Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alice Vilela | -- | 6346 | 2024-01-10 10:55:44 | | | |
| 2 | Rita Xu | Meta information modification | 6346 | 2024-01-11 02:50:53 | | | | |
| 3 | Alice Vilela | Meta information modification | 6346 | 2024-01-17 12:32:15 | | | | |
| 4 | Alice Vilela | -3 word(s) | 6343 | 2024-05-09 18:45:52 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Afonso, S.M.; Inês, A.; Vilela, A. Bio-Dealcoholization of Wines. Encyclopedia. Available online: https://encyclopedia.pub/entry/53673 (accessed on 08 February 2026).
Afonso SM, Inês A, Vilela A. Bio-Dealcoholization of Wines. Encyclopedia. Available at: https://encyclopedia.pub/entry/53673. Accessed February 08, 2026.
Afonso, Sílvia Martins, António Inês, Alice Vilela. "Bio-Dealcoholization of Wines" Encyclopedia, https://encyclopedia.pub/entry/53673 (accessed February 08, 2026).
Afonso, S.M., Inês, A., & Vilela, A. (2024, January 10). Bio-Dealcoholization of Wines. In Encyclopedia. https://encyclopedia.pub/entry/53673
Afonso, Sílvia Martins, et al. "Bio-Dealcoholization of Wines." Encyclopedia. Web. 10 January, 2024.
Copy Citation
The link between viticulture and climate change has become more evident in recent years. The temperature rise has been a crucial factor in the early ripening of grapes, which has resulted in wines with imbalanced acidity, higher alcohol content, and higher pH values. Consumers are seeking high-quality and healthy products, and this trend has extended to wine consumption.
climate change
post-fermentation
membrane separation
heat treatment
1. Climate Change and Wine Quality
Climate change, primarily driven by anthropogenic influence, induces significant transformations in the Earth’s system [1][2], including temperature extremes and modifications in precipitation patterns [3][4]. The latest Assessment Report of the Intergovernmental Panel on Climate Change (IPCC) [5] predicts a continued rise in global surface temperature and a decrease in annual precipitation across emission scenarios, with intensifying and more frequent extreme events globally [4][6]. The Mediterranean region is particularly vulnerable to global warming, with projected severe climate events affecting agriculture and leading to economic and food security challenges [7].
As indicated by Lamonaca et al. [8], detrimental outcomes, such as reductions in agricultural production and economic conditions associated with an increase in food insecurity, are anticipated to exacerbate, significantly affecting the agricultural sector [9][10]. This emphasizes the considerable vulnerability of agriculture to climate change, including viticulture.
In 2022, wine exports from these three European countries amounted to around EUR 23 billion, emphasizing the economic importance of viticulture in Europe and globally, highlighting wine grapes as one of the most valuable fruit crops worldwide [2][11][12]. Additionally, global wine production in 2022 reached 258 million hectoliters, slightly lower than the previous year’s output of 261 million hectoliters. Nevertheless, the vineyard area has decreased by approximately 1.18% over the past five years (from 2018 to 2022) due to extreme weather changes [13], underscoring that climate factors, particularly temperature, exert a more significant influence on vine development and berry composition relative to other factors such as soil or variety [14][15]. However, it is essential to acknowledge the multifaceted nature of these interactions, and the impact can vary based on specific vineyard locations [15].
This demonstrates that, as stated earlier, due to the strong relationship between viticulture and climate, fluctuations in temperature and frost events (early or late frosts) can directly impact grape production and the quality of the wine produced [3][6][16]. Increasing temperatures lead to premature grape ripening, culminating in an undesirable rise in sugar levels and a decrease in organic acids and phenolic compounds. This results in higher alcohol content, reduced acidity, and modified sensory profiles [16][17][18], ultimately altering wine quality and typicity [2][19][20]. Given the potential compromise of crop yields and viticulture productivity under future climatic conditions, the urgent adoption of cultivation approaches and strategies is crucial to mitigate the effects of climate change on wine production and quality.
Moreover, recent data indicate a growing consumer preference for low-alcohol wines (9% to 13%). This preference is associated with increased social awareness of the harmful effects of alcohol on human health (calorific intake and possibility of alcohol-related diseases) as consumers seek a healthier lifestyle [21][22][23][24]. This has led to an increase in the volume of no and low-alcohol beverages of over 7% across the ten major global markets in 2022. This upward trajectory is projected to outpace the growth observed in the past four years, with a forecasted Compound Annual Growth Rate (CAGR) of over 7% from 2022 to 2026. This forecasted rate represents an increase compared to the 5% CAGR recorded from 2018 to 2022 (Figure 1). In this context, wine producers are innovatively lowering alcohol content to meet consumer demands and address challenges posed by high grape sugar concentration during winemaking. Musts with high sugar content usually present difficulty in performing alcoholic fermentation, causing stuck or sluggish fermentation, leading to prolonged and intricate processes or even complete stoppage [22][23][25][26]. These issues arise from the osmotically stressful environment of high sugar concentrations, hindering water absorption and slowing yeast metabolic activity. The resulting alcohol production can reach toxic levels for the yeast, and the must’s nutritional conditions and the potential presence of fermentation inhibitors further contribute to the complications [27]. Furthermore, in some countries, such as the USA, Finland, Sweden, Ireland, and the United Kingdom, exceeding 14.5% (v/v) alcohol content results in higher taxes [22][28][29].
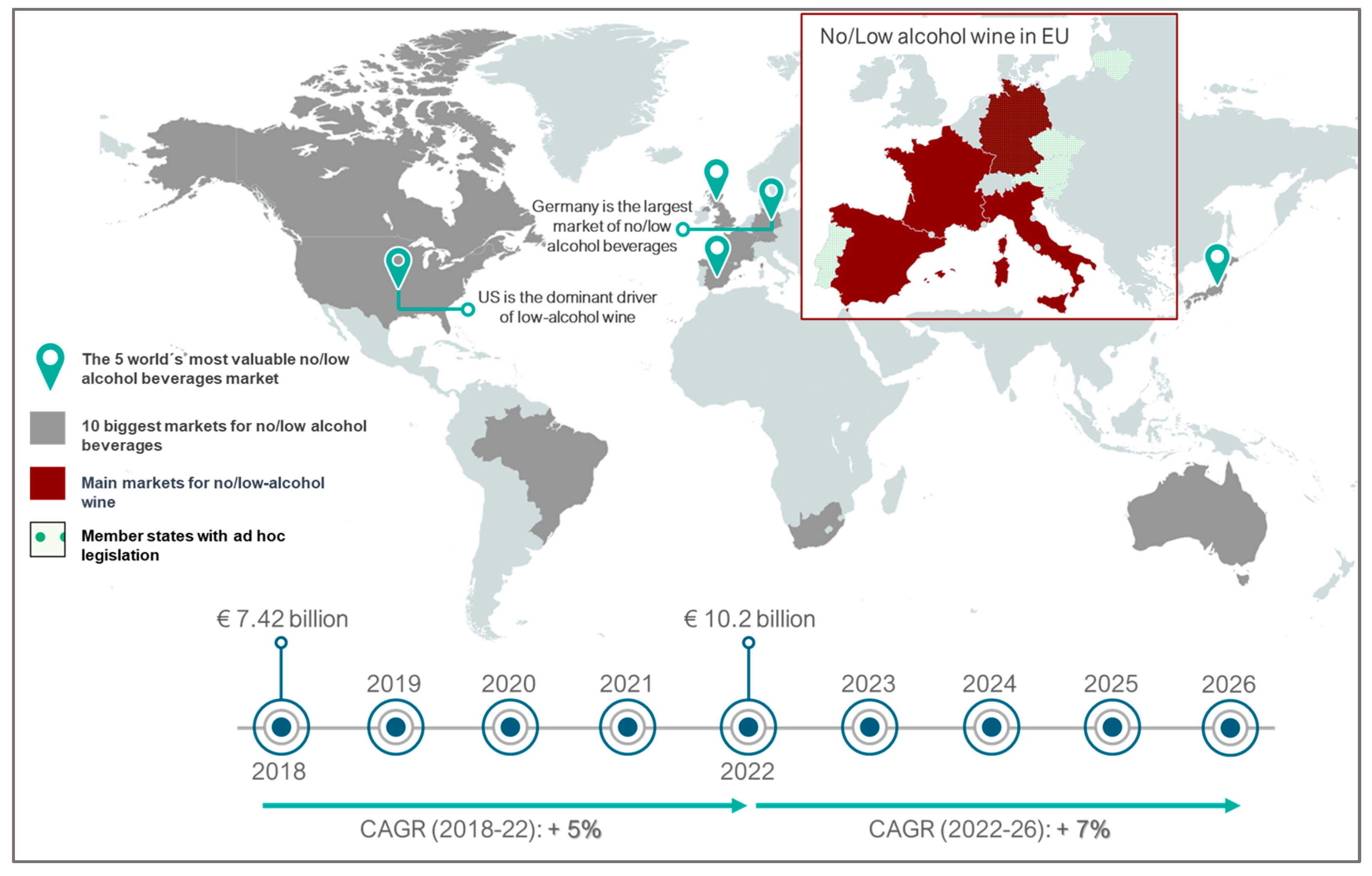
Figure 1. Overview of global market trends for no-alcohol and low-alcohol beverages, including wine.
2. Techniques to Decrease Alcohol Content in Wines
In recent years, consumer preferences have evolved substantially as they have become increasingly demanding, conscious of the characteristics of the goods they choose, and focused on products that promote a healthier lifestyle. This trend extends to the consumption of wine, where consumers are more attentive and concerned about the health risks associated with alcohol consumption. As a result, there is an increasing demand for wines with reduced alcohol content [22][23][24], thus driving the production and sale of such wines. In this regard, winemakers have been actively seeking technological strategies to decrease or eliminate the alcohol content of wines. Furthermore, given the context of climate change, which has significantly contributed to the production of grapes with higher sugar levels and, consequently, an increase in the alcohol content of wines, the search for innovative methods to meet this demand becomes imperative.
These strategies for winemakers to produce wines with reduced alcohol levels can be categorized in several approaches. Still, more commonly, they are separated depending on when they are applied in the wine production process. Some authors [30][31][32] indicate four different categories, while other authors [33][34] divide these strategies into three, as will be discussed in this document (Figure 2).
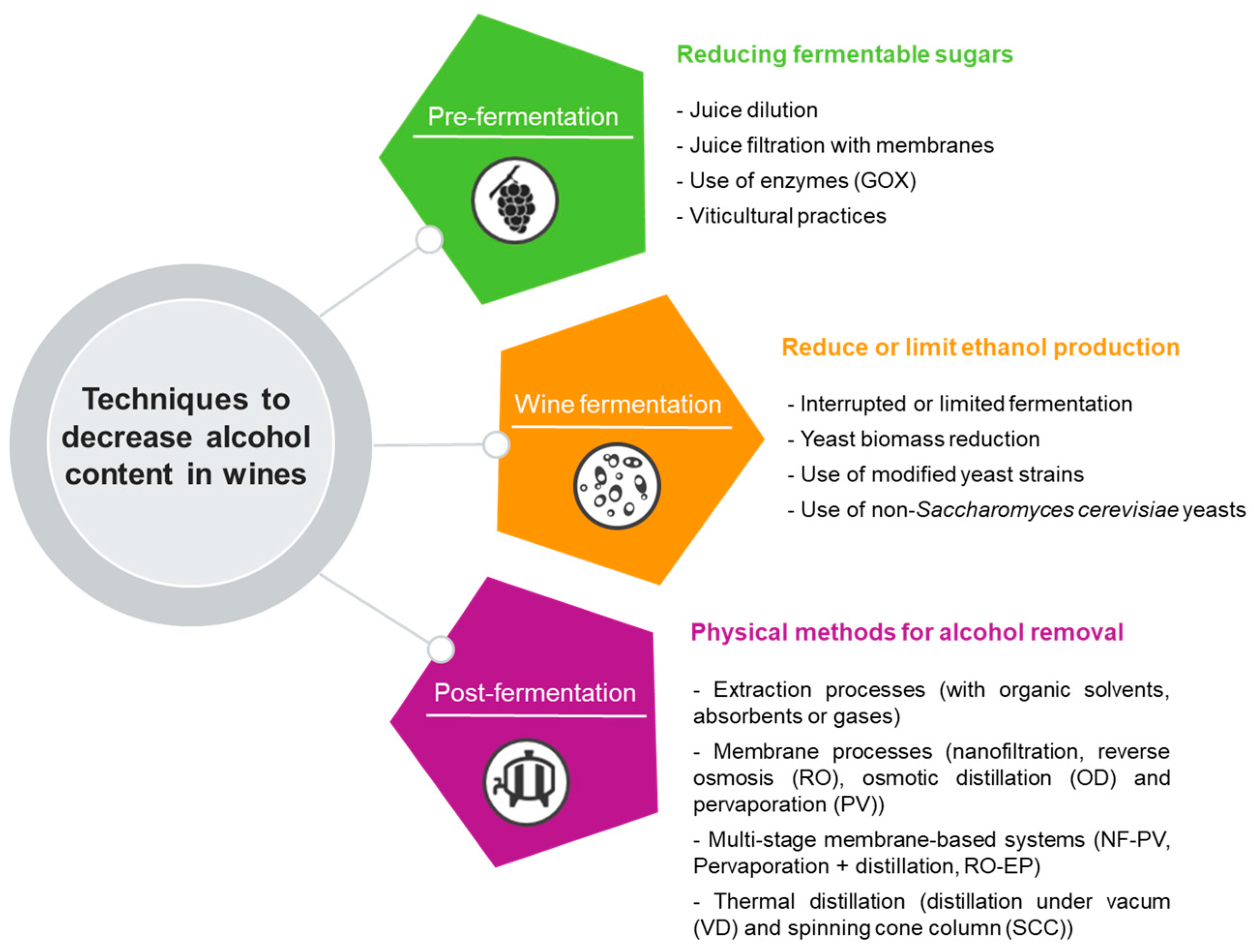
Figure 2. Techniques to decrease alcohol content in wines and fermented beverages.
2.1. Non-Microbial Alcohol Reduction in Wines
2.1.1. Reducing Fermentable Sugars in the Grapes
The first category, used during the pre-fermentation stage, focuses on reducing fermentable sugars. This limits the amount of alcohol produced during fermentation, resulting in wines with lower alcohol content right from the start [34]. It is one of the most common methods for producing wines with lower or reduced alcohol content. Techniques such as juice dilution and juice filtration with membranes or enzymes are employed at this stage. Additionally, viticultural practices such as early harvesting, growth regulators, reducing leaf area to limit photosynthetic rate, and pre-harvest irrigation are cited by Schelezki et al. [35]. Specific viticultural methods can produce a wine with lower alcohol content and higher acidity, which can then be blended with a more mature fermented juice. Although these methods are known to decrease ethanol concentration by 3% (v/v), the resulting wines may have unpleasant, acidic, and unripe flavors [36][37].
Juice Dilution
The addition of water to the must to reduce the concentration of fermentable sugars effectively reduced the characteristics of Cabernet Sauvignon and Shiraz wines. However, it is necessary to find a limit to avoid changes in the sensory profile of wines, as demonstrated in other studies [38].
Juice Filtration with Membranes
Nanofiltration, ultrafiltration, or reverse osmosis membranes retain sugar from the must before fermentation. The filtered juice is then fermented to produce wine with reduced alcohol content. Several studies have investigated this technology’s use and reported positive results that do not affect essential compounds such as polyphenols, malic acid, and tartaric acid, with no significant changes in the sensory profile of the wines [39][40][41][42][43].
Use of Enzymes
The use of enzymes, specifically glucose oxidase (GOX), has shown to be an effective technique in reducing the alcohol content of wines by reducing glucose in grape juice before the fermentation process and has been the subject of numerous studies [44][45][46][47][48]. Derived from the fungus Aspergillus niger, the GOX enzyme promotes the conversion of β-D-glucose into D-glucono-lactone, releasing H2O2, and subsequently catalyzes the conversion of D-glucono-lactone into gluconic acid, producing gluconic acid [45]. These reactions are responsible for the oxidation of fermentable sugars in the juice, thus preventing ethanol formation during fermentation. Applying the GOX enzyme has been shown in assays to decrease alcohol content by approximately 2% v/v [46] to 4.3% v/v [47]. Petkova et al. [48] found a 28% reduction in glucose after enzyme application, preserving some glucose and fructose for yeast fermentation in a second treatment. Additional studies observed not only reduced alcohol content but also decreases in heptyl acetate concentration, certain alcohols with floral notes, and ketones with floral and fruity notes in the wines [44].
Viticultural Practices
Early grape harvesting is another widely used pre-fermentation method to reduce alcohol in wines, involving either early harvesting or blending mature grapes with early-harvested ones. This approach has shown promise, achieving about a 3% v/v decrease in ethanol content [49][50]. In studies with Pinot Noir and Tannat grape varieties, this technique not only lowered alcohol content but also decreased pH and total acidity without affecting other wine constituents [51].
Therefore, regarding viticultural practices, it is essential to carefully consider early harvest to maximize grape flavor compounds for the final wine style. However, this approach may not be appropriate for all wine styles. Early harvest can be beneficial when there is a rapid onset of berry shrivel. Still, options for color enhancement in red wines should be considered depending on how early the harvest is conducted [52][53].
Using growth regulators also appears to be a viable option, as they can reduce the sugar concentration in the berries, resulting in lower wine alcohol content [54][55][56]. Naphthaleneacetic acid, used as an antitranspirant on Syrah grapes during pre-veraison, led to delayed berry ripening. This delay allowed better management of sugar accumulation and resulted in wines with no significant changes in sensory profiles [57].
Grapevine canopy management, a crucial viticultural practice for vineyard balance, significantly influences sugar accumulation in grapes [58]. Reducing leaf area through severe pruning or leaf removal at various growth stages minimizes fermentable sugar accumulation, lowering ethanol content in the resulting wine [33]. Zhang et al. [59] compared basal and apical defoliation, observing lower alcohol content in Shiraz grapes and wines with minimal impact on aromatic properties. Similar outcomes were reported by Poni et al. [60], achieving significantly reduced total soluble solids and alcohol concentration without affecting phenolic composition through leaf removal above the grape cluster zone in Sangiovese after veraison. Other studies have aimed to reduce the alcohol content in wine through leaf area reduction [61][62][63][64]. However, shoot thinning in Carot Noir grapes (a red interspecific hybrid of Vitis) increased soluble solids and alcohol content [65], as it did in Cabernet Sauvignon grapes [66]. Demonstration that the effects of shoot thinning practices are unclear and depend on the grape variety. A previous study on shoot thinning found that shoot-thinned Marechal Foch (a red interspecific hybrid of Vitis) vines showed higher total soluble solids (ᵒBrix) and berry anthocyanin concentrations compared to un-thinned vines [67]. However, the increase in berry anthocyanin did not result in higher anthocyanin concentration in the final wine. Furthermore, shoot thinning did not affect the sensory perception of the “fruitiness” of the wines [67]. In contrast, a study focused on Corot noir and the implementation of shoot thinning provided inconsistent results in grape and wine quality across a two-year evaluation (2008–2009). The evaluation was determined by soluble sugars (ᵒBrix), pH, titratable acidity (TA), wine anthocyanin, berry, and wine tannin content. The study found that shoot thinning increased berry ᵒBrix, wine alcohol concentration, and anthocyanin concentration only in the second year [65].
2.1.2. Reduce or Limit Ethanol after Winemaking
The following category relates to post-fermentation procedures. In this phase, physical methods for alcohol removal, including extraction, membrane separation, or distillation, are applied.
Alcohol Removal via Extraction Methods
Extraction can be carried out using gases, organic solvents (not for wine used commercially), or adsorbents.
The unique properties of carbon dioxide (CO2), including its ability to transform into a supercritical fluid under specific temperature and pressure conditions, make it an effective compound for extracting organic compounds, such as ethanol in wine. In its liquid state within the wine, CO2 has an affinity for the carbon chain of ethanol, facilitating its dissolution. As it transitions back to a gaseous state, it carries the dissolved ethanol, reducing the ethanol content in the wine [68]. According to Ruiz-Rodríguez et al. [69], extraction carried out with supercritical CO2 in white, red, and rosé wines has proven to be an advantageous alternative. Besides removing ethanol from beverages, it leaves no residues in the wines and does not alter their aromatic profile or antioxidant activity. Furthermore, it is an economical, safe, and easy-to-handle solvent [34]. Other organic solvents like pentane and hexane are also used to remove ethanol from wines but may remove other aromatic compounds, affecting the final taste of the wines [70]. Hydrophobic adsorbents like zeolites are used in the production of dealcoholized wine. Nikolaou et al. [71] reported a conversion rate of 69.2% of malic acid content and the production of wines with low ethanol content. According to Akyereko et al. [72], this method enables the production of wines with an ethanol content of 0.5% v/v.
Alcohol Removal through Membrane-Based Processes
The separation through the use of membranes refers to physical separation techniques aimed at reducing or eliminating the alcohol content of wine through a semipermeable membrane. In this method, a natural osmotic pressure occurs due to the difference in concentration between two solutions flowing through the semipermeable membrane (tangentially, in parallel, or circularly). As a result, alcohol and water from the wine move from the more concentrated solution to the less concentrated one, reducing or eliminating ethanol from the wine [40][73][74]. The most commonly used membrane separation techniques include nanofiltration, reverse osmosis (RO), osmotic distillation (OD), and pervaporation (PV).
Besides its application in pre-fermentation to lower the sugar content in the must, nanofiltration can also be employed for alcohol removal from finished wines [40][75][76]. The semipermeable membrane has a size ranging from 1 to 10 nm, allowing the rejection of smaller molecules (such as sugars and proteins) at a pressure of about 75 bar, surpassing ultrafiltration membranes [77]. Some studies demonstrate the advantages and effectiveness of this technique compared to the reverse osmosis technique, which, in addition to reducing the alcohol content of wine, preserves its organoleptic characteristics with fewer losses in anthocyanins at lower pressures, making the process more cost-effective [43][78][79][80].
On the other hand, the RO process involves the application of high pressures, in the range of 60 to 80 bar, under which water and ethanol molecules are forced through the semi-permeable membrane, leaving behind a retentate with the remaining compounds. Simultaneously, a permeate flow is generated, containing higher amounts of water and ethanol due to their smaller molecular size [23][81]. However, adding water to the retentate is necessary to achieve efficient dealcoholization, which becomes a disadvantage of this process since it is generally illegal or restricted in many wine-producing countries. Additionally, this technique has other challenges related to dilution due to potential alterations in the sensory properties of wines and the operation at high pressures [79][82][83].
Another membrane separation technique for producing low-alcohol wine is osmotic distillation (OD), also referred to as evaporative perstraction (EP) or isothermal membrane distillation [34]. This technique is based on two aqueous phases: the wine containing volatile compounds and the water acting as the stripping liquid. Both circulate in countercurrent on opposite sides of a hydrophobic hollow fiber membrane module. The mechanism of ethanol removal in this technique involves the initial evaporation of ethanol due to increased temperature and the difference in partial pressure between ethanol in the wine and the space inside the membrane pores. Subsequently, the ethanol vapor diffuses through the membrane pores and, upon emerging from these pores, mixes with the stripping water solution. When the ethanol vapor comes into contact with the stripping water, it condenses into a liquid state [41][74]. This evaporation, diffusion, and condensation process allows ethanol removal from wine, with values ranging from 1.3% to 10.5% v/v, while preserving some essential components [84][85][86]. Reducing alcohol in wine can negatively affect its quality, causing loss of aroma, oxidation, and spoilage due to microorganisms. Therefore, it is crucial to follow the manufacturer’s recommendations and perform the alcohol reduction with care [86][87][88].
In contrast to OD, vapor permeation (PV) is a separation technique that employs dense and non-porous membranes to separate components from liquid mixtures based on partial evaporation. In this technique, the liquid mixture comes into direct contact with the selective side of the membrane (chemical affinity). In contrast, the permeate is collected in vapor form on the opposite side of the membrane. Separation occurs due to a driving force, a vacuum, a sweep of inert gas (such as nitrogen), or a temperature difference. This technique has three main steps: adsorption of the target component onto the membrane, diffusion of this component through the membrane, and desorption on the permeate side [89][90]. This technique has been explored as a promising method for reducing the alcohol content in wines due to several advantages applied in this context: it allows for the selective removal of ethanol from wine while preserving its aromatic profile, thus avoiding significant losses in sensory quality; it is more energy-efficient compared to traditional distillation; there is a lower possibility of contamination of the final product; it operates at lower temperatures than other dealcoholization methods; it is a “clean” technique as it produces water and ethanol as byproducts that can be reused [89][91][92]. Nevertheless, this technique faces limitations, such as high investment costs, a limited membrane market, low permeation rates at low temperatures, and the need to optimize operating conditions to achieve desired results [33][74][92].
Alcohol Removal: Thermal Processes in Winemaking
Thermal processes are widely employed to reduce the alcohol content in wines. Vacuum distillation (VD) and spinning cone column (SCC) are methods based on the fundamental principle of heating and evaporation.
Vacuum distillation (VD) is another process for producing low-alcohol wines. It is a thermal process that, under vacuum conditions, concerns evaporation, distillation, and condensation [93]. VD entails heating the wine to relatively low temperatures (typically between 15 °C and 20 °C) compared to traditional distillation. Heating is essential for evaporating volatile compounds, particularly ethanol, from the wine. The alcohol vapor is then separated under vacuum conditions and condensed into liquid form, yielding a distillate containing the extracted alcohol. After alcohol removal, the remaining wine has a significantly lower alcohol content, and retention of the aromas and flavors present in the original wine can occur under certain conditions [33]. Furthermore, it allows for flexible adjustment of wine alcohol content based on producer and consumer preferences. The recovered distillate can be added to the dealcoholized portion [33][89]. Nevertheless, studies indicate that while VD enhances volatile compounds, it may significantly reduce esters, alcohols, and terpenes, impacting the wine’s aromatic complexity and sensory profile [34][94].
The SCC technique, widely used in the beverage industry for producing low-alcohol beverages, efficiently preserves aromatic compounds [95]. It involves rotating vertically stacked cones within a column [55]. The alcohol removal occurs in two phases: first, the wine undergoes SCC at a moderate temperature (26–28 °C) and reduced vacuum pressure (0.04 atm) to extract aromatic compounds. In the second phase, at higher pressure and temperature (38 °C), the alcohol content is reduced, resulting in a low-alcohol or non-alcoholic wine, depending on the remaining ethanol. Aromas recovered in the first phase enhance the final wine’s aroma [96][97]. Studies highlight the effectiveness of SCC in reducing wine alcohol content and recovering aromatic compounds from red, white, and rosé wines [74][95][96][97][98]. Furthermore, a study conducted by Puglisi et al. [99] suggests SCC, combined with adsorbents, as a profitable strategy for remediating “smoke taint” in wines from grapes affected by wildfires. However, SCC technology is costly and involves expense management [34].
Multi-Stage Membrane-Based Systems
Recently, an innovative approach has emerged for producing low-alcohol beverages, aiming to prevent the loss of desirable aromatic compounds associated with single-membrane dealcoholization methods (such as NF, PV, RO, and OD) and thermal separation processes [23][74][84][85][93]. This approach uses an integrated membrane and distillation system called a multi-stage membrane-based system [74]. This technique combines two or more alcohol removal methods to remove ethanol from wines and beers. Commonly used multi-stage membrane-based systems include nanofiltration and pervaporation (NF–PV) systems [100], pervaporation units combined with distillation [78], and reverse osmosis-evaporative pertraction (RO–EP) systems [101], with the RO–EP system being the most widely used. These combined systems have proven highly efficient for producing low-alcohol wine, as they not only maintain aroma characteristics similar to the original wine but often achieve improved versions compared to the original product [100][102][103]. Despite some losses of desirable aromatic compounds such as ethyl esters, acetate esters, and monoterpenes [101][104], the ability of these systems to preserve the wine’s aroma and volatiles constitutes a promising strategy in the production of low-alcohol or alcohol-free beverages, meeting the growing consumer demand for healthier and high-quality products.
2.2. Microbial Strategies for Producing Low-Alcohol Wines
The increasing trend of alcohol content in wines, linked to climate change, might result in changes in flavor and complexity and, given the current consumer preferences, might negatively impact commercialization. Therefore, strategies limiting alcohol production or its reduction must be defined. These techniques can be categorized into three fundamental approaches, as mentioned earlier: pre-fermentation, fermentation processes, and post-fermentation techniques [105]. Focusing on microbial strategies, emphasis must be placed on the selection of fermentation microorganisms, their proportion or time of inoculation on grape must, and the conditions during fermentation. The goal is to reduce or restrict ethanol production during the fermentation phase. Specific yeast strains (genetically modified or non-Saccharomyces yeasts) can also be used to reduce yeast biomass (keeping the fermentation rate of fermentable sugars as low as possible) [34], or techniques such as interrupted fermentation are employed for this purpose.
2.2.1. GMO Microorganisms
Recently, genetic modifications or adaptive evolution and selection have developed modified yeast strains capable of reducing wine alcohol content during fermentation [69][78][106][107]. However, their use may pose challenges, such as producing undesired secondary metabolites, like acetaldehyde and acetoin, that can affect wine characteristics [108][109]. Another challenge in using such yeast strains is consumer acceptance of genetically modified organisms in food and beverages.
Given the relative simplicity of the yeast genome, its modification can be achieved. To obtain low-ethanol wine, the logical step would be to limit the expression of the enzyme alcohol dehydrogenase (ADH), Figure 3, which catalyzes the final step in ethanol production during alcoholic fermentation. However, this approach was deemed unpractical because strains with ADH deletion could not grow under anaerobic conditions and the production of higher levels of acetic acid and acetaldehyde [110][111]. Hence, novel targets had to be found to redirect the metabolism from ethanol production to other end-products [32]. However, these changes in the metabolic pathways must be carefully monitored, as other products can impact the overall quality of the wine [112].
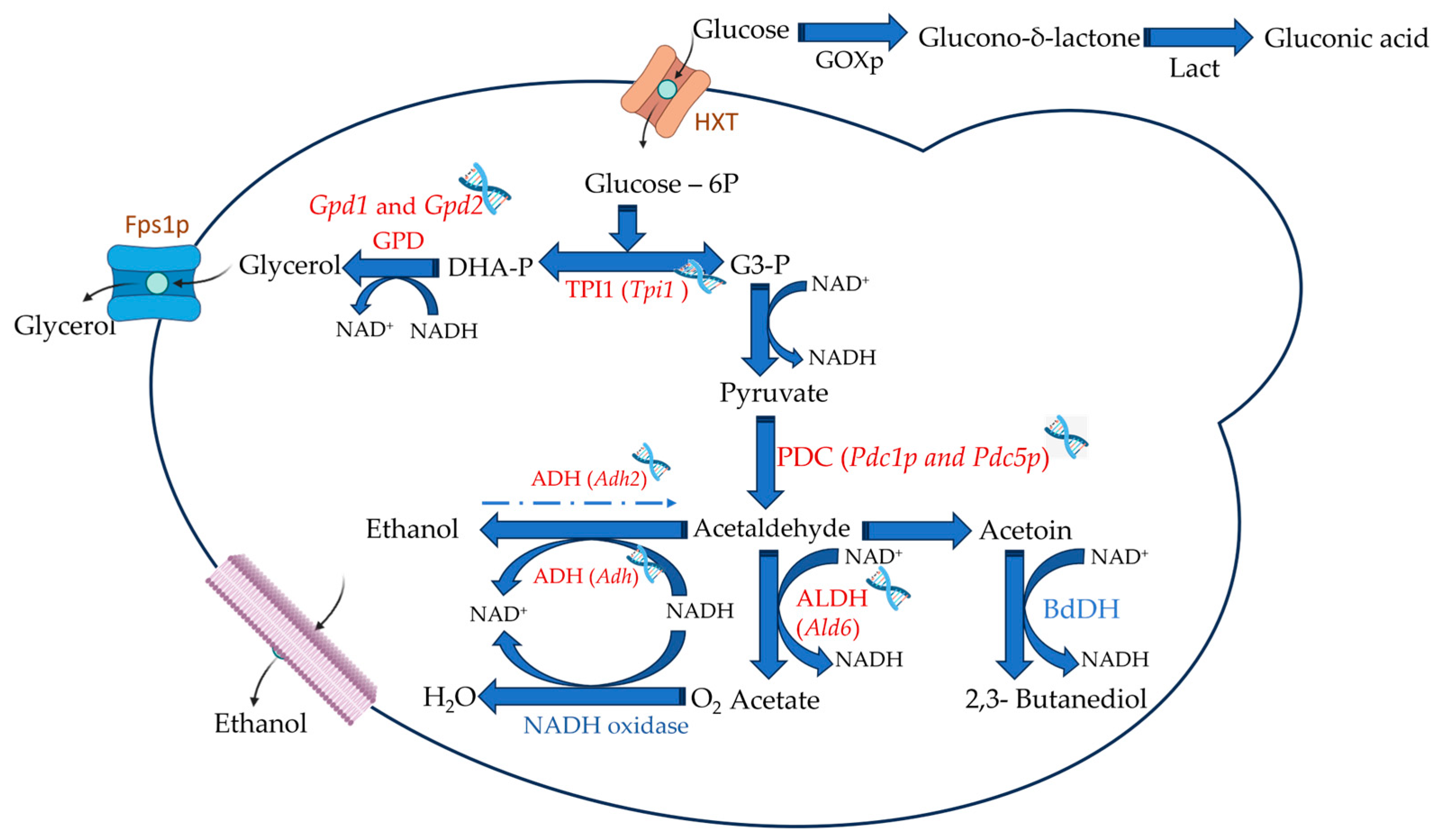
Figure 3. Main target specific enzymes and genes in yeast cells that may lead to lower ethanol yields. HXT—hexose transporter; Fps1p—aquaglyceroporin; GPD—glyceraldehyde-3-phosphate dehydrogenase; TPI1—triosephosphate isomerase; PDC—pyruvate decarboxylase; ADH—alcohol dehydrogenase and ALDH—Aldehyde dehydrogenase. GOXp—Glucose-oxidase—and Lact—lactonase—are expressions, in yeasts, of non-yeast genes.
One of the modifications that can be used to reduce ethanol production is linked to the overexpression of glycerol-3-phosphate dehydrogenase isozymes, namely through Gpd1 and Gpd2 genes [113], Figure 3. Glycerol production usually uses about 4% of grape juice carbon during fermentation by S. cerevisiae, generally in the initial stages of biomass formation [114]. Glycerol has two essential functions: to combat osmotic stress and to maintain the oxidation-reduction balance. The reaction behind glycerol formation is linked to the correction of redox balance within cells [115]. The overexpression of these genes increases glycerol synthesis while decreasing ethanol synthesis [116]. This increase in glycerol production can reach as much as 548%, while the reduction in ethanol can be of great significance, as shown in early works [117]. Other results indicate an increase in glycerol production ranging from 109 to 275% and a reduction in ethanol from 3 to 24%, depending on the experimental medium (yeast extract, peptone, dextrose medium, yeast nitrogen base medium, synthetic medium, grape juice or synthetic Leu-free) or the overexpressed gene (Gpd1 or Gpd2) [112]. However, other metabolites are also more produced and can cause changes in the quality of wine. Of those, succinate, acetate, acetaldehyde, acetoin, and 2,3-butanediol [118][119], Figure 3, must be referred to, as their presence above the sensory threshold may be detrimental to perceived wine quality [112][120]. Mutant yeasts with modifications of GPD need further genetic modifications to avoid excessive production of these metabolites. One modification is the deletion of aldehyde dehydrogenases, namely the Ald6 gene, that contribute to the formation of acetic acid [121]. This modification decreased the formation of acetic acid, with increased glycerol production and lower ethanol yield [119]. However, a subsequent problem arose, as the deletion of Ald6 increased acetoin production, negatively affecting wine aroma. Ehsani and collaborators [122] obtained a strain that produced lower ethanol levels (3%, v/v less) and higher glycerol production with a reduced impact on sensory parameters in the final wine.
Other changes in glycerol metabolism can be achieved by changing the expression of genes linked to Fps1p, an aquaglyceroporin channel that controls the intracellular glycerol concentration [123][124][125]. The production of glycerol is controlled by a regulatory domain located in the N-terminal extension of Fps1p. This domain regulates glycerol transport; when removed, the channel becomes hyperactive. As a result, glycerol continuously leaks out of the cell, and the cell compensates for the loss by producing more glycerol [124][126]. Varela et al. [109] observed that the increased glycerol production, due to deleting the regulatory domain of Fps1p, lowered ethanol formation considerably (Figure 3).
Another gene modification to reduce ethanol production can be deleting Pyruvate decarboxylase (PDC) genes. This enzyme catalyzes the decarboxylation of pyruvate to acetaldehyde and CO2, being three genes known in S. cerevisiae (Pdc1, Pdc5, and Pdc6) up-regulated by the transcription factor Pdc2p; however, only Pdc1p and Pdc5p are known to be active in yeast during fermentation [127], Figure 3. Modifying the PDC genes resulted in diverse outcomes. Deleting Pdc1 resulted in lower activity and increased pyruvate levels, which are undesirable, considering microbial stability and balance of sulfur dioxide. Still, no reduction in ethanol, while deleting Pdc2, led to a considerable decrease in ethanol levels and increased glycerol production while maintaining sufficient pyruvate decarboxylase activity to support glucose growth [117]. Further changes in this set of genes were performed by Cuello et al. [128], which resulted in a strain with reduced ethanol production without effect on other fermentation kinetics.
Another gene editing that can be used to reduce the production of ethanol is modifying the expression of triose-phosphate isomerase, encoded by the Tpi1, which is the enzyme catalyzing the interconversion between dihydroxyacetone phosphate and glyceraldehyde 3-phosphate, the two products following the breakdown of fructose 1,6-bisphosphate [111], Figure 3. The lack of this gene resulted in high amounts of glycerol, with a reduced ethanol yield [129]. However, the deletion of the Tpi1 gene caused the inability of this strain to grow in a glucose medium, probably due to the reduced content of NADH, which is produced during the conversion of glyceraldehyde 3-phosphate into pyruvate [130][131]. A complete loss of activity of the TPI1 enzyme seems like it would be more feasible, as a reduction in yeast growth with attendant fermentation problems is likely to occur. Still, a partial reduction in its activity could provide an opportunity for low alcohol-producing yeast. This partial reduction can be achieved by changing regulatory genes, like Gcr1 and Gcr2 (transcription factor for glycolytic genes), that can reduce the expression of Tpi1 [132], with mutations in other genes, like Reb1 (an essential gene that maps on chromosome), Rap1 (Repressor Activator Protein), and Grc1 (component of the minus-end located γ-tubulin ring complex) also able to reduce TPI1 activity [133]. Avoiding glucose repression of respiration has also been a target for producing low-alcohol wines. This approach relied on the use of a chimeric glucose transporter, comprised of the amino-terminal part of HXT and the carboxy-terminal region of Hxt7 (HXT1, 2, 3, 4, 6, and 7 are intramembrane transporter proteins known to be involved in the transport of glucose) [134]. Using a yeast strain with deleted Hxt1, 2, 3, 4, 6, and 7 resulted in a respiratory phenotype with low ethanol production [135], even though results were achieved using low sugar concentrations medium (5%, w/v) compared to grape juice. This shifts the metabolism from the Pasteur effect (under low O2 concentrations, yeasts conduct alcoholic fermentation forming ethanol and CO2, or, under high O2 concentrations, aerobic fermentation occurs with lower glucose consumption) to the Crabtree effect, where, in the presence of high sugar content (about 200 g/L), regardless of O2 presence, fermentation can occur [136].
A different approach is the expression, in yeasts, of non-yeast genes. Reducing the content of sugars in grape juice before fermentation can be achieved by glucose oxidase (GOXp), an oxygen-dependent dehydrogenase that catalyzes the first step of a two-step process associated with converting glucose to gluconic acid [112]. Besides treating grape juice with GOX to reduce the sugar content, a strategy that has several limitations, even though it can reduce up to 40% of ethanol production [137], the introduction of the gene encoding this enzyme has already been performed in S. cerevisiae [138]. Microvinifications of Chardonnay juice samples resulted in wines containing 1.8–2.0% less alcohol, possibly due to the use of glucose to produce D-glucono-δ-lactone and gluconic acid by GOX (Figure 3). Other authors overexpressed the gene noxE from Lactoccocus lactis, encoding H2O-forming NADH oxidase, NoxEp, that uses NADH, oxidizing it when oxygen is available [139]. The introduction of this NADH oxidase can decrease the available intracellular NADH pool, affecting alcohol dehydrogenase (ADH), hence resulting in reduced ethanol formation [32]. This resulted in using only half the available sugar but with paralleled changes in other metabolic pathways, increasing acetaldehyde production, impairing growth, and fermentation performance. All these genetic manipulation approaches to low ethanol-producing yeast, as well as others that might arise, will be dependent on the acceptability of both the industry and consumers of using such yeast, pushing current research on the search for non-GMO alternatives [31].
2.2.2. Yeast Selection for Low Alcohol Production
One alternative to avoid GMO yeasts, designed for low-ethanol production, is to isolate those that naturally present that trait. However, this effort for S. cerevisiae can be complex, as biochemical and physiological characteristics and the underlying genetics of this yeast have been pushed by natural selection to favor the yield of ethanol [31][140]. This has resulted in a slight variation in this phenotype [141], with currently available S. cerevisiae wine yeasts resulting in similar ethanol production when fermenting the same must [142]. Hence, the option would be selecting non-Saccharomyces (NS) yeasts that preferentially consume sugars by respiration rather than fermentation [136]. Therefore, evaluation of ethanol production variation among NS yeasts has been addressed. Using non-Saccharomyces yeasts has garnered significant interest from the scientific community and winemakers, as the available data state (e.g., [143][144][145]). These yeasts can divert carbon or sugar metabolism into other pathways, thus avoiding ethanol production during fermentation [143][144][145][146][147]. Several studies have shown significantly reduced wine ethanol levels when using these yeasts. For instance, Magyar and Toth [148] identified Saccharomyces uvarum, Candida stellata, and C. zemplinina strains with exciting properties. These strains produced, in laboratory fermentations, similar residual concentrations of sugars but with considerable chances in alcohol production, namely for C. zemplinina, with approximately half the alcohol content that was recorded for S. cerevisiae.
Another exciting work was performed by Gobbi et al. [149], using Zygosaccharomyces bailii, Z. sapae, Z. bisporus, C. zemplinina, C. stellata, Hanseniaspora uvarum, Saccharomycodes ludwigii, Dekkera bruxellensis, and S. cerevisiae, for fermentation tests with grape juices. Results showed significantly low ethanol production in Z. bailii, Z. sapae, Z. bisporus, and C. zemplinina, but more prominently when using H. uvarum, confirming data observed by other authors [150][151]. Low ethanol production has also been reported for strains of Metschnikowia pulcherrima, Schizosaccharomyces malidevorans and C. stellata [146], Torulaspora delbrueckii [150], Pichia kudriavzevii and Z. bailii [152]. Schizosaccharomyces pombe reduced 0.65% of ethanol in the fermentation of white Airén grapes [153], and some Saccharomyces species can also provide low ethanol-producing strains. A reduction of 0.7% was achieved with the use of S. uvarum [154], with S. kudriavzevii also presenting interesting results [155]. However, some adverse effects have also been linked to the use of these alternative strains if partial aeration strategies during fermentation are applied to allow the use of sugar to be consumed via respiration rather than alcoholic fermentation, namely the formation of undesirable volatile compounds, including acetic acid [152][156][157], even though positive effects on sensory characteristics also occur [32].
Additionally, non-Saccharomyces yeasts play a multifaceted role in wine fermentation, potentially enhancing its sensory profile and aromas and contributing to wine stability and complexity [143][144][145][146][147][149][157][158][159][160][161][162]. Some non-Saccharomyces possess antimicrobial properties, including the production of Killer factors (mycocins), which inhibit the growth of undesirable yeasts (Zygosaccharomyces genus, Brettanomyces bruxellensis, among others), providing an additional advantage [163]. These “Killer factors”, such as CpKT1 and CpKT2 produced by Candida pyralidae, have demonstrated effectiveness in controlling the population of undesired yeast strains, such as B. bruxellensis, in winemaking conditions, without adversely affecting the fermentation processes of S. cerevisiae or the tested lactic acid bacteria [164]. Other “Killer factors”, such as KTCf20 and Pikt (produced by Wickerhamomyces anomalus), Kwkt (produced by Kluyveromyces wickerhamii), PMKT and PMKT2 (produced by Pichia membranifaciens), have also demonstrated potential in controlling unwanted yeast strains in vinification environments [165][166][167][168].
2.2.3. Co-Inoculations and Sequential Inoculations (Non-Saccharomyces and S. cerevisiae)
Considering some of the previously referred advantages and drawbacks of using different yeasts, two approaches to reducing the ethanol content in wine are co-inoculation of those yeasts or their sequential introduction in fermentation. The first approach (co-inoculation) involves concurrent inoculations of non-Saccharomyces or other Saccharomyces non-cerevisiae yeasts at high cell concentration with S. cerevisiae, and the second approach (sequential inoculation) consists of the start of fermentation with non-Saccharomyces or other Saccharomyces non-cerevisiae yeasts, occurring for a given duration and inoculating S. cerevisiae to take over and complete the fermentation [169].
The critical factors affecting fermentation and oenological outcomes of this approach are the time leading to the inoculation of S. cerevisiae (in sequential fermentations) and the ratio of S. cerevisiae and other yeast [160]. Besides ethanol changes, non-Saccharomyces or other Saccharomyces non-cerevisiae yeasts are essential due to their contribution to wine aroma and flavor, with several yeasts already described as contributors to that profile. Padilla et al. [160] and Ivit et al. [144] point out several yeasts as having great oenological interest and used in co- or sequential inoculations, which will be briefly reviewed here.
One of the most important genera is Hanseniaspora, which comprises at least ten species, H. uvarum and H. guilliermondii being the most common. Several Hanseniaspora species have been tested in sequential or co-inoculated fermentation with S. cerevisiae, with a recorded reduction in the ethanol content of wines. Reductions of around 1% were achieved using sequential inoculation of H. uvarum and S. cerevisiae in synthetic grape juice [158], and, in white (Sauvignon blanc) and red (Pinotage) musts, reductions in ethanol were also achieved of around 1.3% and 0.8%, for white and red musts, respectively [170]. Furthermore, three strains of H. uvarum, in sequential or co-inoculated fermentations with S. cerevisiae, resulted in lower ethanol concentration when compared to fermentations with the latter only [171]. Also, in synthetic grape juice, a reduction in alcohol was recorded with sequential inoculation of H. osmophyla and S. cerevisiae [158] and white (Sauvignon blanc) and red (Pinotage) musts; the use of H. opuntiae also resulted in less production of ethanol [170]. However, some studies point out the increase in acetic acid when fermentations are performed using Hanseniaspora yeast strains [171].
Another important yeast already known to have essential winemaking traits is Schizosaccharomyces pombe. Besides being able to moderate wine acidity by metabolizing malic acid, this strain enhances the color of red wine and reduces Ochratoxin A, biogenic amines, and ethyl carbamate [145]. In addition, some S. pombe strains used in sequential fermentation resulted in lower ethanol content [153], even though a lack of reduction or even increase in alcohol has been reported when using S. pombe, or even the presence of unsuitable aroma produced by the fermentative metabolism of S. pombe [172].
An alternative non-Saccharomyces yeast of great importance is Metschnikowia pulcherrima. This non-Saccharomyces yeast is commercially available from many suppliers and is known to improve several organoleptic characteristics of wines [173]. Furthermore, the production of wines with lower ethanol in sequential fermentations with S. cerevisiae has been reported in several works, either with grape juice [174], synthetic grape must [175], Chardonnay and Shiraz musts [146], and in white grape must (mixture of Malvasia and Viura varieties) [156]. A reduction of up to 1.6% in ethanol was recorded in Shiraz wines [146], with a drop of alcohol further confirmed in later works [176]. The use of M. pulcherrima and S. uvarum mixed inoculum, sequentially used with S. cerevisiae, reduced 1.7% v/v of ethanol compared to wine fermented with S. cerevisiae [176]. When immobilized, the sequential inoculation of M. pulcherrima could also reduce ethanol content in synthetic or natural grape juice [158]. However, results are linked to several conditions, namely aeration regimes, that must be carefully monitored [156][177].
Lachancea thermotolerans (previously Kluyveromyces thermotolerans) is a commercially available yeast that positively influences wine’s sensory profile and total acidity [161]. Besides organoleptic advantages, reduction in ethanol content has been achieved in sequential or co-inoculation. A fermentation started with L. thermotolerans and a sequential inoculation, after two days, with S. cerevisiae, led to a reduction in ethanol up to 0.7% v/v [159][178][179]. Mixed or sequential fermentation with L. thermotolerans and S. cerevisiae also reduced alcohol production [180]. Further works prove that sequential fermentations with L. thermotolerans and S. cerevisiae can reduce the ethanol content in must of Tempranillo grapes. Mixed fermentations of S. pombe and L. thermotolerans can lower the ethanol content in wine but may also increase the acetaldehyde content [143]. The sensory threshold for acetaldehyde ranges from 100–125 mg/L. Typically, table wines have acetaldehyde levels below 75 mg/L immediately after fermentation. However, if the levels exceed 125 mg/L, it can result in unpleasant odors such as ‘over-ripe bruised apples’, ‘stuck ferment’ character, or ‘sherry’ and ‘nut-like’ characters.
Torulaspora delbrueckii was one of the first commercially accessible non-Saccharomyces yeasts, as they had similar fermentation patterns as S. cerevisiae and were able to enhance aroma composition and positively impacting properties for traditional methods of sparkling wine [181]. Several studies have proven that its use in mixed or sequential fermentation reduced ethanol. Most of these studies refer to reductions of 0.5% or below [177][182][183][184][185][186]. Higher reductions of ethanol were recorded in other works, like 1% less alcohol using Chardonnay [162] or less than 1.5% using chemically defined grape juice [176]. However, to achieve higher levels of alcohol reduction, T. delbrueckii must be used in regular fermentations with high aeration processes [187].
Another yeast commonly studied due to positive contributions during fermentations is Starmerella bombicola (formerly known as Candida stellata). Early works by Soden et al. [188] with mixed and sequential fermentations with C. stellata and S. cerevisiae resulted in less alcohol than the mono-inoculated S. cerevisiae control. Further works with sequential fermentations using Starmerella bombicola and S. cerevisiae in Chardonnay juice yielded lower ethanol concentrations when compared to S. cerevisiae fermentations [188]. Immobilizing Starmerella bombicola is a practical approach to reducing the final ethanol content in grape must. Studies have shown that using this yeast species in the Trebbiano Toscano grape-must and the Verdicchio grape-must significantly reduce the final ethanol content [158][189].
References
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. Climate Change 2021—The Physical Science Basis: Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; pp. 3–32.
- Dinis, L.T.; Bernardo, S.; Yang, C.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Mediterranean viticulture in the context of climate change. Ciência Téc. Vitiv. 2022, 37, 139–158.
- Adão, F.; Campos, J.C.; Santos, J.A.; Malheiro, A.C.; Fraga, H. Relocation of bioclimatic suitability of Portuguese grapevine varieties under climate change scenarios. Front. Plant Sci. 2023, 14, 974020.
- Fonseca, A.; Fraga, H.; Santos, J.A. Exposure of Portuguese viticulture to weather extremes under climate change. Clim. Serv. 2023, 30, 100357.
- IPCC. Working Group II Contribution to the IPCC Sixth Assessment Report. In Climate Change 2022: Impacts, Adaptation and Vulnerability; IPCC: Geneva, Switzerland, 2022.
- van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parlker, A.; de Reséguier, L.; Ollat, N. An update on the impact of climate change in viticulture and potential adaptations. Agronomy 2019, 9, 514.
- Arfini, F. Mediterranean agriculture facing climate change: Challenges and policies. Bio-Based Appl. Econ. 2021, 10, 87–88.
- Lamonaca, E.; Santeramo, F.G.; Seccia, A. Climate changes and new productive dynamics in the global wine sector. Bio-Based Appl. Econ. 2021, 10, 123–135.
- Deschênes, O.; Greenstone, M. The economic impacts of climate change: Evidence from agricultural output and random fluctuations in weather. Am. Econ. Rev. 2007, 97, 354–385.
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318.
- Macedo, A.; Gouveia, S.; Rebelo, J.; Santos, J.; Fraga, H. International trade, non-tariff measures and climate change: Insights from Port wine exports. J. Econ. Stud. 2021, 48, 1228–1243.
- Bai, H.; Gambetta, G.A.; Wang, Y.; Kong, J.; Long, Q.; Fan, P.; Duan, W.; Liang, Z.; Dai, Z. Historical long-term cultivar× climate suitability data to inform viticultural adaptation to climate change. Sci. Data 2022, 9, 271.
- State of the World Vine and Wine Sector in 2022. Available online: https://www.oiv.int/sites/default/files/documents/OIV_State_of_the_world_Vine_and_Wine_sector_in_2022_2 (accessed on 1 November 2023).
- Parker, A.K.; de Cortázar-Atauri, I.G.; Gény, L.; Spring, J.L.; Destrac, A.; Schultz, H.; Van Leeuwen, C. Temperature-based grapevine sugar ripeness modelling for a wide range of Vitis vinifera L. cultivars. Agric. For. Meteorol. 2020, 285, 107902.
- Van Leeuwen, C.; Friant, P.; Chone, X.; Tregoat, O.; Koundouras, S.; Dubourdieu, D. Influence of climate, soil, and cultivar on terroir. Am. J. Enol. Vitic. 2004, 55, 207–217.
- Jones, G.V.; Davis, R.E. Climate influences on grapevine phenology, grape composition, and wine production and quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261.
- De Orduna, R.M. Climate change associated effects on grape and wine quality and production. Int. Food Res. J. 2010, 43, 1844–1855.
- Clemente, N.; Santos, J.A.; Fontes, N.; Graça, A.; Gonçalves, I.; Fraga, H. Grapevine Sugar Concentration Model (GSCM): A decision support tool for the douro superior winemaking region. Agronomy 2022, 12, 1404.
- Gutierrez-Gamboa, G.; Perez-Alvarez, E.P.; Rubio-Breton, P.; Garde-Cerdan, T. Changes on grape volatile composition through elicitation with methyl jasmonate, chitosan, and a yeast extract in Tempranillo (Vitis vinifera L.) grapevines. Sci. Hortic. Amst. 2019, 244, 257–262.
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.T.; Correia, C.; Schultz, H.R. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092.
- Saliba, A.J.; Ovington, L.A.; Moran, C.C. Consumer demand for low-alcohol wine in an Australian sample. Int. J. Wine Res. 2013, 5, 1–8.
- Jordão, A.M.; Vilela, A.; Cosme, F. From sugar of grape to alcohol of wine: Sensorial impact of alcohol in wine. Beverages 2015, 1, 292–310.
- Longo, R.; Blackman, J.W.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Changes in volatile composition and sensory attributes of wines during alcohol content reduction. J. Sci. Food Agric. 2017, 97, 8–16.
- Teslić, N.; Zinzani, G.; Parpinello, G.P.; Versari, A. Climate change trends, grape production, and potential alcohol concentration in wine from the “Romagna Sangiovese” appellation area (Italy). Theor. Appl. Climatol. 2018, 131, 793–803.
- Sadras, V.O.; Moran, M.A. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and Cabernet Franc. Aust. J. Grape Wine Res. 2012, 18, 115–122.
- King, E.S.; Dunn, R.L.; Heymann, H. The influence of alcohol on the sensory perception of red wines. Food Qual. Prefer. 2013, 28, 235–243.
- Eliodório, K.P.; Cunha, G.C.D.G.; Müller, C.; Lucaroni, A.C.; Giudici, R.; Walker, G.M.; Alves, S.L., Jr.; Basso, T.O. Chapter Three—Advances in yeast alcoholic fermentations for the production of bioethanol, beer and wine. In Advances in Applied Microbiology; Geoffrey, M.G., Sima, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 109, pp. 61–119. ISBN 9780128176221. ISSN 0065-2164.
- Angus, C.; Holmes, J.; Meier, P.S. Comparing alcohol taxation throughout the European Union. Addiction 2019, 114, 1489–1494.
- Rehm, J.; Neufeld, M.; Room, R.; Sornpaisarn, B.; Štelemėkas, M.; Swahn, M.H.; Lachenmeier, D.W. The impact of alcohol taxation changes on unrecorded alcohol consumption: A review and recommendations. Int. J. Drug Policy 2022, 99, 103420.
- Xynas, B.; Barnes, C. Yeast or water: Producing wine with lower alcohol levels in a warming climate: A review. J. Sci. Food Agric. 2023, 103, 3249–3260.
- Varela, C.; Dry, P.R.; Kutyna, D.R.; Francis, I.L.; Henschke, P.A.; Curtin, C.D.; Chambers, P.J. Strategies for reducing alcohol concentration in wine. Aust. J. Grape Wine Res. 2015, 21, 670–679.
- Varela, J.; Varela, C. Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr. Opin. Biotechnol. 2019, 56, 88–96.
- Sam, F.E.; Ma, T.Z.; Salifu, R.; Wang, J.; Jiang, Y.M.; Zhang, B.; Han, S.Y. Techniques for dealcoholization of wines: Their impact on wine phenolic composition, volatile composition, and sensory characteristics. Foods 2021, 10, 2498.
- Ma, T.Z.; Sam, F.E.; Zhang, B. Low-Alcohol and nonalcoholic wines: Production methods, compositional changes, and aroma improvement. In Recent Advances in Grapes and Wine Production-New Perspectives for Quality Improvement; IntechOpen: London, UK, 2022.
- Schelezki, O.J.; Deloire, A.; Jeffery, D.W. Substitution or dilution? Assessing Pre-fermentative water implementation to produce lower alcohol shiraz wines. Molecules 2020, 25, 2245.
- Filimon, V.R.; Filimon, R.; Nechita, A.; Bora, F.D.; Cotea, V.V. Compositional characteristics of low-alcohol wines obtained by staggered grape harvesting technology. Lucr. Ştiinţifice 2021, 64, 69–74.
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.-M.; Zamora, F. Use of Unripe Grapes Harvested during Cluster Thinning as a Method for Reducing Alcohol Content and pH of Wine. Aust. J. Grape Wine Res. 2011, 17, 230–238.
- Teng, B.; Petrie, P.R.; Smith, P.A.; Bindon, K.A. Comparison of water addition and early-harvest strategies to decrease alcohol concentration in Vitis vinifera cv. Shiraz wine: Impact on wine phenolics, tannin composition and colour properties. Aust. J. Grape Wine Res. 2020, 26, 158–171.
- Salgado, C.M.; Fernández-Fernández, E.; Palacio, L.; Hernández, A.; Prádanos, P. Alcohol reduction in red and white wines by nanofiltration of musts before fermentation. Food Bioprocess Proc. 2015, 96, 285–295.
- Mira, H.; De Pinho, M.N.; Guiomar, A.; Geraldes, V. Membrane processing of grape must for control of the alcohol content in fermented beverages. J. Membr. Sci. Res. 2017, 3, 308–312.
- Cassano, A.; Mecchia, A.; Drioli, E. Analyses of hydrodynamic resistances and operating parameters in the ultrafiltration of grape must. J. Food Eng. 2008, 89, 171–177.
- Filimon, R.V.; Nechita, B.; Bora, F.D.; Nechita, A.; Filimon, R. Analysis of volatile compounds in low alcoholic wines obtained by reverse osmosis of grape must. Adv. Agric. Bot. 2021, 13, 1–7.
- Ivić, I.; Kopjar, M.; Jukić, V.; Bošnjak, M.; Maglica, M.; Mesić, J.; Pichler, A. Aroma profile and chemical composition of reverse osmosis and nanofiltration concentrates of red wine Cabernet Sauvignon. Molecules 2021, 26, 874.
- Mangas, R.; González, M.R.; Martín, P.; Rodríguez-Nogales, J.M. Impact of glucose oxidase treatment in high sugar and pH musts on volatile composition of white wines. LWT 2023, 184, 114975.
- Khatami, S.H.; Vakili, O.; Ahmadi, N.; Soltani Fard, E.; Mousavi, P.; Khalvati, B.; Maleksabet, A.; Savardashtaki, A.; Taheri-Anganeh, M.; Movahedpour, A. Glucose oxidase: Applications, sources, and recombinant production. Biotechnol. Appl. Biochem. 2022, 69, 939–950.
- Röcker, J.; Schmitt, M.; Pasch, L.; Ebert, K.; Grossmann, M. The use of glucose oxidase and catalase for the enzymatic reduction of the potential ethanol content in wine. Food Chem. 2016, 210, 660–670.
- Pickering, G. The Production of Reduced-Alcohol Wine Using Glucose Oxidase. Ph.D. Thesis, Lincoln University, Lincoln, UK, 1997.
- Petkova, V.; Mladenoska, I.; Dimitrovski, D.; Stafilov, T.; Stefova, M. Pre-fermentative treatment of grape juice and must from vranec variety with a glucose oxidase from Aspergillus niger. In Experimental and Computational Investigations in Engineering: Proceedings of the International Conference of Experimental and Numerical Investigations and New Technologies, CNNTech 2020; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 83–90.
- de Toda, F.M.; Balda, P. Decreasing the alcohol level in quality red wines by the “double harvest” technique. In Proceedings of the 17th International Symposium Giesco, Asti, Italy, 29 August–2 September 2011.
- Asproudi, A.; Ferrandino, A.; Bonello, F.; Vaudano, E.; Pollon, M.; Petrozziello, M. Key norisoprenoid compounds in wines from early-harvested grapes in view of climate change. Food Chem. 2018, 268, 143–152.
- Piccardo, D.; Favre, G.; Pascual, O.; Canals, J.; Zamora, F.; González-Neves, G. Influence of the use of unripe grapes to reduce ethanol content and pH on the color, polyphenol and polysaccharide composition of conventional and hot macerated Pinot Noir and Tannat wines. Eur. Food Res. Technol. 2019, 245, 1321–1335.
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Harvesting and blending options for lower alcohol wines: A sensory and chemical investigation. J. Sci. Food Agric. 2018, 98, 33–42.
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Volatile and sensory profiling of Shiraz wine in response to alcohol management: Comparison of harvest timing versus technological approaches. Food Res. Int. 2018, 109, 561–571.
- Palliotti, A.; Poni, S.; Berrios, J.G.; Bernizzoni, F. Vine Performance and Grape Composition as Affected by Early-Season Source Limitation Induced with Anti-Transpirants in Two Red Vitis vinifera L. Cultivars. Aust. J. Grape Wine Res. 2010, 16, 426–433.
- Filimon, V.R.; Tudose Sandu Ville, Ș.; Bora, F.D.; Tudor, G.; Filimon, R.; Nechita, A.; Damian, D. Methods for Producing Low-Alcohol Wine I. Viticultural and Pre-Fermentation Strategies. 2020. Available online: https://www.uaiasi.ro/revista_horti/files/Nr1_2020/vol%2063_1_2020%20(15).pdf (accessed on 1 November 2023).
- Tyagi, K.; Maoz, I.; Lapidot, O.; Kochanek, B.; Butnaro, Y.; Shlisel, M.; Lerno, L.; Ebeler, S.; Lichter, A. Effects of gibberellin and cytokinin on phenolic and volatile composition of Sangiovese grapes. Sci. Hortic. 2022, 295, 110860.
- Böttcher, C.; Harvey, K.; Forde, C.G.; Boss, P.K.; Davies, C. Auxin treatment of pre-veraison grape (Vitis Vinifera L.) berries both delays ripening and increases the synchronicity of sugar accumulation. Aust. J. Grape Wine Res. 2011, 17, 1–8.
- Ozturk, B.; Anli, E. Different techniques for reducing alcohol levels in wine: A review. In Proceedings of the BIO Web of Conferences, Mendoza, Argentina, 9–14 November 2014; EDP Sciences: Lejulis, France, 2014; Volume 3, p. 02012.
- Zhang, P.; Wu, X.; Needs, S.; Liu, D.; Fuentes, S.; Howell, K. The influence of apical and basal defoliation on the canopy structure and biochemical composition of Vitis vinifera cv. Shiraz grapes and wine. Front. Chem. 2017, 5, 48.
- Poni, S.; Gatti, M.; Bernizzoni, F.; Civardi, S.; Bobeica, N.; Magnanini, E.; Palliotti, A. Late leaf removal aimed at delaying ripening in cv. S angiovese: Physiological assessment and vine performance. Aust. J. Grape Wine Res. 2013, 19, 378–387.
- Caccavello, G.; Giaccone, M.; Scognamiglio, P.; Forlani, M.; Basile, B. Influence of intensity of post-veraison defoliation or shoot trimming on vine physiology, yield components, berry and wine composition in Aglianico grapevines. Aust. J. Grape Wine Res. 2016, 22, 226–239.
- Palliotti, A.; Cini, R.; Silvestroni, O.; Leoni, F.; Poni, S. Effects of late mechanized leaf removal above the clusters zone to delay grape ripening in ‘Sangiovese’ vines. In Proceedings of the I International Workshop on Vineyard Mechanization and Grape and Wine Quality 978, Piacenza, Italy, 27–29 June 2012; pp. 301–307.
- Parker, A.K.; Hofmann, R.W.; Van Leeuwen, C.; McLachlan, A.R.; Trought, M.C. Manipulating the leaf area to fruit mass ratio alters the synchrony of total soluble solids accumulation and titratable acidity of grape berries. Aust. J. Grape Wine Res. 2015, 21, 266–276.
- Cincotta, F.; Verzera, A.; Prestia, O.; Tripodi, G.; Lechhab, W.; Sparacio, A.; Condurso, C. Influence of leaf removal on grape, wine and aroma compounds of Vitis vinifera L. cv. Merlot under Mediterranean climate. Eur. Food Res. Technol. 2022, 248, 403–413.
- Sun, Q.; Sacks, G.L.; Lerch, S.D.; Heuvel, J.E.V. Impact of shoot and cluster thinning on yield, fruit composition, and wine quality of Corot noir. Am. J. Enol. Vitic. 2012, 63, 49–56.
- Torres, N.; Martínez-Lüscher, J.; Porte, E.; Yu, R.; Kurtural, S.K. Impacts of leaf removal and shoot thinning on cumulative daily light intensity and thermal time and their cascading effects of grapevine (Vitis vinifera L.) berry and wine chemistry in warm climates. Food Chem. 2021, 343, 128447.
- Sun, Q.; Sacks, G.; Lerch, S.; Vanden-Heuvel, J.E. Impact of shoot thinning and harvest date on yield components, fruit composition, and wine quality of Marechal Foch. Am. J. Enol. Vitic. 2011, 62, 32–41.
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical carbon dioxide applications in food processing. Food Eng. Rev. 2021, 13, 570–591.
- Ruiz-Rodríguez, A.; Fornari, T.; Jaime, L.; Vázquez, E.; Amador, B.; Nieto, J.A.; Reglero, G. Supercritical CO2 extraction applied toward the production of a functional beverage from wine. J. Supercrit. Fluids 2012, 61, 92–100.
- Raventós, M.; Duarte, S.; Alarcón, R. Application and possibilities of supercritical CO2 extraction in food processing industry: An overview. Food Sci. Technol. Int. 2002, 8, 269–284.
- Nikolaou, A.; Kandylis, P.; Kanellaki, M.; Kourkoutas, Y. Winemaking using immobilized kefir cells on natural zeolites. LWT 2020, 133, 110043.
- Akyereko, Y.G.; Wireko-Manu, F.D.; Alemawor, F.; Adzanyo, M. Effects of production methods on flavour characteristics of nonalcoholic wine. J. Food Qual. 2021, 2021, 3014793.
- Cassano, A.; Conidi, C.; Drioli, E. A comprehensive review of membrane distillation and osmotic distillation in agro-food applications. J. Membr. Sci. Res. 2020, 6, 304–318.
- Sam, F.E.; Ma, T.; Liang, Y.; Qiang, W.; Atuna, R.A.; Amagloh, F.K.; Han, S. Comparison between membrane and thermal dealcoholization methods: Their impact on the chemical parameters, volatile composition, and sensory characteristics of wines. Membranes 2021, 11, 957.
- Banvolgyi, S.; Savaş Bahçeci, K.; Vatai, G.; Bekassy, S.; Bekassy-Molnar, E. Partial dealcoholization of red wine by nanofiltration and its effect on anthocyanin and resveratrol levels. Food Sci. Technol. Int. 2016, 22, 677–687.
- Conidi, C.; Castro-Muñoz, R.; Cassano, A. Nanofiltration in beverage industry. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 525–548.
- Mangindaan, D.; Khoiruddin, K.; Wenten, I.G. Beverage dealcoholization processes: Past, present, and future. Trends Food Sci. Technol. 2018, 71, 36–45.
- Catarino, M.; Mendes, A. Dealcoholizing wine by membrane separation processes. Innov. Food Sci. Emerg. Technol. 2011, 12, 330–337.
- Gonçalves, F.; Ribeiro, R.; Neves, L.; Lemperle, T.; Lança, M.; Ricardo da Silva, J.; Laureano, O. Alcohol reduction in wine by nanofiltration. Some comparisons with reverse osmosis technique. In Proceedings of the 1st Oenoviti International Symposium—Alcohol Level Reduction in Wine, Bordeaux, France, 6 September 2013; VIGNE et vin Publications Internationales: Bordeaux, France; pp. 64–67.
- Labanda, J.; Vichi, S.; Llorens, J.; López-Tamames, E. Membrane separation technology for the reduction of alcoholic degree of a white model wine. LWT 2009, 42, 1390–1395.
- Massot, A.; Mietton-Peuchot, M.; Peuchot, C.; Milisic, V. Nanofiltration and reverse osmosis in winemaking. Desalination 2008, 231, 283–289.
- Meillon, S.; Viala, D.; Medel, M.; Urbano, C.; Guillot, G.; Schlich, P. Impact of partial alcohol reduction in Syrah wine on perceived complexity and temporality of sensations and link with preference. Food Qual. Pref. 2010, 21, 732–740.
- Ambrosi, A.; Motke, M.B.; Souza-Silva, É.A.; Zini, C.A.; McCutcheon, J.R.; Cardozo, N.S.M.; Tessaro, I.C. Beer dealcoholization by forward osmosis diafiltration. Innov. Food Sci. Emerg. Technol. 2020, 63, 102371.
- Corona, O.; Liguori, L.; Albanese, D.; Di Matteo, M.; Cinquanta, L.; Russo, P. Quality and volatile compounds in red wine at different degrees of dealcoholization by membrane process. Eur. Food Res. Technol. 2019, 245, 2601–2611.
- Liguori, L.; Russo, P.; Albanese, D.; Di Matteo, M. Evolution of quality parameters during red wine dealcoholization by osmotic distillation. Food Chem. 2013, 140, 68–75.
- Liguori, L.; Albanese, D.; Crescitelli, A.; Di Matteo, M.; Russo, P. Impact of dealcoholization on quality properties in white wine at various alcohol content levels. J. Food Sci. Technol. 2019, 56, 3707–3720.
- Lisanti, M.T.; Gambuti, A.; Genovese, A.; Piombino, P.; Moio, L. Partial dealcoholization of red wines by membrane contactor technique: Effect on sensory characteristics and volatile composition. Food Bioproc. Technol. 2013, 6, 2289–2305.
- Fedrizzi, B.; Nicolis, E.; Camin, F.; Bocca, E.; Carbognin, C.; Scholz, M.; Barbieri, P.; Finato, F.; Ferrarini, R. Stable isotope ratios and aroma profile changes induced due to innovative wine dealcoholisation approaches. Food Bioproc. Technol. 2014, 7, 62–70.
- Sun, X.; Dang, G.; Ding, X.; Shen, C.; Liu, G.; Zuo, C.; Chen, X.; Xing, W.; Jin, W. Production of alcohol-free wine and grape spirit by pervaporation membrane technology. Food Bioprod. Process. 2020, 123, 262–273.
- Crespo, J.G.; Brazinha, C. Fundamentals of pervaporation. In Pervaporation, Vapour Permeation and Membrane Distillation; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–17.
- Figoli, A.; Santoro, S.; Galiano, F.; Basile, A. Pervaporation membranes: Preparation, characterization, and application. In Pervaporation, Vapour Permeation and Membrane Distillation; Woodhead Publishing: Cambridge, UK, 2015; pp. 19–63.
- Castro-Muñoz, R. Pervaporation-based membrane processes for the production of non-alcoholic beverages. J. Food Sci. Technol. 2019, 56, 2333–2344.
- Motta, S.; Guaita, M.; Petrozziello, M.; Ciambotti, A.; Panero, L.; Solomita, M.; Bosso, A. Comparison of the physicochemical and volatile composition of wine fractions obtained by two different dealcoholization techniques. Food Chem. 2017, 221, 1–10.
- Gómez-Plaza, E.; López-Nicolás, J.M.; López-Roca, J.M.; Martínez-Cutillas, A. Dealcoholization of wine. Behaviour of the aroma components during the process. LWT Food Sci. Technol. 1999, 32, 384–386.
- Belisario-Sánchez, Y.Y.; Taboada-Rodriguez, A.; Marin-Iniesta, F.; Lopez-Gomez, A. Dealcoholized wines by spinning cone column distillation: Phenolic compounds and antioxidant activity measured by the 1,1-diphenyl-2-picrylhydrazyl method. J. Agric. Food Chem. 2009, 57, 6770–6778.
- Belisario-Sánchez, Y.Y.; Taboada-Rodríguez, A.; Marín-Iniesta, F.; Iguaz-Gainza, A.; López-Gómez, A. Aroma recovery in wine dealcoholization by SCC distillation. Food Bioproc. Technol. 2012, 5, 2529–2539.
- Di Giacomo, G.; Romano, P. Advanced fractionation process for wine-based products diversification. J. Food Sci. Technol. 2021, 58, 4685–4692.
- Paredes, D.A.F.; Sánchez, R.J.; Morero, B.; Fernández, M.B.; Espinosa, J. Enriching the conceptual modelling approach with environmental considerations: Application to the partial dealcoholization of wines. Sep. Purif. Technol. 2023, 308, 122950.
- Puglisi, C.; Ristic, R.; Saint, J.; Wilkinson, K. Evaluation of Spinning Cone Column Distillation as a Strategy for Remediation of Smoke Taint in Juice and Wine. Molecules 2022, 27, 8096.
- Salgado, C.M.; Fernández-Fernández, E.; Palacio, L.; Carmona, F.J.; Hernández, A.; Prádanos, P. Application of pervaporation and nanofiltration membrane processes for the elaboration of full flavored low alcohol white wines. Food Bioprod. Process. 2017, 101, 11–21.
- Pham, D.T.; Ristic, R.; Stockdale, V.J.; Jeffery, D.W.; Tuke, J.; Wilkinson, K. Influence of partial dealcoholization on the composition and sensory properties of Cabernet Sauvignon wines. Food Chem. 2020, 325, 126869.
- Pham, D.T.; Stockdale, V.J.; Wollan, D.; Jeffery, D.W.; Wilkinson, K.L. Compositional consequences of partial dealcoholization of red wine by reverse osmosis-evaporative perstraction. Molecules 2019, 24, 1404.
- Esteras-Saz, J.; de la Iglesia, Ó.; Kumakiri, I.; Peña, C.; Escudero, A.; Téllez, C.; Coronas, J. Pervaporation of the low ethanol content extracting stream generated from the dealcoholization of red wine by membrane osmotic distillation. J. Ind. Eng. Chem. 2023, 122, 231–240.
- Russo, P.; Liguori, L.; Corona, O.; Albanese, D.; Matteo, M.D.; Cinquanta, L. Combined membrane process for dealcoholization of wines. Osmotic distillation and reverse osmosis. Chem. Eng. Trans. 2019, 75, 7–12.
- Liguori, L.; Russo, P.; Albanese, D.; Di Matteo, M. Production of low-alcohol beverages: Current status and perspectives. In Food Processing for Increased Quality and Consumption; Academic Press: Cambridge, MA, USA, 2018; pp. 347–382.
- Rossouw, D.; Heyns, E.H.; Setati, M.E.; Bosch, S.; Bauer, F.F. Adjustment of trehalose metabolism in wine Saccharomyces cerevisiae strains to modify ethanol yields. Appl. Environ. Microbiol. 2013, 79, 5197–5207.
- Tilloy, V.; Cadière, A.; Ehsani, M.; Dequin, S. Reducing alcohol levels in wines through rational and evolutionary engineering of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2015, 213, 49–58.
- Puškaš, V.S.; Miljić, U.D.; Djuran, J.J.; Vučurović, V.M. The aptitude of commercial yeast strains for lowering the ethanol content of wine. Nutr. Food Sci. 2020, 8, 1489–1498.
- Varela, C.; Kutyna, D.R.; Solomon, M.R.; Black, C.A.; Borneman, A.; Henschke, P.A.; Pretorius, I.S.; Chambers, P.J. Evaluation of gene modification strategies for the development of low-alcohol-wine yeasts. Appl. Environ. Microbiol. 2012, 78, 6068–6077.
- Drewke, C.; Thielen, J.; Ciriacy, M. Ethanol formation in adh0 mutants reveals the existence of a novel acetaldehyde-reducing activity in Saccharomyces cerevisiae. J. Bacteriol. 1990, 172, 3909–3917.
- Gonzalez, R.; Guindal, A.M.; Tronchoni, J.; Morales, P. Biotechnological approaches to lowering the ethanol yield during wine fermentation. Biomolecules 2021, 11, 1569.
- Kutyna, D.R.; Varela, C.; Henschke, P.A.; Chambers, P.J.; Stanley, G.A. Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci. Technol. 2010, 21, 293–302.
- Goold, H.D.; Kroukamp, H.; Williams, T.C.; Paulsen, I.T.; Varela, C.; Pretorius, I.S. Yeast’s balancing act between ethanol and glycerol production in low-alcohol wines. J. Microbiol. Biotechnol. 2017, 10, 264–278.
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.B.; Lonvaud, A.A.; Darriet, P.; Towey, J. The Microbiology of Wine and Vinifications. In Handbook of Enology; Wiley: Hoboken, NJ, USA, 2006; pp. 208–209.
- Scanes, K.T.; Hohmann, S.; Prior, B.A. Glycerol production by the yeast Saccharomyces cerevisiae and its relevance to wine: A review. S. Afr. J. Enol. Vitic. 1998, 19, 17–24.
- Lopes, M.; De, B.; Ur-Rehman, A.; Gockowiak, H.; Heinrich, A.J.; Langridge, P.; Henschke, P.A. Fermentation properties of a wine yeast over-expressing the Saccharomyces cerevisiae Glycerol 3-Phosphate Dehydrogenase Gene (GPD2). Aust. J. Grape Wine Res. 2000, 6, 208–215.
- Nevoigt, E.; Stahl, U. Reduced pyruvate decarboxylase and increased glycerol-3-phosphate dehydrogenase levels enhance glycerol production in Saccharomyces cerevisiae. Yeast 1996, 12, 1331–1337.
- Cambon, B.; Monteil, V.; Remize, F.; Camarasa, C.; Dequin, S. Effects of GPD1 overexpression in Saccharomyces cerevisiae commercial wine yeast strains lacking ALD6 genes. Appl. Environ. Microbiol. 2006, 72, 4688–4694.
- Eglinton, J.M.; Heinrich, A.J.; Pollnitz, A.P.; Langridge, P.; Henschke, P.A.; de Barros Lopes, M. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeast 2002, 19, 295–301.
- Boidron, J.N.; Chatonnet, P.; Pons, M. Influence du bois sur certaines substances odorantesdes vin. OENO One 1988, 22, 275–294.
- Saint-Prix, F.; Bonquist, L.; Dequin, S. Functional analysis of the ALD gene family of Saccharomyces cerevisiae during anaerobic growth on glucose: The NADP+-dependent Ald6p and Ald5p isoforms play a major role in acetate formation. Microbiology 2004, 150, 2209–2220.
- Ehsani, M.; Fernández, M.R.; Biosca, J.A.; Julien, A.; Dequin, S. Engineering of 2,3-butanediol dehydrogenase to reduce acetoin formation by glycerol-overproducing, low-alcohol Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2009, 75, 3196–3205.
- Luyten, K.; Albertyn, J.; Skibbe, W.F.; Prior, B.A.; Ramos, J.; Thevelein, J.M.; Hohmann, S. Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J. 1995, 14, 1360–1371.
- Tamás, M.J.; Luyten, K.; Sutherland, F.C.W.; Hernandez, A.; Albertyn, J.; Valadi, H.; Hohmann, S. Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol. Microbiol. 1999, 31, 1087–1104.
- Tamás, M.J.; Rep, M.; Thevelein, J.M.; Hohmann, S. Stimulation of the yeast high osmolarity glycerol (HOG) pathway: Evidence for a signal generated by a change in turgor rather than by water stress. FEBS Lett. 2000, 472, 159–165.
- Remize, F.; Barnavon, L.; Dequin, S. Glycerol export and glycerol-3-phosphate dehydrogenase, but not glycerol phosphatase, are rate limiting for glycerol production in Saccharomyces cerevisiae. Metab. Eng. 2001, 3, 301–312.
- Flikweert, M.T.; de Swaaf, M.; van Dijken, J.P.; Pronk, J.T. Growth requirements of pyruvate-decarboxylase-negative Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1999, 174, 73–79.
- Cuello, R.A.; Flores Montero, K.J.; Mercado, L.A.; Combina, M.; Ciklic, I.F. Construction of low-ethanol–wine yeasts through partial deletion of the Saccharomyces cerevisiae PDC2 gene. AMB Express 2017, 7, 67.
- Compagno, C.; Boschi, F.; Ranzi, B.M. Glycerol production in a triose phosphate isomerase deficient mutant of Saccharomyces cerevisiae. Biotechnol. Prog. 1996, 12, 591–595.
- Compagno, C.; Brambilla, L.; Capitanio, D.; Boschi, F.; Maria Ranzi, B.; Porro, D. Alterations of the glucose metabolism in a triose phosphate isomerase-negative Saccharomyces cerevisiae mutant. Yeast 2001, 18, 663–670.
- Overkamp, K.M.; Bakker, B.M.; Kötter, P.; Luttik, M.A.; Van Dijken, J.P.; Pronk, J.T. Metabolic engineering of glycerol production in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2002, 68, 2814–2821.
- Uemura, H.; Fraenkel, D.G. Glucose metabolism in gcr mutants of Saccharomyces cerevisiae. J. Bacteriol. 1999, 181, 4719–4723.
- Scott, E.W.; Baker, H.V. Concerted action of the transcriptional activators REB1, RAP1, and GCR1 in the high-level expression of the glycolytic gene TPI. Mol. Cell Biol. 1993, 13, 543–550.
- Johnston, M.; Kim, J.H. Glucose as a hormone: Receptor-mediated glucose sensing in the yeast Saccharomyces cerevisiae. Biochem. Soc. Trans. 2005, 33, 247–252.
- Henricsson, C.; de Jesus Ferreira, M.C.; Hedfalk, K.; Elbing, K.; Larsson, C.; Bill, R.M.; Gustafsson, L. Engineering of a novel Saccharomyces cerevisiae wine strain with a respiratory phenotype at high external glucose concentrations. Appl. Environ. Microbiol. 2005, 71, 6185–6192.
- Fazio, N.A.; Russo, N.; Foti, P.; Pino, A.; Caggia, C.; Randazzo, C.L. Inside current winemaking challenges: Exploiting the potential of conventional and unconventional yeasts. Microorganisms 2023, 11, 1338.
- Schmidtke, L.M.; Blackman, J.W.; Agboola, S.O. Production technologies for reduced alcoholic wines. J. Food Sci. 2012, 77, R25–R41.
- Malherbe, D.F.; Du Toit, M.; Cordero Otero, R.R.; Van Rensburg, P.; Pretorius, I.S. Expression of the Aspergillus niger glucose oxidase gene in Saccharomyces cerevisiae and its potential applications in wine production. Appl. Microbiol. Biotechnol. 2003, 61, 502–511.
- Heux, S.; Sablayrolles, J.M.; Cachon, R.; Dequin, S. Engineering a Saccharomyces cerevisiae wine yeast that exhibits reduced ethanol production during fermentation under controlled microoxygenation conditions. Appl. Environ. Microbiol. 2006, 72, 5822–5828.
- Piškur, J.; Rozpędowska, E.; Polakova, S.; Merico, A.; Compagno, C. How did Saccharomyces evolve to become a good brewer? Trends Genet. 2006, 22, 183–186.
- Varela, C.; Kutyna, D.; Henschke, P.A.; Chambers, P.J.; Herderich, M.J.; Pretorius, I.S. Taking control of alcohol. Aust. N. Z. Wine Ind. J. 2008, 23, 41–43.
- Palacios, A.; Raginel, F.; Ortiz-Julien, A. Can the selection of Saccharomyces cerevisiae yeast lead to variations in the final alcohol degree of wines? Aust. N. Z. Grapegrow. Winemak. 2007, 527, 71–75.
- Del Fresno, J.M.; Morata, A.; Loira, I.; Bañuelos, M.A.; Escott, C.; Benito, S.; Chamoro, C.; Suárez-Lepe, J.A. Use of non-Saccharomyces in single-culture, mixed and sequential fermentation to improve red wine quality. Eur. Food Res. Technol. 2017, 243, 2175–2185.
- Ivit, N.N.; Longo, R.; Kemp, B. The Effect of non-Saccharomyces and Saccharomyces non-Cerevisiae yeasts on ethanol and glycerol levels in wine. Fermentation 2020, 6, 77.
- Borren, E.; Tian, B. The Important Contribution of Non-Saccharomyces yeasts to the aroma complexity of wine: A Review. Foods 2021, 10, 13.
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678.
- Gonzalez, R.; Quiros, M.; Morales, P. Yeast respiration of sugars by non-Saccharomyces yeast species: A promising and barely explored approach to lowering alcohol content of wines. Trends Food Sci. Technol. 2013, 29, 55–61.
- Magyar, I.; Toth, T. Comparative evaluation of some oenological properties in wine strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 94–100.
- Gobbi, M.; De Vero, L.; Solieri, L.; Comitini, F.; Oro, L.; Giudici, P.; Ciani, M. Fermentative aptitude of non-Saccharomyces wine yeast for reduction in the ethanol content in wine. Eur. Food Res. Technol. 2014, 239, 41–48.
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245.
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 2017, 252, 1–9.
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015, 205, 7–15.
- Benito, S.; Palomero, F.; Morata, A.; Calderón, F.; Palmero, D.; Suárez-Lepe, J.A. Physiological features of Schizosaccharomyces pombe of interest in making of white wines. Eur. Food Res. Technol. 2013, 236, 29–36.
- Muratore, G.; Asmundo, C.N.; Lanza, C.M.; Caggia, C.; Licciardello, F.; Restuccia, C. Influence of Saccharomyces uvarum on volatile acidity, aromatic and sensory profile of Malvasia delle Lipari wine. Food Technol. Biotechnol. 2007, 45, 101–106.
- Arroyo-López, F.N.; Pérez-Torrado, R.; Querol, A.; Barrio, E. Modulation of the glycerol and ethanol syntheses in the yeast Saccharomyces kudriavzevii differs from that exhibited by Saccharomyces cerevisiae and their hybrid. Food Microbiol. 2010, 27, 628–637.
- Morales, P.; Rojas, V.; Quirós, M.; Gonzalez, R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotechnol. 2015, 99, 3993–4003.
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247.
- Canonico, L.; Comitini, F.; Oro, L.; Ciani, M. Sequential fermentation with selected immobilized non-Saccharomyces yeast for reduction of ethanol content in wine. Front. Microbiol. 2016, 7, 278.
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Rocker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717.
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411.
- Vilela, A. Lachancea thermotolerans, the non-Saccharomyces yeast that reduces the volatile acidity of wines. Fermentation 2018, 4, 56.
- Canonico, L.; Galli, E.; Ciani, E.; Comitini, F.; Ciani, M. Exploitation of three non-conventional yeast species in the brewing process. Microorganisms 2019, 7, 11.
- Alonso, A.; Belda, I.; Santos, A.; Navascués, E.; Marquina, D. Advances in the control of the spoilage caused by Zygosaccharomyces species on sweet wines and concentrated grape musts. Food Control 2015, 51, 129–134.
- Mehlomakulu, N.N.; Setati, M.E.; Divol, B. Characterization of novel killer toxins secreted by wine-related non-Saccharomyces yeasts and their action on Brettanomyces spp. Int. J. Food Microbiol. 2014, 188, 83–91.
- Santos, A.; San Mauro, M.; Bravo, E.; Marquina, D. PMKT2, a new killer toxin from Pichia membranifaciens, and its promising biotechnological properties for control of the spoilage yeast Brettanomyces bruxellensis. Microbiology 2009, 155, 624–634.
- Oro, L.; Ciani, M.; Bizzaro, D.; Comitini, F. Evaluation of damage induced by Kwkt and Pikt zymocins against Brettanomyces/Dekkera spoilage yeast, as compared to sulphur dioxide. J. Appl. Microbiol. 2016, 121, 207–214.
- Oro, L.; Feliziani, E.; Ciani, M.; Romanazzi, G.; Comitini, F. Volatile organic compounds from Wickerhamomyces anomalus, Metschnikowia pulcherrima and Saccharomyces cerevisiae inhibit growth of decay causing fungi and control postharvest diseases of strawberries. Int. J. Food Microbiol. 2018, 265, 18–22.
- Comitini, F.; Canonico, L.; Agarbati, A.; Ciani, M. Biocontrol and probiotic function of non-Saccharomyces yeasts: New insights in agri-food industry. Microorganisms 2023, 11, 1450.
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017, 8, 995.
- Rossouw, D.; Bauer, F. Exploring the phenotypic space of non-Saccharomyces wine yeast biodiversity. Food Microbiol. 2016, 55, 32–46.
- Tristezza, M.; Tufariello, M.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. The oenological potential of Hanseniaspora uvarum in simultaneous and sequential co-fermentation with Saccharomyces cerevisiae for industrial wine production. Front. Microbiol. 2016, 7, 670.
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333.
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in Wine Biotechnology. Fermentation 2019, 5, 63.
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and phenotypic characterization of Metschnikowia pulcherrima strains from Douro wine region. Fermentation 2018, 4, 8.
- Quirós, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014, 181, 85–91.
- Contreras, A.; Curtin, C.; Varela, C. Yeast population dynamics reveal a potential ‘collaboration’ between Metschnikowia pulcherrima and Saccharomyces uvarum for the production of reduced alcohol wines during Shiraz fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 1885–1895.
- Tronchoni, J.; Curiel, J.A.; Sáenz-Navajas, M.P.; Morales, P.; De-La-Fuente-Blanco, A.; Fernández-Zurbano, P.; Ferreira, V.; Gonzalez, R. Aroma profiling of an aerated fermentation of natural grape must with selected yeast strains at pilot scale. Food Microbiol. 2018, 70, 214–223.
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281.
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2018, 24, 166–180.
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality, and composition of Airén wines fermented by sequential inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135.
- Ramírez, M.; Velázquez, R. The yeast Torulaspora delbrueckii: An interesting but difficult-to-use tool for winemaking. Fermentation 2018, 4, 94.
- Izquierdo Canas, P.M.; García Romero, E.; Huertas Nebreda, B.; Gómez Alonso, S.; Gómez-Alonso, S.; Collins, V.J.; Corona, G. Enhancement of flavour properties in wines using sequential inoculations of non. VITIS-J. Grapevine Res. 2011, 50, 177–182.
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food Res. Technol. 2012, 235, 303–313.
- Puertas, B.; Jiménez, M.J.; Cantos-Villar, E.; Cantoral, J.M.; Rodríguez, M. Use of Torulaspora delbrueckii and Saccharomyces cerevisiae in semi-industrial sequential inoculation to improve quality of palomino and chardonnay wines in warm climates. J. Appl. Microbiol. 2017, 122, 733–746.
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in varietal thiol (3-SH and 4-MSP) release in wine sequential fermentations. Int. J. Food Microbiol. 2017, 257, 183–191.
- Čuš, F.; Jenko, M. The influence of yeast strains on the composition and sensory quality of Gewürztraminer wine. Food Technol. Biotechnol. 2013, 51, 547–553.
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094.
- Soden, A.; Francis, I.L.; Oakey, H.; Henschke, P.A. Effects of co-fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of Chardonnay wine. Aust. J. Grape Wine Res. 2000, 6, 21–30.
- Ferraro, L.; Fatichenti, F.; Ciani, M. Pilot scale vinification process using immobilized Candida stellata cells and Saccharomyces cerevisiae. Process Biochem. 2000, 35, 1125–1129.
More
Information
Subjects:
Biotechnology & Applied Microbiology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
4 times
(View History)
Update Date:
09 May 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




