Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jianqin Lu | -- | 2930 | 2024-01-10 03:01:44 | | | |
| 2 | Lindsay Dong | Meta information modification | 2930 | 2024-01-12 00:45:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jiang, Y.; Li, W.; Wang, Z.; Lu, J. Pharmaceutical Applications of Liposomes. Encyclopedia. Available online: https://encyclopedia.pub/entry/53635 (accessed on 08 February 2026).
Jiang Y, Li W, Wang Z, Lu J. Pharmaceutical Applications of Liposomes. Encyclopedia. Available at: https://encyclopedia.pub/entry/53635. Accessed February 08, 2026.
Jiang, Yanhao, Wenpan Li, Zhiren Wang, Jianqin Lu. "Pharmaceutical Applications of Liposomes" Encyclopedia, https://encyclopedia.pub/entry/53635 (accessed February 08, 2026).
Jiang, Y., Li, W., Wang, Z., & Lu, J. (2024, January 10). Pharmaceutical Applications of Liposomes. In Encyclopedia. https://encyclopedia.pub/entry/53635
Jiang, Yanhao, et al. "Pharmaceutical Applications of Liposomes." Encyclopedia. Web. 10 January, 2024.
Copy Citation
Liposomes have been extensively developed and used for various clinical applications such as in pharmaceutical, cosmetic, and dietetic fields, due to its versatility, biocompatibility, and biodegradability, as well as the ability to enhance the therapeutic index of free drugs. However, some challenges remain unsolved, including liposome premature leakage, manufacturing irreproducibility, and limited translation success.
liposome
drug delivery
disease treatments
1. Introduction
Cancer has brought a critical burden to the economy and society. GLOBOCAN (the World Health Organization’s International Agency for Research on Cancer Global Cancer Observatory) 2020 reported an estimation of 19 million new cancer cases and 10 million cancer deaths occurred worldwide [1]. Currently, cancer treatments are still mainly proceeded by surgery, radiotherapy, and chemotherapy, although gene therapy and immunotherapy have been brought up as novel methods with a higher therapeutic index. However, some challenges remain unsolved even with the advanced therapies, such as low solubility, poor pharmacokinetics, non-specific biodistribution, and systemic toxicities [2][3]. Therefore, targeted delivery of therapeutics to specific sites has been an active area of research in the last couple of decades. Of note, several drug delivery platforms have been reported, and some are being used in clinical settings, including antibody-drug conjugates, polymers, as well as liposomes [4][5][6]. Of those, liposomes are a promising drug delivery vehicle due to their biocompatibility and biodegradability, good stability, as well as the ability to encapsulate both hydrophobic and hydrophilic contents [7]. When the first liposome was described by Bangham et al. in 1964 [8], it had grown to be a great interest in cosmetic, dietetic, and pharmaceutical areas [9][10][11].
Due to the natural properties of liposome, the major components are lipids and fatty acids comprising phospholipids, which can spontaneously self-assemble into a lipid bilayer with an aqueous core. The phospholipid bilayer is similar to the construction of the cell membrane. Therefore, liposomes are considered to be biocompatible and biodegradable [7]. Because of the presence of a lipid membrane and a hydrophilic interior, liposomes can be used to deliver both hydrophilic and hydrophobic molecules. With that, liposomes have been further researched of their benefits as a drug delivery platform.
2. Characterization and Major Components of Liposomes
Several ways can be used to classify liposomes, including size, lamellarity, and method of preparation [12][13]. Scholars define liposomes by their size and lamellarity. These two factors also dominate the drug encapsulation efficiency and ADME (absorption, distribution, metabolism, and elimination) of the drug [7][14][15]. By lamellarity, liposomes can be defined as: a unilamellar vesicle (ULV), with one bilayer membrane; an oligolamellar vesicle (OLV), with 2–5 bilayer membranes; or a multilamellar vesicle (MLV), with five or more bilayer membranes. Furthermore, ULV can be classified by its size, including small unilamellar vesicle (SUV) ranging from 20 to 100 nm; large unilamellar vesicle (LUV) with a size larger than 100 nm; and giant unilamellar vesicle (GUV) with a size bigger than 1000 nm [16]. Generally, ULV is formed by a phospholipid bilayer and an aqueous core. More uniquely, several ULVs with gradually smaller sizes caging inside each other compose the MLV, which resembles an onion, and each lipid bilayer is separated by an aqueous layer [17].
Three dominant components that contribute to the formation, stability, and functionality of liposomes include phospholipids, cholesterol, and polyethylene glycol (PEG).
3. Pharmaceutical Applications of Liposomes
Owing to its biocompatibility, biodegradability, nontoxicity, and favorable physical properties for convenient modifications of surface charge and its size, since the 1990s, there have been more than a dozen U.S. FDA-approved liposomal or lipid-based nanodrugs (Table 1) with numerous more under preclinical and clinical development.
Table 1. U.S. FDA-approved liposomal/lipid-based nanodrugs.
| Name | Clinical Approval Year | Liposomal Composition | Drug Encapsulated | Drug Type | Route of Administration | Company | References |
|---|---|---|---|---|---|---|---|
| Doxil | 1995 | HSPC:Cholesterol:DSPE-PEG2000 | Doxorubicin | Chemotherapeutic | I.V. | Johnson & Johnson, Milpitas, CA, USA | [18][19] |
| Abelcet | 1995 | DMPC:DMPG | Amphotericin B | Antifungal | I.V. | Leadiant Biosciences. Inc., Rockville, MD, USA | [20][21] |
| DaunoXome | 1996 | DSPC:Cholesterol | Daunorubicin | Chemotherapeutic | I.V. | Galen US, Inc., Souderton, PA, USA | [18][22] |
| Amphotec | 1996 | Cholesteryl sulphate:Amphotericin B | Amphotericin B | Antifungal | I.V. | Sequus Pharmaceuticals Inc., Menlo Park, CA, USA | [18] |
| Inflexal V | 1997 | 70% Lecithin, 20% Cephalin and 10% Phospholipids | Influenza virus antigen, strain A and B | Vaccine | I.M. | Sun Pharmaceutical Industries Ltd., Princeton, NJ, USA | [18][23] |
| Ambisome | 1997 | HSPC:DSPG:Cholesterol:Amphotericin B | Amphotericin B | Antifungal | I.V. | Fujisawa Healthcare, Inc. and Gilead Sciences, Inc., Foster City, CA, USA | [18] |
| Myocet | 2000 | EPG:Cholesterol | Doxorubicin | Chemotherapeutic | I.V. | Zeneus Pharma Ltd., Oxford, UK | [18][24] |
| Visudyne | 2000 | Verteporfin:DMPC and EPG | Verteporfin | Photosensitizer | I.V. | Novartis International AG, Basel, Switzerland | [18] |
| DepoDur | 2004 | DOPC:DPPG:Cholesterol:Tricaprylin and Triolein | Morphine sulfate | Narcotic Analgesic | Epidural | Pacira Pharmaceuticals, Inc., Watford, UK | [18][25] |
| Mepact | 2004 | DOPS:POPC | Mifamurtide | Immunomodulator/Antitumor | I.V. | Takeda Pharmaceutical Limited, Tokyo, Japan | [18] |
| Exparel | 2011 | DEPC:DPPG:Cholesterol:Tricaprylin | Bupivacaine | Anesthetic | I.V. | Pacira Pharmaceuticals, Inc., Parsippany-Troy Hills, NJ, USA | [18] |
| Onivyde | 2015 | DSPC:MPEG-2000:DSPE | Irinotecan | Chemotherapeutic | I.V. | Merrimack Pharmaceuticals, Inc., Cambridge, MA, USA | [18][26] |
| Vyxeos | 2017 | DSPC:DSPG:Cholesterol | Daunorubicin + Cytarabine | Antineoplastic | I.V. | Jazz Pharmaceuticals, Inc., Dublin, Ireland | [27] |
| Onpattro | 2018 | Cholesterol, DLin-MC3-DMA:DSPC:PEG2000-C-DMG | Patisiran | RNAi agent | I.V. | Alnylam Pharmaceuticals, Cambridge, MA, USA | [28] |
| Comirnaty | 2021 | ALC-0315:ALC-0159:cholesterol:DSPC | Nucleoside-modified mRNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 | Vaccine | I.M. | Pfizer-BioNTech, Mainz, Germany | [29] |
| Spikevax | 2022 | SM-102:mPEG2000-DMG:Cholesterol:DSPC | Nucleoside-modified mRNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 | Vaccine | I.M. | Moderna, Cambridge, MA, USA | [30] |
3.1. Anti-Cancer
Cancer is a disease which is flourishing rapidly throughout the world. The ultimate goal of cancer therapy is to destroy all the malignant cells. Conventional chemotherapy, as one of the most common cancer treatments, employs cytotoxic agents that target rapidly proliferating cells, especially like cancer cells [31]. For instance, anthracyclines, including Daunorubicin, Doxorubicin, Epirubicin, Idarubicin, Mitoxantrone, and Valrubicin, are used as chemotherapeutic agents for treatment of numerous types of cancers [32]. Yet, chemotherapies have been associated with severe unwanted systemic toxicities, off-target effect, and rapidly emerging drug resistance [33]. Most of the chemotherapeutic drugs are not selective to cancer cells, which indicate that they not only target cancer cells, but also can be randomly distributed to healthy organs. As reported, detrimental effects to the central nervous system (CNS) are recognized as cognition dysfunction during chemotherapy for a non-CNS cancer [34].
3.1.1. Doxil
The liposome has been the most successful in therapeutic delivery as evidenced by numerous FDA-approved liposomal nanodrugs (e.g., Doxil, DaunoXome, Depocyt, Myocet, Mepact, and Onivyde, etc.) for diverse diseases management (e.g., cancers). Doxil, the first FDA-approved nanodrug delivery system using pegylated liposomes to encapsulate doxorubicin, consists of three major components: the high-transition-temperature (Tm) phospholipid hydrogenated soy phosphatidylcholine (HSPC; Tm 52.5 °C); cholesterol; and N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (MPEG-DSPE) [18].
3.1.2. Onivyde
Onivyde, also known as an irinotecan liposome injection, is used for patients with metastatic adenocarcinoma of the pancreas with cancer progression after the gemcitabine-based therapy, usually in combination with fluorouracil and leucovorin [35]. The Onivyde liposomal vesicles comprise three key components: distearoylphosphatidylcholine (DSPC), cholesterol, and methoxy-terminated polyethylene glycol (MW2000)-distearoylphosphatidylethanolamine (MPEG-2000-DSPE) [36]. The efficacy and safety of Onivyde were evaluated in a global, randomized, open-label NAPOLI-1 clinical trial involving patients with metastatic pancreatic cancer who experienced disease progression after gemcitabine treatment [37]. The clinical results confirmed that liposomal irinotecan, Onivyde, significantly extends the lifespan of patients compared to free drugs.
3.1.3. Liposome-Peptide Conjugated Drugs
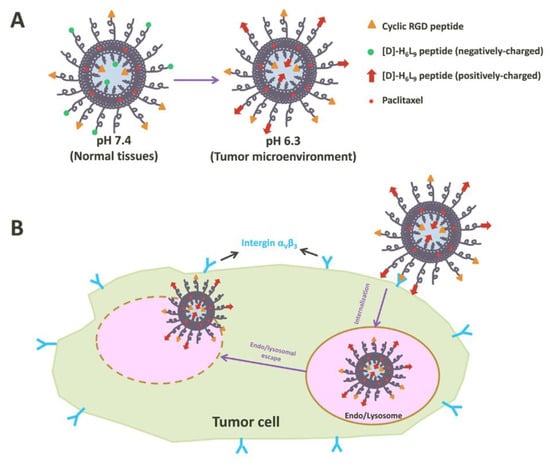
Peptides play a critical role in genes and drugs delivery, classified into two types: cell-penetrating peptides and cell-targeting peptides (Figure 1) [38]. Peptides exhibit advantageous properties, being biocompatible and well-tolerated, with modifiable features such as hydrophobicity, charge, solubility, and stability [39]. While most of the cell-penetrating peptides are cationic peptides and possess the ability for cellular uptake without inducing cytotoxicity, they lack selectivity and receptor-dependence, thereby limiting tissue specificity and tumor targeting [40]. As the need for enhanced peptide targeting and selectivity emerged, liposomes have been introduced as a delivery platform, forming an engineered combination known as liposome–peptide conjugates [39][40]. These conjugates showcase remarkable performance improvements in cellular uptake, tumor penetration, extended circulation time, and enhanced site-specific targeting, surpassing both liposomal drugs and free drugs [41].

Figure 1. Illustration of tumor cell penetration with a peptide-decorated liposome [42]. (A) The Structure of peptide-decorated liposomes under different pH environments. (B) Within tumors, the peptide-decorated liposomes could target integrin αVβ3 and initiate internalization and further intertumoral activities.
3.2. Anti-Fungal
There are two forms of fungi existing in nature, yeasts and molds [43]. Most fungi do not live dependent on animals or human beings. Yet, some groups are exterior pathogens in humans, such as Candida spp., Aspergillus spp., Cryptococcus spp., Fusarium spp., Mucorales, and endemic mycosis [44], and these cause superficial, subcutaneous, or systemic infections. Additionally, a severe, systemic fungal infection with yeasts or molds is clinically described with invasive fungal infection. Although some infections, like superficial infections, are not life-threatening, the consequences could be severe and affect the patient’s quality of life [45]. On the other hand, in immunocompromised patients, for example, bone marrow and organ transplant patients, systemic fungal infections are associated with high mortality rates [46][47].
3.2.1. Amphotericin B and Ambisome
Amphotericin B is one of the most widespread therapeutic polyene antifungals [48]. According to the Infectious Diseases Society of America (IDSA) [49] and the European Confederation of Medical Mycology (ECMM) [50], Amphotericin B is still recommended as first line treatment polyene antifungals used for severe cryptococcosis, disseminated histoplasmosis, and mucormycosis. However, a number of studies show that Amphotericin B treatments of systemic mycosis caused by species such as Aspergillus terreus [51], Scedosporium spp. [52], and Candida auris [53] are not always effective, which results from the intrinsic or acquired drug resistance [54]. Moreover, the intrinsic host toxicity of Amphotericin B is another clinical concern [54].
To date, several liposomal formulations for anti-fungal infections have been approved by the FDA, including Abelcet, Ambisome, and Amphotec. Ambisome was developed by Astellas Pharma USA for the treatment of serious, life-threatening fungal infections, and also for Amphotericin B intolerance or renal-impaired patients who were infected with invasive systemic infections caused by Aspergillus, Candida, or Cryptococcus [18]. Structurally, the lipid bilayer of Ambisome is composed of hydrogenated soy phosphatidylcholine (HSPC), cholesterol, 1,2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG), and Amphotericin B [55].
3.2.2. Nystatin and Nyotran
Like Amphotericin B, Nystatin is a polyene antibiotic. However, due to its systemic toxicity and low intestinal permeability, the therapeutic application of Nystatin has been limited to topical use in mucocutaneous (oral) and cutaneous (vaginal) forms of candidiasis [56].
To overcome these limitations, lipid-based nanotechnologies have been applied to Nystatin as a multilamellar liposome, known as Nyotran, used in treating systemic fungal infections [57]. With the liposomal formulation, Nyotran shows reduced toxicity, improved pharmacokinetics, and better tolerability [58]. Another clinical report also stated that Nyotran was active in some patients in which Amphotericin B treatment failed [59].
3.2.3. Inhaled Liposomal Antimicrobial Medications
Besides the most common routes of liposomal drug delivery, such as oral, typical, and parenteral, the use of inhaled liposomal dosage form for treating respiratory diseases and/or infections has been used increasingly in clinical practice [60]. Inhaled liposomal dosage forms offer potential advantages for the treatment of respiratory infections including targeted delivery, reduced systemic toxicity, improved efficacy, and minimized side effects [61][62][63].
Amphotericin B, as the common treatment for pulmonary fungal infections, is limited by high mortality in achieving the minimum inhibitory concentration in the lung [64]. To address these challenges, clinical studies have explored the administration of a liposomal amphotericin B parenteral formulation alone or with amphotericin B deoxycholate through nebulization [65]. Nebulization is the method that converts medications into fine mist for inhalation, which has shown promise in enhancing the delivery of amphotericin B and combating pulmonary fungal infections. Apart from the nebulization, pressurized metered-dose inhalers (pMDIs) and dry powder inhalers (DPIs) are also being used for drug delivery, but each device requires different formulations to ensure successful drug delivery into the lung [66][67][68].
3.3. Pain Management
Acute pain mostly happens following tissue damage associated with surgery, also known as acute postoperative pain, and chronic pain would persist during the healing process for at least three months after the surgery [69]. Additionally, chronic pain could produce an enormous financial burden for the patients [70]. While chronic pain is not life-threatening, it may have a lasting impact on functioning and influence the quality of life of the patients.
An ideal postoperative pain management should use a multidisciplinary approach to interfere with different pain propagation and perception mechanisms [71]. Moreover, an effective pain management control method is to shorten the inpatient time, avoid opioid dependence or addiction, and reduce the mortality [72]. In addition, regional and local anesthesia play an important role in postoperative pain control, as they block the afferent neural stimuli from the surgical area in order to reach the effective analgesic effect [73]. Local anesthetics, such as bupivacaine, provide more successful pain control than opioids and are widely used for preemptive infiltration during the postoperative period with prolonged duration of action [74].
3.3.1. Exparel
Two FDA-approved liposome formulations (DepoDur and Exparel) have been used for pain management. Exparel is a multivesicular liposomal formulation of bupivacaine being developed for wound infiltration in patients with hemorrhoidectomy and bunionectomy [75]. Exparel is composed of dierucoylphosphatidylcholine (DEPC), which is a novel phospholipid excipient, and other lipid components, including DPPG, cholesterol, and tricaprylin.
3.3.2. Liposomal Cannabidiol
Cannabidiol (CBD), a phytocannabinoid discovered in 1940, can be used to treat a number of diseases, such as Alzheimer’s disease, Parkinson’s disease, and chronic pain [76][77][78]. The traditional form of CBD has low oral bioavailability and off-targeting effects, thus impeding its optimal therapeutic index [79]. Nanocarriers have been used for the targeted delivery of various phytocompounds, including CBD, and can improve the stability of phytocompounds, enhance bioavailability, and increase solubility and permeability [80].
3.4. Vaccination
Conventional or classical vaccines are based on the use of whole or killed bacteria or viruses to mimic their natural interaction with human immune systems [81][82]. Vaccines remain the most cost-efficient way to defend infectious diseases. Nonetheless, several challenges are yet to be solved, such as the identification of the antigen candidates, ability to induce appropriate immune responses for protection, cross-protection against different strains of pathogens, and route of administration [83]. In vaccine development, the ability of initiating the innate and adaptive immune responses is essential. To elicit a sufficient immune response against the antigens, choosing the appropriate immunostimulatory molecules (e.g., adjuvants) and the efficient delivery platform matters. The adjuvants could not only help prolong the exposure time of the vaccine molecules to the antigen-presenting cells (APCs) but could also interact with APCs and trigger immune responses by themselves [84][85]. Liposomes were first investigated as vaccine adjuvants and a delivery platform in 1974 [86]. Due to their flexibility and versatility, immuno-stimulation induced by liposome-carried vaccines can be modified by various factors including liposome composition, size and homogeneity, charge, and location of antigens and/or adjuvants [87]. It is well noted that the versatility of liposomes in cargo selection plays a pivotal role in vaccine delivery system [88]. Water-soluble antigens such as proteins, peptides, and nucleic acids are encapsulated in the aqueous core of the liposomes, while the lipophilic substrates such as adjuvants, glycolipids, and lipopeptides are entrapped in the lipid bilayers of the liposomes. The antigens could also associate with the surface of the liposome by absorption or covalent binding [89]. Regardless of where the antigens are present (in/on liposomes), the immune responses can be induced by the liposomes, which are phagocytosed by the macrophage and the antigens are processed and presented on the macrophage surface with either the MHCI (major histocompatibility class I) complex if antigens end up in the cytoplasm or the MHCII if antigens end up in the lysosomes. Consequently, the antigen peptides on the MHC complex are recognized by the cytotoxic T lymphocytes (CTLs) and bind to the T cells. Specific cytokines are secreted from the T cells, interacting with B cells and then stimulating B cells to produce antibodies [90].
4. Conclusions
The pharmaceutical applications of liposomes are not limited to what have been mentioned above. Liposomal drugs have also been used for photodynamic therapy [91], bacterial infections [92], and cardiovascular diseases [93]. In addition, liposomes have been explored for nanotechnologies as signal enhancers in medical diagnosis [94], solubilizers for various ingredients, and penetration enhancers in cosmetics [95].
Because of the unique characteristics of liposomes, they have also been developed as carriers for brain delivery of bioactive constituents and used for treatments of various central nervous systems disorders such as Alzheimer’s disease [96], ischemic disease [97], and Parkinson’s disease [98][99]. However, to fulfill their clinical translation, the liposomal formulations are required to undergo additional studies to further prove their effectiveness, such as to evaluate the combinations of bioactive molecules, measure the dosage of bioactive molecules administered, and perform assessment in patients with different central nervous system disorders [100].
The liposome exhibits various advantages, such as reducing the side effects, improving the pharmacokinetics, and enhancing the delivery efficiency to target sites as compared to free (unentrapped) drugs. However, liposomes still face some challenges. One critical issue is drug leakage from the liposome during the circulation before it is navigated to the tumor site [101]. Unwanted leakage would not only yield suboptimal circulation times but also release the cytotoxic agents prematurely, damaging the healthy organs/tissues [101]. The primary cause of liposomes’ drug leakage is serum proteins, such as lipoproteins, which can interrupt the integrity of liposome bilayers [101][102].
While liposomes have shown potential to mitigate systemic toxicity associated with delivered therapeutic agents, systematic evaluation of side effects stemmed by liposomal nanocarriers in preclinical and clinical settings remains crucial [103][104]. Organ toxicity is a major concern of liposomal nanodrugs [105] because they prefer to accumulate in certain organs, such as the liver and spleen, affecting the tissue-specific functionality and potentially causing toxicities [106]. In addition, liposomes may interact with cell membranes, which can alter cell permeability and integrity, ultimately causing cellular damage [107]. Addressing these safety concerns requires strategic refinement and optimization. The formulation of liposomes plays a pivotal role in liposome-induced cytotoxicity. By further optimizing and modifying liposome composition, size, and surface properties, interactions with specific organs could be minimized. Additionally, efforts in enhancing the targeting specificity of liposomal dosage form are research directions that can greatly limit the off-target organ distribution, thus further reducing the systemic toxicities.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Hoffman, L.M.; Van Zanten, S.E.M.V.; Colditz, N.; Baugh, J.; Chaney, B.; Hoffmann, M.; Lane, A.; Fuller, C.; Miles, L.; Hawkins, C. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): A collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. J. Clin. Oncol. 2018, 36, 1963.
- Bureš, J.; Kohoutová, D.; Zavoral, M. Gastrointestinal toxicity of systemic oncology immunotherapy. Klin. Onkol. 2022, 35, 346–357.
- Shastry, M.; Jacob, S.; Rugo, H.S.; Hamilton, E. Antibody-drug conjugates targeting TROP-2: Clinical development in metastatic breast cancer. Breast 2022, 66, 169–177.
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685.
- Cheng, Y.; Ji, Y. RGD-modified polymer and liposome nanovehicles: Recent research progress for drug delivery in cancer therapeutics. Eur. J. Pharm. Sci. 2019, 128, 8–17.
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102.
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252.
- Bilal, M.; Iqbal, H.M. New insights on unique features and role of nanostructured materials in cosmetics. Cosmetics 2020, 7, 24.
- Nkanga, C.I.; Bapolisi, A.M.; Okafor, N.I.; Krause, R.W.M. General perception of liposomes: Formation, manufacturing and applications. In Liposomes-Advances and Perspectives; IntechOpen: London, UK, 2019.
- Kiaie, S.H.; Mojarad-Jabali, S.; Khaleseh, F.; Allahyari, S.; Taheri, E.; Zakeri-Milani, P.; Valizadeh, H. Axial pharmaceutical properties of liposome in cancer therapy: Recent advances and perspectives. Int. J. Pharm. 2020, 581, 119269.
- Vemuri, S.; Rhodes, C.T. Preparation and characterization of liposomes as therapeutic delivery systems: A review. Pharm. Acta Helv. 1995, 70, 95–111.
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851.
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365.
- Lasic, D.D. The mechanism of vesicle formation. Biochem. J. 1988, 256, 1–11.
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New developments in liposomal drug delivery. Chem. Rev. 2015, 115, 10938–10966.
- Touti, R.; Noun, M.; Guimberteau, F.; Lecomte, S.; Faure, C. What is the fate of multi-lamellar liposomes of controlled size, charge and elasticity in artificial and animal skin? Eur. J. Pharm. Biopharm. 2020, 151, 18–31.
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12.
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134.
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975.
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorganic. Med. Chem. 2009, 17, 2950–2962.
- Fassas, A.; Anagnostopoulos, A. The use of liposomal daunorubicin (DaunoXome) in acute myeloid leukemia. Leuk. Lymphoma 2005, 46, 795–802.
- Mischler, R.; Metcalfe, I.C. Inflexal®V a trivalent virosome subunit influenza vaccine: Production. Vaccine 2002, 20, B17–B23.
- Gardikis, K.; Tsimplouli, C.; Dimas, K.; Micha-Screttas, M.; Demetzos, C. New chimeric advanced Drug Delivery nano Systems (chi-aDDnSs) as doxorubicin carriers. Int. J. Pharm. 2010, 402, 231–237.
- Carvalho, B.; Roland, L.M.; Chu, L.F.; Campitelli, V.A., III; Riley, E.T. Single-dose, extended-release epidural morphine (DepoDur™) compared to conventional epidural morphine for post-cesarean pain. Anesth. Analg. 2007, 105, 176–183.
- Fujita, K.-I.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234.
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D. FDA Approval Summary:(Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid LeukemiaFDA Approval:(Daunorubicin and Cytarabine). Clin. Cancer Res. 2019, 25, 2685–2690.
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087.
- Faro-Viana, J.; Bergman, M.-L.; Gonçalves, L.A.; Duarte, N.; Coutinho, T.P.; Borges, P.C.; Diwo, C.; Castro, R.; Matoso, P.; Malheiro, V.; et al. Population homogeneity for the antibody response to COVID-19 BNT162b2/Comirnaty vaccine is only reached after the second dose across all adult age ranges. Nat. Commun. 2022, 13, 140.
- Kirste, I.; Hortsch, S.; Grunert, V.P.; Legault, H.; Maglinao, M.; Eichenlaub, U.; Kashlan, B.; Pajon, R.; Jochum, S. Quantifying the Vaccine-Induced Humoral Immune Response to Spike-Receptor Binding Domain as a Surrogate for Neutralization Testing Following mRNA-1273 (Spikevax) Vaccination Against COVID-19. Infect. Dis. Ther. 2023, 12, 177–191.
- Qiu, R.; Qian, F.; Wang, X.; Li, H.; Wang, L. Targeted delivery of 20 (S)-ginsenoside Rg3-based polypeptide nanoparticles to treat colon cancer. Biomed. Microdevices 2019, 21, 18.
- Cai, F.; Luis, M.A.F.; Lin, X.; Wang, M.; Cai, L.; Cen, C.; Biskup, E. Anthracycline-induced cardiotoxicity in the chemotherapy treatment of breast cancer: Preventive strategies and treatment. Mol. Clin. Oncol. 2019, 11, 15–23.
- Yang, B.; Song, B.-P.; Shankar, S.; Guller, A.; Deng, W. Recent advances in liposome formulations for breast cancer therapeutics. Cell. Mol. Life Sci. 2021, 78, 5225–5243.
- Bompaire, F.; Durand, T.; Léger-Hardy, I.; Psimaras, D.; Ricard, D. Chemotherapy-related cognitive impairment or «chemobrain»: Concept and state of art. Geriatr. Psychol. Neuropsychiatr. Vieil. 2017, 15, 89–98.
- Milano, G.; Innocenti, F.; Minami, H. Liposomal irinotecan (Onivyde): Exemplifying the benefits of nanotherapeutic drugs. Cancer Sci. 2022, 113, 2224–2231.
- Hong, K.; Drummond, D.C.; Kirpotin, D. Liposomes Useful for Drug Delivery. U.S. Patent US20160030341A1, 4 February 2016.
- Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557.
- Sis, M.J.; Webber, M.J. Drug delivery with designed peptide assemblies. Trends Pharmacol. Sci. 2019, 40, 747–762.
- Dissanayake, S.; Denny, W.A.; Gamage, S.; Sarojini, V. Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J. Control. Release 2017, 250, 62–76.
- Deng, X.; Mai, R.; Zhang, C.; Yu, D.; Ren, Y.; Li, G.; Cheng, B.; Li, L.; Yu, Z.; Chen, J. Discovery of novel cell-penetrating and tumor-targeting peptide-drug conjugate (PDC) for programmable delivery of paclitaxel and cancer treatment. Eur. J. Med. Chem. 2021, 213, 113050.
- Zhang, Y.; Zhang, L.; Hu, Y.; Jiang, K.; Li, Z.; Lin, Y.-Z.; Wei, G.; Lu, W. Cell-permeable NF-κB inhibitor-conjugated liposomes for treatment of glioma. J. Control. Release 2018, 289, 102–113.
- Zhang, Q.; Lu, L.; Zhang, L.; Shi, K.; Cun, X.; Yang, Y.; Liu, Y.; Gao, H.; He, Q. Dual-functionalized liposomal delivery system for solid tumors based on RGD and a pH-responsive antimicrobial peptide. Sci. Rep. 2016, 6, 19800.
- Dellière, S.; Rivero-Menendez, O.; Gautier, C.; Garcia-Hermoso, D.; Alastruey-Izquierdo, A.; Alanio, A. Emerging mould infections: Get prepared to meet unexpected fungi in your patient. Med. Mycol. 2020, 58, 156–162.
- Chandrasekar, P. Management of invasive fungal infections: A role for polyenes. J. Antimicrob. Chemother. 2011, 66, 457–465.
- Pathakumari, B.; Liang, G.; Liu, W. Immune defence to invasive fungal infections: A comprehensive review. Biomed. Pharmacother. 2020, 130, 110550.
- Senoner, T.; Breitkopf, R.; Treml, B.; Rajsic, S. Invasive Fungal Infections after Liver Transplantation. J. Clin. Med. 2023, 12, 3238.
- Scolarici, M.; Jorgenson, M.; Saddler, C.; Smith, J. Fungal infections in liver transplant recipients. J. Fungi 2021, 7, 524.
- Stone, N.R.H.; Bicanic, T.; Salim, R.; Hope, W. Liposomal Amphotericin B (AmBisome®): A Review of the Pharmacokinetics, Pharmacodynamics, Clinical Experience and Future Directions. Drugs 2016, 76, 485–500.
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50.
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421.
- Vahedi-Shahandashti, R.; Dietl, A.M.; Binder, U.; Nagl, M.; Würzner, R.; Lass-Flörl, C. Aspergillus terreus and the Interplay with Amphotericin B: From Resistance to Tolerance? Antimicrob. Agents Chemother. 2022, 66, e0227421.
- Paajanen, J.; Halme, M.; Palomäki, M.; Anttila, V.-J. Disseminated Scedosporium apiospermum central nervous system infection after lung transplantation: A case report with successful recovery. Med. Mycol. Case Rep. 2019, 24, 37–40.
- Escandón, P.; Chow, N.A.; Caceres, D.H.; Gade, L.; Berkow, E.L.; Armstrong, P.; Rivera, S.; Misas, E.; Duarte, C.; Moulton-Meissner, H. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin. Infect. Dis. 2019, 68, 15–21.
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and other polyenes—Discovery, clinical use, mode of action and drug resistance. J. Fungi 2020, 6, 321.
- Frézard, F.; Aguiar, M.M.G.; Ferreira, L.A.M.; Ramos, G.S.; Santos, T.T.; Borges, G.S.M.; Vallejos, V.M.R.; De Morais, H.L.O. Liposomal Amphotericin B for Treatment of Leishmaniasis: From the Identification of Critical Physicochemical Attributes to the Design of Effective Topical and Oral Formulations. Pharmaceutics 2023, 15, 99.
- Sousa, F.; Nascimento, C.; Ferreira, D.; Reis, S.; Costa, P. Reviving the interest in the versatile drug nystatin: A multitude of strategies to increase its potential as an effective and safe antifungal agent. Adv. Drug Deliv. Rev. 2023, 199, 114969.
- Anim, V.C.E. Antifungal drug therapy in avian species. Vet. Clin. Exot. Anim. Pract. 2003, 6, 337–350.
- Belakhov, V.; Garabadzhiu, A.; Chistyakova, T. Polyene Macrolide Antibotic Derivatives: Preparation, Overcoming Drug Resistance, and Prospects for Use in Medical Practice. Pharm. Chem. J. 2019, 52, 890–901.
- Johnson, E.M.; Ojwang, J.O.; Szekely, A.; Wallace, T.L.; Warnock, D.W. Comparison of in vitro antifungal activities of free and liposome-encapsulated nystatin with those of four amphotericin B formulations. Antimicrob. Agents Chemother. 1998, 42, 1412–1416.
- Krishna, S.S.; Sudheesh, M.; Viswanad, V. Liposomal drug delivery to the lungs: A post COVID-19 scenario. J. Liposome Res. 2023, 33, 410–424.
- Forest, V.; Pourchez, J. Nano-delivery to the lung-by inhalation or other routes and why nano when micro is largely sufficient? Adv. Drug Deliv. Rev. 2022, 183, 114173.
- Dhiman, N.; Sarvaiya, J.; Mohindroo, P. A drift on liposomes to proliposomes: Recent advances and promising approaches. J. Liposome Res. 2022, 32, 317–331.
- Vuong, N.N.; Hammond, D.; Kontoyiannis, D.P. Clinical Uses of Inhaled Antifungals for Invasive Pulmonary Fungal Disease: Promises and Challenges. J. Fungi 2023, 9, 464.
- Alabdullah, M.N.; Yousfan, A. Is low dose of liposomal amphotericin B effective in management of acute invasive fungal rhinosinusitis? Our conclusions from Al-Mowassat University Hospital, Syria: A prospective observational study. BMC Infect. Dis. 2023, 23, 196.
- Muthu, V.; Gogineni, R.R.; Agarwal, R.; Prasad, K.T.; Sehgal, I.S.; Dhooria, S.; Aggarwal, A.N.; Rudramurthy, S.M.; Singh, H.; Garg, M. Treatment of pulmonary mucormycosis with adjunctive nebulized amphotericin B (MUCONAB trial): Results of an open-label randomized controlled trial. Mycoses 2023, 66, 688–696.
- de Pablo, E.; O’Connell, P.; Fernández-García, R.; Marchand, S.; Chauzy, A.; Tewes, F.; Dea-Ayuela, M.; Kumar, D.; Bolás, F.; Ballesteros, M. Targeting lung macrophages for fungal and parasitic pulmonary infections with innovative amphotericin B dry powder inhalers. Int. J. Pharm. 2023, 635, 122788.
- Celi, S.S.; Fernández-García, R.; Afonso-Urich, A.I.; Ballesteros, M.P.; Healy, A.M.; Serrano, D.R. Co-Delivery of a High Dose of Amphotericin B and Itraconazole by Means of a Dry Powder Inhaler Formulation for the Treatment of Severe Fungal Pulmonary Infections. Pharmaceutics 2023, 15, 2601.
- Saalbach, K.P. Nasal and pulmonary routes of drug delivery. In Novel Platforms for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 569–606.
- Schug, S.A.; Lavand’homme, P.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D. The IASP classification of chronic pain for ICD-11: Chronic postsurgical or posttraumatic pain. Pain 2019, 160, 45–52.
- El-Tallawy, S.N.; Nalamasu, R.; Pergolizzi, J.V.; Gharibo, C. Pain management during the COVID-19 pandemic. Pain Ther. 2020, 9, 453–466.
- Hollmann, M.W.; Rathmell, J.P.; Lirk, P. Optimal postoperative pain management: Redefining the role for opioids. Lancet 2019, 393, 1483–1485.
- Imani, F.; Zaman, B.; De Negri, P. Postoperative pain management: Role of dexmedetomidine as an adjuvant. Anesthesiol. Pain Med. 2020, 10, e112176.
- Ghai, B.; Jafra, A.; Bhatia, N.; Chanana, N.; Bansal, D.; Mehta, V. Opioid sparing strategies for perioperative pain management other than regional anaesthesia: A narrative review. J. Anaesthesiol. Clin. Pharmacol. 2022, 38, 3.
- Gu, J.-H.; Liu, C.-C.; Xie, J.-L.; Ma, B.; Cui, S.-M.; Yang, G.-Z.; He, S.-C. The Local Anesthetic Bupivacaine Inhibits the Progression of Non-Small Cell Lung Cancer by Inducing Autophagy Through Akt/mTOR Signaling. Front. Oncol. 2021, 11, 616445.
- US Food and Drug Administration. FDA Label Approved on 10/28/2011 (PDF) for EXPAREL; US Food and Drug Administration: Silver Spring, MD, USA, 2014.
- Li, H.; Liu, Y.; Tian, D.; Tian, L.; Ju, X.; Qi, L.; Wang, Y.; Liang, C. Overview of cannabidiol (CBD) and its analogues: Structures, biological activities, and neuroprotective mechanisms in epilepsy and Alzheimer’s disease. Eur. J. Med. Chem. 2020, 192, 112163.
- Patricio, F.; Morales-Andrade, A.A.; Patricio-Martínez, A.; Limón, I.D. Cannabidiol as a therapeutic target: Evidence of its neuroprotective and neuromodulatory function in Parkinson’s disease. Front. Pharmacol. 2020, 11, 595635.
- Boyaji, S.; Merkow, J.; Elman, R.N.M.; Kaye, A.D.; Yong, R.J.; Urman, R.D. The role of cannabidiol (CBD) in chronic pain management: An assessment of current evidence. Curr. Pain Headache Rep. 2020, 24, 4.
- Perucca, E.; Bialer, M. Critical aspects affecting cannabidiol oral bioavailability and metabolic elimination, and related clinical implications. CNS Drugs 2020, 34, 795–800.
- Jha, N.K.; Arfin, S.; Jha, S.K.; Kar, R.; Dey, A.; Gundamaraju, R.; Ashraf, G.M.; Gupta, P.K.; Dhanasekaran, S.; Abomughaid, M.M. Re-establishing the comprehension of phytomedicine and nanomedicine in inflammation-mediated cancer signaling. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022; pp. 1086–1104.
- Canouï, E.; Launay, O. History and principles of vaccination. Rev. Des Mal. Respir. 2019, 36, 74–81.
- Iwasaki, A.; Omer, S.B. Why and how vaccines work. Cell 2020, 183, 290–295.
- Renukaradhya, G.J.; Narasimhan, B.; Mallapragada, S.K. Respiratory nanoparticle-based vaccines and challenges associated with animal models and translation. J. Control. Release 2015, 219, 622–631.
- Song, C.; Li, F.; Wang, S.; Wang, J.; Wei, W.; Ma, G. Recent advances in particulate adjuvants for cancer vaccination. Adv. Ther. 2020, 3, 1900115.
- Wu, N.; Chen, Q.; Zou, Y.; Miao, C.; Ma, G.; Wu, J. Chitosan particle-emulsion complex adjuvants: The effect of particle distribution on the immune intensity and response type. Carbohydr. Polym. 2023, 309, 120673.
- Andersson, L.; Häyry, P.; Bach, M.; Bach, J. Differences in the effects of adult thymectomy on T-cell mediated responses in vitro. Nature 1974, 252, 252–254.
- Tretiakova, D.; Vodovozova, E. Liposomes as adjuvants and vaccine delivery systems. Biochem. Suppl. Ser. A Membr. Cell Biol. 2022, 16, 1–20.
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 2021, 38, 101142.
- Di, J.; Xie, F.; Xu, Y. When liposomes met antibodies: Drug delivery and beyond. Adv. Drug Deliv. Rev. 2020, 154, 151–162.
- Kato, T.; Fahrmann, J.F.; Hanash, S.M.; Vykoukal, J. Extracellular vesicles mediate B cell immune response and are a potential target for cancer therapy. Cells 2020, 9, 1518.
- Cheng, X.; Gao, J.; Ding, Y.; Lu, Y.; Wei, Q.; Cui, D.; Fan, J.; Li, X.; Zhu, E.; Lu, Y. Multi-functional liposome: A powerful theranostic nano-platform enhancing photodynamic therapy. Adv. Sci. 2021, 8, 2100876.
- Wang, D.-Y.; Van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Lipid-based antimicrobial delivery-systems for the treatment of bacterial infections. Front. Chem. 2020, 7, 872.
- Pala, R.; Anju, V.; Dyavaiah, M.; Busi, S.; Nauli, S.M. Nanoparticle-mediated drug delivery for the treatment of cardiovascular diseases. Int. J. Nanomed. 2020, 15, 3741–3769.
- Sforzi, J.; Palagi, L.; Aime, S. Liposome-based bioassays. Biology 2020, 9, 202.
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984.
- Hernandez, C.; Shukla, S. Liposome based drug delivery as a potential treatment option for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1190.
- Che, J.; Najer, A.; Blakney, A.K.; McKay, P.F.; Bellahcene, M.; Winter, C.W.; Sintou, A.; Tang, J.; Keane, T.J.; Schneider, M.D. Neutrophils enable local and non-invasive liposome delivery to inflamed skeletal muscle and ischemic heart. Adv. Mater. 2020, 32, 2003598.
- Lin, C.-Y.; Lin, Y.-C.; Huang, C.-Y.; Wu, S.-R.; Chen, C.-M.; Liu, H.-L. Ultrasound-responsive neurotrophic factor-loaded microbubble-liposome complex: Preclinical investigation for Parkinson’s disease treatment. J. Control. Release 2020, 321, 519–528.
- Kahana, M.; Weizman, A.; Gabay, M.; Loboda, Y.; Segal-Gavish, H.; Gavish, A.; Barhum, Y.; Offen, D.; Finberg, J.; Allon, N. Liposome-based targeting of dopamine to the brain: A novel approach for the treatment of Parkinson’s disease. Mol. Psychiatry 2021, 26, 2626–2632.
- Srivastava, S.; Srivastava, S.; Singh, M.R.; Singh, D.; Tekwani, B.L. Chapter 11—Novel perspectives for delivery of bioactives through blood–brain barrier and treatment of brain diseases. In Advances and Avenues in the Development of Novel Carriers for Bioactives and Biological Agents; Singh, M.R., Singh, D., Kanwar, J.R., Chauhan, N.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 317–341.
- Chen, Z.-J.; Yang, S.-C.; Liu, X.-L.; Gao, Y.; Dong, X.; Lai, X.; Zhu, M.-H.; Feng, H.-Y.; Zhu, X.-D.; Lu, Q. Nanobowl-supported liposomes improve drug loading and delivery. Nano Lett. 2020, 20, 4177–4187.
- Rommasi, F.; Esfandiari, N. Liposomal nanomedicine: Applications for drug delivery in cancer therapy. Nanoscale Res. Lett. 2021, 16, 95.
- Aloss, K.; Hamar, P. Recent Preclinical and Clinical Progress in Liposomal Doxorubicin. Pharmaceutics 2023, 15, 893.
- Kommineni, N.; Chaudhari, R.; Conde, J.; Tamburaci, S.; Cecen, B.; Chandra, P.; Prasad, R. Engineered Liposomes in Interventional Theranostics of Solid Tumors. ACS Biomater. Sci. Eng. 2023, 9, 4527–4557.
- de Oliveira Silva, J.; Fernandes, R.S.; de Alcântara Lemos, J.; Cassali, G.D.; de Paula Sabino, A.; Townsend, D.M.; Oliveira, M.C.; de Barros, A.L.B. Evaluation of acute toxicity and in vitro antitumor activity of a novel doxorubicin-loaded folate-coated pH-sensitive liposome. Biomed. Pharmacother. 2023, 165, 115280.
- Oros-Pantoja, R.; Córdoba-Adaya, J.C.; Torres-García, E.; Morales-Avila, E.; Aranda-Lara, L.; Santillán-Benítez, J.G.; Sánchez-Holguín, M.; Hernández-Herrera, N.O.; Otero, G.; Isaac-Olivé, K. Preclinical evaluation of early multi-organ toxicity induced by liposomal doxorubicin using 67Ga-citrate. Nanotoxicology 2022, 16, 247–264.
- Ranjbar, S.; Zhong, X.-B.; Manautou, J.; Lu, X. A holistic analysis of the intrinsic and delivery-mediated toxicity of siRNA therapeutics. Adv. Drug Deliv. Rev. 2023, 201, 115052.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
538
Revisions:
2 times
(View History)
Update Date:
12 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




