D.; Schurtz D.R.; Stillman T.F.; Tice D.M., et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21(7):931-7. Epub 2010/06/16. doi: 10.1177/0956797610374741. PubMed PMID: 20548058.
- Durso, G.R.O.; Luttrell A.; Way B.M. Over-the-Counter Relief From Pains and Pleasures Alike: Acetaminophen Blunts Evaluation Sensitivity to Both Negative and Positive Stimuli. Psychol Sci. 2015;26(6):750-8. Epub 04/10. doi: 10.1177/0956797615570366. PubMed PMID: 25862546.
- Randles, D.; Kam J.W.Y.; Heine S.J.; Inzlicht M.; Handy T.C. Acetaminophen attenuates error evaluation in cortex. Social Cognitive and Affective Neuroscience. 2016;11(6):899-906. doi: 10.1093/scan/nsw023.
- Frye, R.E.; Sequeira J.M.; Quadros E.V.; James S.J.; Rossignol D.A. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol Psychiatry. 2013;18(3):369-81. Epub 01/10. doi: 10.1038/mp.2011.175. PubMed PMID: 22230883.
- Guengerich, F.P. A history of the roles of cytochrome P450 enzymes in the toxicity of drugs. Toxicological Research. 2021;37(1):1-23. doi: 10.1007/s43188-020-00056-z.
- Albano, E.; Rundgren M.; Harvison P.J.; Nelson S.D.; Moldéus P. Mechanisms of N-acetyl-p-benzoquinone imine cytotoxicity. Mol Pharmacol. 1985;28(3):306-11. Epub 1985/09/01. PubMed PMID: 4033631.
- Mitchell, J.R.; Jollow D.J.; Potter W.Z.; Davis D.C.; Gillette J.R.; Brodie B.B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187(1):185-94. Epub 1973/10/01. PubMed PMID: 4746326.
- Mitchell, J.R.; Jollow D.J.; Potter W.Z.; Gillette J.R.; Brodie B.B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187(1):211-7. Epub 1973/10/01. PubMed PMID: 4746329.
- Du, K.; Farhood A.; Jaeschke H. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch Toxicol. 2017;91(2):761-73. Epub 2016/03/24. doi: 10.1007/s00204-016-1692-0. PubMed PMID: 27002509; PubMed Central PMCID: PMCPMC5033665.
- Siddiqui, M.F.; Elwell C.; Johnson M.H. Mitochondrial Dysfunction in Autism Spectrum Disorders. Autism-open access. 2016;6(5). Epub 2016/12/09. doi: 10.4172/2165-7890.1000190. PubMed PMID: 27928515; PubMed Central PMCID: PMCPMC5137782.
- Philippot, G.; Hosseini K.; Yakub A.; Mhajar Y.; Hamid M.; Buratovic S.; Fredriksson R. Paracetamol (Acetaminophen) and its Effect on the Developing Mouse Brain. Frontiers in toxicology. 2022;4:867748. Epub 2022/04/09. doi: 10.3389/ftox.2022.867748. PubMed PMID: 35391823; PubMed Central PMCID: PMCPMC8981466.
- Kanno, S.I.; Tomizawa A.; Yomogida S.; Hara A. Glutathione peroxidase 3 is a protective factor against acetaminophen‑induced hepatotoxicity in vivo and in vitro. Int J Mol Med. 2017;40(3):748-54. Epub 2017/07/06. doi: 10.3892/ijmm.2017.3049. PubMed PMID: 28677736; PubMed Central PMCID: PMCPMC5547967.
- McCarthy, M.M.; Wright C.L. Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder. Biol Psychiatry. 2017;81(5):402-10. Epub 2016/11/23. doi: 10.1016/j.biopsych.2016.10.004. PubMed PMID: 27871670; PubMed Central PMCID: PMCPMC5285451.
- Donovan, A.P.; Basson M.A. The neuroanatomy of autism - a developmental perspective. J Anat. 2017;230(1):4-15. Epub 2016/09/14. doi: 10.1111/joa.12542. PubMed PMID: 27620360; PubMed Central PMCID: PMCPMC5192959.
- Casanova, M.F.; Sokhadze E.M.; Casanova E.L.; Opris I.; Abujadi C.; Marcolin M.A.; Li X. Translational Neuroscience in Autism: From Neuropathology to Transcranial Magnetic Stimulation Therapies. The Psychiatric clinics of North America. 2020;43(2):229-48. Epub 2020/05/23. doi: 10.1016/j.psc.2020.02.004. PubMed PMID: 32439019; PubMed Central PMCID: PMCPMC7245584.
- Dong, D.; Zielke H.R.; Yeh D.; Yang P. Cellular stress and apoptosis contribute to the pathogenesis of autism spectrum disorder. Autism research : official journal of the International Society for Autism Research. 2018;11(7):1076-90. Epub 2018/05/16. doi: 10.1002/aur.1966. PubMed PMID: 29761862; PubMed Central PMCID: PMCPMC6107407.
- Lv, M.N.; Zhang H.; Shu Y.; Chen S.; Hu Y.Y.; Zhou M. The neonatal levels of TSB, NSE and CK-BB in autism spectrum disorder from Southern China. Translational neuroscience. 2016;7(1):6-11. Epub 2017/01/27. doi: 10.1515/tnsci-2016-0002. PubMed PMID: 28123815; PubMed Central PMCID: PMCPMC5017591.
- Stancioiu, F.; Bogdan R.; Dumitrescu R. Neuron-Specific Enolase (NSE) as a Biomarker for Autistic Spectrum Disease (ASD). Life (Basel, Switzerland). 2023;13(8). Epub 2023/08/26. doi: 10.3390/life13081736. PubMed PMID: 37629593; PubMed Central PMCID: PMCPMC10455327.
- Anvik, J.O. Acetaminophen toxicosis in a cat. The Canadian veterinary journal = La revue veterinaire canadienne. 1984;25(12):445-7. Epub 1984/12/01. PubMed PMID: 17422485; PubMed Central PMCID: PMCPMC1790690.
- Savides, M.C.; Oehme F.W.; Nash S.L.; Leipold H.W. The toxicity and biotransformation of single doses of acetaminophen in dogs and cats. Toxicol Appl Pharmacol. 1984;74(1):26-34. Epub 1984/06/15. doi: 10.1016/0041-008x(84)90266-7. PubMed PMID: 6729821.
- Court, M.H. Feline drug metabolism and disposition: pharmacokinetic evidence for species differences and molecular mechanisms. The Veterinary clinics of North America Small animal practice. 2013;43(5):1039-54. Epub 2013/07/31. doi: 10.1016/j.cvsm.2013.05.002. PubMed PMID: 23890237; PubMed Central PMCID: PMCPMC3811070.
- Lautz, L.S.; Jeddi M.Z.; Girolami F.; Nebbia C.; Dorne J. Metabolism and pharmacokinetics of pharmaceuticals in cats (Felix sylvestris catus) and implications for the risk assessment of feed additives and contaminants. Toxicology letters. 2021;338:114-27. Epub 2020/12/01. doi: 10.1016/j.toxlet.2020.11.014. PubMed PMID: 33253781.
- Miller, R.P.; Roberts R.J.; Fischer L.J. Acetaminophen elimination kinetics in neonates, children, and adults. Clin Pharmacol Ther. 1976;19(3):284-94. Epub 1976/03/01. doi: 10.1002/cpt1976193284. PubMed PMID: 1261167.
- Cook, S.F.; Stockmann C.; Samiee-Zafarghandy S.; King A.D.; Deutsch N.; Williams E.F.; Wilkins D.G.; Sherwin C.M.; van den Anker J.N. Neonatal Maturation of Paracetamol (Acetaminophen) Glucuronidation, Sulfation, and Oxidation Based on a Parent-Metabolite Population Pharmacokinetic Model. Clin Pharmacokinet. 2016;55(11):1395-411. Epub 2016/10/21. doi: 10.1007/s40262-016-0408-1. PubMed PMID: 27209292; PubMed Central PMCID: PMCPMC5572771.
- Wastesson, J.W.; Martikainen J.E.; Zoëga H.; Schmidt M.; Karlstad Ø.; Pottegård A. Trends in Use of Paracetamol in the Nordic Countries. Basic Clin Pharmacol Toxicol. 2018;123(3):301-7. Epub 2018/03/13. doi: 10.1111/bcpt.13003. PubMed PMID: 29527817.
- Delobel-Ayoub, M.; Saemundsen E.; Gissler M.; Ego A.; Moilanen I.; Ebeling H.; Rafnsson V.; Klapouszczak D.; Thorsteinsson E.; Arnaldsdóttir K.M., et al. Prevalence of Autism Spectrum Disorder in 7-9-Year-Old Children in Denmark, Finland, France and Iceland: A Population-Based Registries Approach Within the ASDEU Project. J Autism Dev Disord. 2020;50(3):949-59. Epub 2019/12/10. doi: 10.1007/s10803-019-04328-y. PubMed PMID: 31813107.
- McFadden, J. Razor sharp: The role of Occam's razor in science. Ann N Y Acad Sci. 2023;1530(1):8-17. doi: https://doi.org/10.1111/nyas.15086.
- Kristin Gustavson; Eivind Ystrom; Helga Ask; Fartein Ask Torvik; Mady Hornig; Ezra Susser; W. Ian Lipkin; Angela Lupattelli; Camilla Stoltenberg; Per Magnus; Siri Mjaaland; Ragna Bugge Askeland; Kjersti Mæhlum Walle; Michaeline Bresnahan; Hedvig Nordeng; Ted Reichborn‐Kjennerud; Acetaminophen use during pregnancy and offspring attention deficit hyperactivity disorder – a longitudinal sibling control study. JCPP Adv.. 2021, 1, e12020.
- Per Damkier; Erika B. Gram; Michael Ceulemans; Alice Panchaud; Brian Cleary; Christina Chambers; Corinna Weber-Schoendorfer; Debra Kennedy; Ken Hodson; Kimberly S. Grant; Orna Diav-Citrin; Sarah G. Običan; Svetlana Shechtman; Sura Alwan; Acetaminophen in Pregnancy and Attention-Deficit and Hyperactivity Disorder and Autism Spectrum Disorder. Obstet. Gynecol.. 2024, 145, 168-176.
- Alemany, S.; Avella-García, C.; Liew, Z.; et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. . Eur J Epidemiol. . 2021, 36, 993-1004.
- Ahlqvist, V.H.; Sjöqvist, H.; Dalman, C. et al. Acetaminophen Use During Pregnancy and Children's Risk of Autism, ADHD, and Intellectual Disability. . JAMA. 2024, 331, 1205-14.
- Frisch, M.; Simonsen J. Ritual circumcision and risk of autism spectrum disorder in 0- to 9-year-old boys: national cohort study in Denmark. J R Soc Med. 2015;108(7):266-79. doi: 10.1177/0141076814565942. PubMed PMID: PMC4530408.
- Miners, J.O.; Robson R.A.; Birkett D.J. Paracetamol metabolism in pregnancy. Br J Clin Pharmacol. 1986;22(3):359-62. Epub 1986/09/01. doi: 10.1111/j.1365-2125.1986.tb02901.x. PubMed PMID: 3768250; PubMed Central PMCID: PMCPMC1401133.
- Cresteil, T. Onset of xenobiotic metabolism in children: toxicological implications. Food Addit Contam. 1998;15 Suppl:45-51. Epub 1998/05/29. doi: 10.1080/02652039809374614. PubMed PMID: 9602911.
- O'Hara, K.; Wright I.M.; Schneider J.J.; Jones A.L.; Martin J.H. Pharmacokinetics in neonatal prescribing: evidence base, paradigms and the future. Br J Clin Pharmacol. 2015;80(6):1281-8. Epub 2015/08/11. doi: 10.1111/bcp.12741. PubMed PMID: 26256466; PubMed Central PMCID: PMCPMC4693494.
- Tan, C.; Frewer V.; Cox G.; Williams K.; Ure A. Prevalence and Age of Onset of Regression in Children with Autism Spectrum Disorder: A Systematic Review and Meta-analytical Update. Autism research : official journal of the International Society for Autism Research. 2021;14(3):582-98. Epub 2021/01/26. doi: 10.1002/aur.2463. PubMed PMID: 33491292.
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic to translational science. 2019;4(7):845-54. Epub 2020/01/31. doi: 10.1016/j.jacbts.2019.10.008. PubMed PMID: 31998852; PubMed Central PMCID: PMCPMC6978558.
- Workman, A.D.; Charvet C.J.; Clancy B.; Darlington R.B.; Finlay B.L. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013;33(17):7368-83. Epub 2013/04/26. doi: 10.1523/jneurosci.5746-12.2013. PubMed PMID: 23616543; PubMed Central PMCID: PMCPMC3928428.
- Lubrano, R.; Paoli S.; Bonci M.; Di Ruzza L.; Cecchetti C.; Falsaperla R.; Pavone P.; Matin N.; Vitaliti G.; Gentile I. Acetaminophen administration in pediatric age: an observational prospective cross-sectional study. Ital J Pediatr. 2016;42:20-. doi: 10.1186/s13052-016-0219-x. PubMed PMID: 26920747.
- May, A.; Bauchner H. Fever Phobia: The Pediatrician’s Contribution. Pediatrics. 1992;90.
- Bilenko, N.; Tessler H.; Okbe R.; Gorodischer R. Determinants of antipyretic misuse in children up to 5 years of age: A cross-sectional study. Clinical Therapeutics. 2006;28:783-93.
- Poirier, M.P.; Collins E.P.; McGuire E. Fever phobia: a survey of caregivers of children seen in a pediatric emergency department. Clinical pediatrics. 2010;49(6):530-4. Epub 2010/05/22. doi: 10.1177/0009922809355312. PubMed PMID: 20488812.
- ProPublica. Use Only As Directed2013 June 28, 2018 [cited 2013 September 20, 2013]. Available from: https://www.propublica.org/article/tylenol-mcneil-fda-use-only-as-directed.
- Walsh, A.; Edwards H.; Fraser J. Over-the-counter medication use for childhood fever: A cross-sectional study of Australian parents. Journal of Paediatrics and Child Health. 2007;43(9):601-6. doi: 10.1111/j.1440-1754.2007.01161.x.
- Li, S.F.; Lacher B.; Crain E.F. Acetaminophen and ibuprofen dosing by parents. Pediatric emergency care. 2000;16(6):394-7. Epub 2001/01/04. PubMed PMID: 11138879.
- Alomar, M.; Alenazi F.; Alruwaili N. Accuracy of acetaminophen dosing in children by caregivers in Saudi Arabia. Ann Saudi Med. 2011;31(5):513-7. doi: 10.4103/0256-4947.84630. PubMed PMID: PMC3183687.
- Betz, M.G.; Grunfeld A.F. 'Fever phobia' in the emergency department: a survey of children's caregivers. European journal of emergency medicine : official journal of the European Society for Emergency Medicine. 2006;13(3):129-33. Epub 2006/05/09. doi: 10.1097/01.mej.0000194401.15335.c7. PubMed PMID: 16679875.
- Heubi, J.E.; Barbacci M.B.; Zimmerman H.J. Therapeutic misadventures with acetaminophen: Hepatoxicity after multiple doses in children. The Journal of Pediatrics. 1998;132:22-7.
- Arikan, Z.; Teksam O.; Kara A.; Kale G. Determining causes and frequency of misdosing of antipyretics in patients presenting with fever to pediatric emergency2012. 114-8 p.
- Yavuz, E.; Yayla E.; Cebeci S.E.; Kirimli E.; Gumustakim R.S.; Cakir L.; Dogan S. Parental beliefs and practices regarding childhood fever in Turkish primary care. Nigerian journal of clinical practice. 2017;20(1):93-8. Epub 2016/12/14. doi: 10.4103/1119-3077.181318. PubMed PMID: 27958254.




