Please note this is a comparison between Version 1 by Jianqin Lu and Version 2 by Lindsay Dong.
Liposomes have been extensively developed and used for various clinical applications such as in pharmaceutical, cosmetic, and dietetic fields, due to its versatility, biocompatibility, and biodegradability, as well as the ability to enhance the therapeutic index of free drugs. However, some challenges remain unsolved, including liposome premature leakage, manufacturing irreproducibility, and limited translation success.
- liposome
- drug delivery
- disease treatments
1. Introduction
Cancer has brought a critical burden to the economy and society. GLOBOCAN (the World Health Organization’s International Agency for Research on Cancer Global Cancer Observatory) 2020 reported an estimation of 19 million new cancer cases and 10 million cancer deaths occurred worldwide [1]. Currently, cancer treatments are still mainly proceeded by surgery, radiotherapy, and chemotherapy, although gene therapy and immunotherapy have been brought up as novel methods with a higher therapeutic index. However, some challenges remain unsolved even with the advanced therapies, such as low solubility, poor pharmacokinetics, non-specific biodistribution, and systemic toxicities [2][3][2,3]. Therefore, targeted delivery of therapeutics to specific sites has been an active area of research in the last couple of decades. Of note, several drug delivery platforms have been reported, and some are being used in clinical settings, including antibody-drug conjugates, polymers, as well as liposomes [4][5][6][4,5,6]. Of those, liposomes are a promising drug delivery vehicle due to their biocompatibility and biodegradability, good stability, as well as the ability to encapsulate both hydrophobic and hydrophilic contents [7]. When the first liposome was described by Bangham et al. in 1964 [8], it had grown to be a great interest in cosmetic, dietetic, and pharmaceutical areas [9][10][11][9,10,11].
Due to the natural properties of liposome, the major components are lipids and fatty acids comprising phospholipids, which can spontaneously self-assemble into a lipid bilayer with an aqueous core. The phospholipid bilayer is similar to the construction of the cell membrane. Therefore, liposomes are considered to be biocompatible and biodegradable [7]. Because of the presence of a lipid membrane and a hydrophilic interior, liposomes can be used to deliver both hydrophilic and hydrophobic molecules. With that, liposomes have been further researched of their benefits as a drug delivery platform.
2. Characterization and Major Components of Liposomes
Several ways can be used to classify liposomes, including size, lamellarity, and method of preparation [12][13][12,13]. Scholars define liposomes by their size and lamellarity. These two factors also dominate the drug encapsulation efficiency and ADME (absorption, distribution, metabolism, and elimination) of the drug [7][14][15][7,14,15]. By lamellarity, liposomes can be defined as: a unilamellar vesicle (ULV), with one bilayer membrane; an oligolamellar vesicle (OLV), with 2–5 bilayer membranes; or a multilamellar vesicle (MLV), with five or more bilayer membranes. Furthermore, ULV can be classified by its size, including small unilamellar vesicle (SUV) ranging from 20 to 100 nm; large unilamellar vesicle (LUV) with a size larger than 100 nm; and giant unilamellar vesicle (GUV) with a size bigger than 1000 nm [16]. Generally, ULV is formed by a phospholipid bilayer and an aqueous core. More uniquely, several ULVs with gradually smaller sizes caging inside each other compose the MLV, which resembles an onion, and each lipid bilayer is separated by an aqueous layer [17]. Three dominant components that contribute to the formation, stability, and functionality of liposomes include phospholipids, cholesterol, and polyethylene glycol (PEG).3. Pharmaceutical Applications of Liposomes
Owing to its biocompatibility, biodegradability, nontoxicity, and favorable physical properties for convenient modifications of surface charge and its size, since the 1990s, there have been more than a dozen U.S. FDA-approved liposomal or lipid-based nanodrugs (Table 13) with numerous more under preclinical and clinical development.Table 13.
U.S. FDA-approved liposomal/lipid-based nanodrugs.
| Name | Clinical Approval Year | Liposomal Composition | Drug Encapsulated | Drug Type | Route of Administration | Company | References |
|---|---|---|---|---|---|---|---|
| Doxil | 1995 | HSPC:Cholesterol:DSPE-PEG2000 | Doxorubicin | Chemotherapeutic | I.V. | Johnson & Johnson, Milpitas, CA, USA | [18][19][101,102] |
| Abelcet | 1995 | DMPC:DMPG | Amphotericin B | Antifungal | I.V. | Leadiant Biosciences. Inc., Rockville, MD, USA | [20][21][103,104] |
| DaunoXome | 1996 | DSPC:Cholesterol | Daunorubicin | Chemotherapeutic | I.V. | Galen US, Inc., Souderton, PA, USA | [18][22][101,105] |
| Amphotec | 1996 | Cholesteryl sulphate:Amphotericin B | Amphotericin B | Antifungal | I.V. | Sequus Pharmaceuticals Inc., Menlo Park, CA, USA | [18][101] |
| Inflexal V | 1997 | 70% Lecithin, 20% Cephalin and 10% Phospholipids | Influenza virus antigen, strain A and B | Vaccine | I.M. | Sun Pharmaceutical Industries Ltd., Princeton, NJ, USA | [18][23][101,106] |
| Ambisome | 1997 | HSPC:DSPG:Cholesterol:Amphotericin B | Amphotericin B | Antifungal | I.V. | Fujisawa Healthcare, Inc. and Gilead Sciences, Inc., Foster City, CA, USA | [18][101] |
| Myocet | 2000 | EPG:Cholesterol | Doxorubicin | Chemotherapeutic | I.V. | Zeneus Pharma Ltd., Oxford, UK | [18][24][101,107] |
| Visudyne | 2000 | Verteporfin:DMPC and EPG | Verteporfin | Photosensitizer | I.V. | Novartis International AG, Basel, Switzerland | [18][101] |
| DepoDur | 2004 | DOPC:DPPG:Cholesterol:Tricaprylin and Triolein | Morphine sulfate | Narcotic Analgesic | Epidural | Pacira Pharmaceuticals, Inc., Watford, UK | [18][25][101,108] |
| Mepact | 2004 | DOPS:POPC | Mifamurtide | Immunomodulator/Antitumor | I.V. | Takeda Pharmaceutical Limited, Tokyo, Japan | [18][101] |
| Exparel | 2011 | DEPC:DPPG:Cholesterol:Tricaprylin | Bupivacaine | Anesthetic | I.V. | Pacira Pharmaceuticals, Inc., Parsippany-Troy Hills, NJ, USA | [18][101] |
| Onivyde | 2015 | DSPC:MPEG-2000:DSPE | Irinotecan | Chemotherapeutic | I.V. | Merrimack Pharmaceuticals, Inc., Cambridge, MA, USA | [18][26][101,109] |
| Vyxeos | 2017 | DSPC:DSPG:Cholesterol | Daunorubicin + Cytarabine | Antineoplastic | I.V. | Jazz Pharmaceuticals, Inc., Dublin, Ireland | [27][110] |
| Onpattro | 2018 | Cholesterol, DLin-MC3-DMA:DSPC:PEG2000-C-DMG | Patisiran | RNAi agent | I.V. | Alnylam Pharmaceuticals, Cambridge, MA, USA | [28][111] |
| Comirnaty | 2021 | ALC-0315:ALC-0159:cholesterol:DSPC | Nucleoside-modified mRNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 | Vaccine | I.M. | Pfizer-BioNTech, Mainz, Germany | [29][112] |
| Spikevax | 2022 | SM-102:mPEG2000-DMG:Cholesterol:DSPC | Nucleoside-modified mRNA encoding the viral spike (S) glycoprotein of SARS-CoV-2 | Vaccine | I.M. | Moderna, Cambridge, MA, USA | [30][113] |
3.1. Anti-Cancer
Cancer is a disease which is flourishing rapidly throughout the world. The ultimate goal of cancer therapy is to destroy all the malignant cells. Conventional chemotherapy, as one of the most common cancer treatments, employs cytotoxic agents that target rapidly proliferating cells, especially like cancer cells [31][114]. For instance, anthracyclines, including Daunorubicin, Doxorubicin, Epirubicin, Idarubicin, Mitoxantrone, and Valrubicin, are used as chemotherapeutic agents for treatment of numerous types of cancers [32][115]. Yet, chemotherapies have been associated with severe unwanted systemic toxicities, off-target effect, and rapidly emerging drug resistance [33][116]. Most of the chemotherapeutic drugs are not selective to cancer cells, which indicate that they not only target cancer cells, but also can be randomly distributed to healthy organs. As reported, detrimental effects to the central nervous system (CNS) are recognized as cognition dysfunction during chemotherapy for a non-CNS cancer [34][117].3.1.1. Doxil
The liposome has been the most successful in therapeutic delivery as evidenced by numerous FDA-approved liposomal nanodrugs (e.g., Doxil, DaunoXome, Depocyt, Myocet, Mepact, and Onivyde, etc.) for diverse diseases management (e.g., cancers). Doxil, the first FDA-approved nanodrug delivery system using pegylated liposomes to encapsulate doxorubicin, consists of three major components: the high-transition-temperature (Tm) phospholipid hydrogenated soy phosphatidylcholine (HSPC; Tm 52.5 °C); cholesterol; and N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (MPEG-DSPE) [18][101].3.1.2. Onivyde
Onivyde, also known as an irinotecan liposome injection, is used for patients with metastatic adenocarcinoma of the pancreas with cancer progression after the gemcitabine-based therapy, usually in combination with fluorouracil and leucovorin [35][125]. The Onivyde liposomal vesicles comprise three key components: distearoylphosphatidylcholine (DSPC), cholesterol, and methoxy-terminated polyethylene glycol (MW2000)-distearoylphosphatidylethanolamine (MPEG-2000-DSPE) [36][126]. The efficacy and safety of Onivyde were evaluated in a global, randomized, open-label NAPOLI-1 clinical trial involving patients with metastatic pancreatic cancer who experienced disease progression after gemcitabine treatment [37][127]. The clinical results confirmed that liposomal irinotecan, Onivyde, significantly extends the lifespan of patients compared to free drugs.3.1.3. Liposome-Peptide Conjugated Drugs
Peptides play a critical role in genes and drugs delivery, classified into two types: cell-penetrating peptides and cell-targeting peptides (Figure 18) [38][128]. Peptides exhibit advantageous properties, being biocompatible and well-tolerated, with modifiable features such as hydrophobicity, charge, solubility, and stability [39][129]. While most of the cell-penetrating peptides are cationic peptides and possess the ability for cellular uptake without inducing cytotoxicity, they lack selectivity and receptor-dependence, thereby limiting tissue specificity and tumor targeting [40][130]. As the need for enhanced peptide targeting and selectivity emerged, liposomes have been introduced as a delivery platform, forming an engineered combination known as liposome–peptide conjugates [39][40][129,130]. These conjugates showcase remarkable performance improvements in cellular uptake, tumor penetration, extended circulation time, and enhanced site-specific targeting, surpassing both liposomal drugs and free drugs [41][131].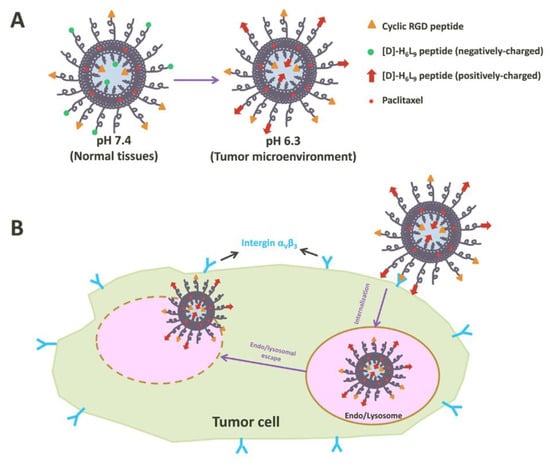
Figure 18. Illustration of tumor cell penetration with a peptide-decorated liposome [42][136]. (A) The Structure of peptide-decorated liposomes under different pH environments. (B) Within tumors, the peptide-decorated liposomes could target integrin αVβ3 and initiate internalization and further intertumoral activities.
3.2. Anti-Fungal
There are two forms of fungi existing in nature, yeasts and molds [43][137]. Most fungi do not live dependent on animals or human beings. Yet, some groups are exterior pathogens in humans, such as Candida spp., Aspergillus spp., Cryptococcus spp., Fusarium spp., Mucorales, and endemic mycosis [44][138], and these cause superficial, subcutaneous, or systemic infections. Additionally, a severe, systemic fungal infection with yeasts or molds is clinically described with invasive fungal infection. Although some infections, like superficial infections, are not life-threatening, the consequences could be severe and affect the patient’s quality of life [45][139]. On the other hand, in immunocompromised patients, for example, bone marrow and organ transplant patients, systemic fungal infections are associated with high mortality rates [46][47][140,141].
3.2.1. Amphotericin B and Ambisome
Amphotericin B is one of the most widespread therapeutic polyene antifungals [48][142]. According to the Infectious Diseases Society of America (IDSA) [49][143] and the European Confederation of Medical Mycology (ECMM) [50][144], Amphotericin B is still recommended as first line treatment polyene antifungals used for severe cryptococcosis, disseminated histoplasmosis, and mucormycosis. However, a number of studies show that Amphotericin B treatments of systemic mycosis caused by species such as Aspergillus terreus [51][145], Scedosporium spp. [52][146], and Candida auris [53][147] are not always effective, which results from the intrinsic or acquired drug resistance [54][148]. Moreover, the intrinsic host toxicity of Amphotericin B is another clinical concern [54][148].
To date, several liposomal formulations for anti-fungal infections have been approved by the FDA, including Abelcet, Ambisome, and Amphotec. Ambisome was developed by Astellas Pharma USA for the treatment of serious, life-threatening fungal infections, and also for Amphotericin B intolerance or renal-impaired patients who were infected with invasive systemic infections caused by Aspergillus, Candida, or Cryptococcus [18][101]. Structurally, the lipid bilayer of Ambisome is composed of hydrogenated soy phosphatidylcholine (HSPC), cholesterol, 1,2-distearoyl-sn-glycero-3-phosphoglycerol (DSPG), and Amphotericin B [55][151].
 Encyclopedia
Encyclopedia
