Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rachid Skouta | -- | 1839 | 2024-01-09 13:49:38 | | | |
| 2 | Jessie Wu | Meta information modification | 1839 | 2024-01-10 02:37:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Adesina, A.; Skouta, R. N-Phenylquinoneimine. Encyclopedia. Available online: https://encyclopedia.pub/entry/53611 (accessed on 08 February 2026).
Adesina A, Skouta R. N-Phenylquinoneimine. Encyclopedia. Available at: https://encyclopedia.pub/entry/53611. Accessed February 08, 2026.
Adesina, Adebimpe, Rachid Skouta. "N-Phenylquinoneimine" Encyclopedia, https://encyclopedia.pub/entry/53611 (accessed February 08, 2026).
Adesina, A., & Skouta, R. (2024, January 09). N-Phenylquinoneimine. In Encyclopedia. https://encyclopedia.pub/entry/53611
Adesina, Adebimpe and Rachid Skouta. "N-Phenylquinoneimine." Encyclopedia. Web. 09 January, 2024.
Copy Citation
The N-phenylquinoneimine scaffold is a versatile synthetic platform that has gained significant attention in the field of drug discovery due to its structural diversity and capacity to interact with biologically relevant targets.
diversity-orientated synthesis
biological activity
DNA
reactive metabolites
drugs
1. Introduction
Diversity-orientated synthesis (DOS) continues to grow as an area of importance in the disciplines of organic synthesis and chemical biology [1][2][3]. One important area that should benefit significantly from DOS is drug discovery. The existing chemical space can be expanded with new synthetic molecules, with the hope of identifying novel and better drug and probe molecules [4]. Arguably, one of the most promising synthetic strategies for generating collections of new compounds with increased molecular complexity and diversity via DOS involves the sequencing of multicomponent reactions with subsequent transformations, including cyclisations, couplings, and refunctionalisations [5].
DOS requires a planning algorithm to deliver an efficient but divergent route. Although DOS aims to achieve a diverse and non-focused coverage of biologically active chemical space, the results of DOS may find use in other fields in future years. Complexity-generating reactions are again important for efficiency (multicomponent-coupling, cascade and tandem complexity-generating reactions are the most valuable); however, pathways need to be identified that give structurally diverse targets. In order to achieve the highest levels of structural diversity, (i) the building blocks, (ii) the stereochemistry, (iii) the functional groups and, most importantly, (iv) the molecular framework must be varied. The key to the structural complexity is the complexity-generating reactions, while the key to the structural diversity comprises the branch points and building blocks [3]. The identification in the forward direction of pairwise relationships, where the product of one complexity-generating reaction is the substrate for another, can lead to high levels of molecular complexity in a very efficient manner [2].
2. N-Phenylquinoneimine and Its Pharmacological Significance
N-Phenylquinoneimine (NPQ 1, Figure 1), due to its α,β-keto and α,β-imino functionalities, is highly reactive and offers great potential for regioselective reactions. NPQs are highly coloured compounds [6][7][8] and constitute a core structure in several important natural products, Refs. [9][10], some of which are key abiotic and biological compounds, which intercalate with DNA [11]. New avenues for molecular sensors [12] and ligands, Refs. [13][14] for drug delivery [15][16] and controlled material growth have been provided through many of these hybrid materials. NPQs can be used as building blocks to access useful synthetic compounds that can serve as potential key intermediates in cascade reactions [17]. Since many natural products and druglike compounds include heterocyclic subunits, the ability to synthesise efficiently diverse heterocyclic compounds is critical. NPQs can be used to access aromatic heterocyclic structures largely used as scaffolds for generating combinatorial libraries in drug-discovery research [18]. Sulfones that can be synthesised from multistep reactions of NPQs are found in many medicines and drug candidates under development for the treatment of a host of diseases impacting human health worldwide [19]. NPQs represent a new frontier for the design and generation of molecular diversity and complexity [20][21].
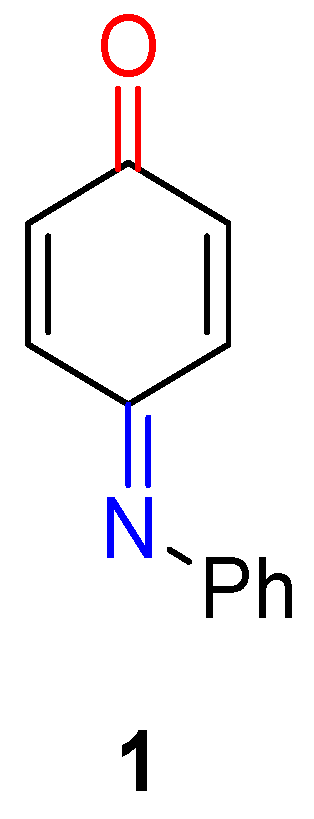
Figure 1. Structure of N-phenylquinoneimine 1 (NPQ).
3. Quinoneimines in Natural Products and Dyes
Natural products and their structural analogues have historically made a major contribution to pharmacotherapy, especially for cancer and infectious diseases [22][23], but also in other therapeutic areas, including cardiovascular diseases and multiple sclerosis [24][25][26]. Natural products are characterised by enormous scaffold diversity and structural complexity [27].
Quinoneimines are highly coloured dyes [6][7][8][28][29] and constitute a core structure in several important natural products [9][10][30][31][32][33][34]. Some of these quinoneimines have been reported as growth-promoting substances with a low molecular weight, isolated from microorganisms [20]. Exfoliazone, Questiomycin A, N-Acetylquestiomycin A, and Acetylmichigazone have been isolated from Streptomyces exfoliates BT-38 [9][10], and Venezuelines A–G from Streptomyces venezuelae [30]. Chandrananimycins A–C were isolated from the culture broth of a marine Actinomadura sp. Isolate M045. They contain the phenoxazin-3-one chromophore, which is part of complex natural products like actinomycin, aurantin, and cryptomycin and is responsible for their colour [31]. Chandrananimycin D, pitucamycin, grixazone B, and benzerramycin A–C have been reported from a Streptomyces griseus strain isolated from an old building with moisture damage [32][33]. Cinnabarin and Cinnabarinic acid have been isolated as fungal pigments [34] (Figure 2).
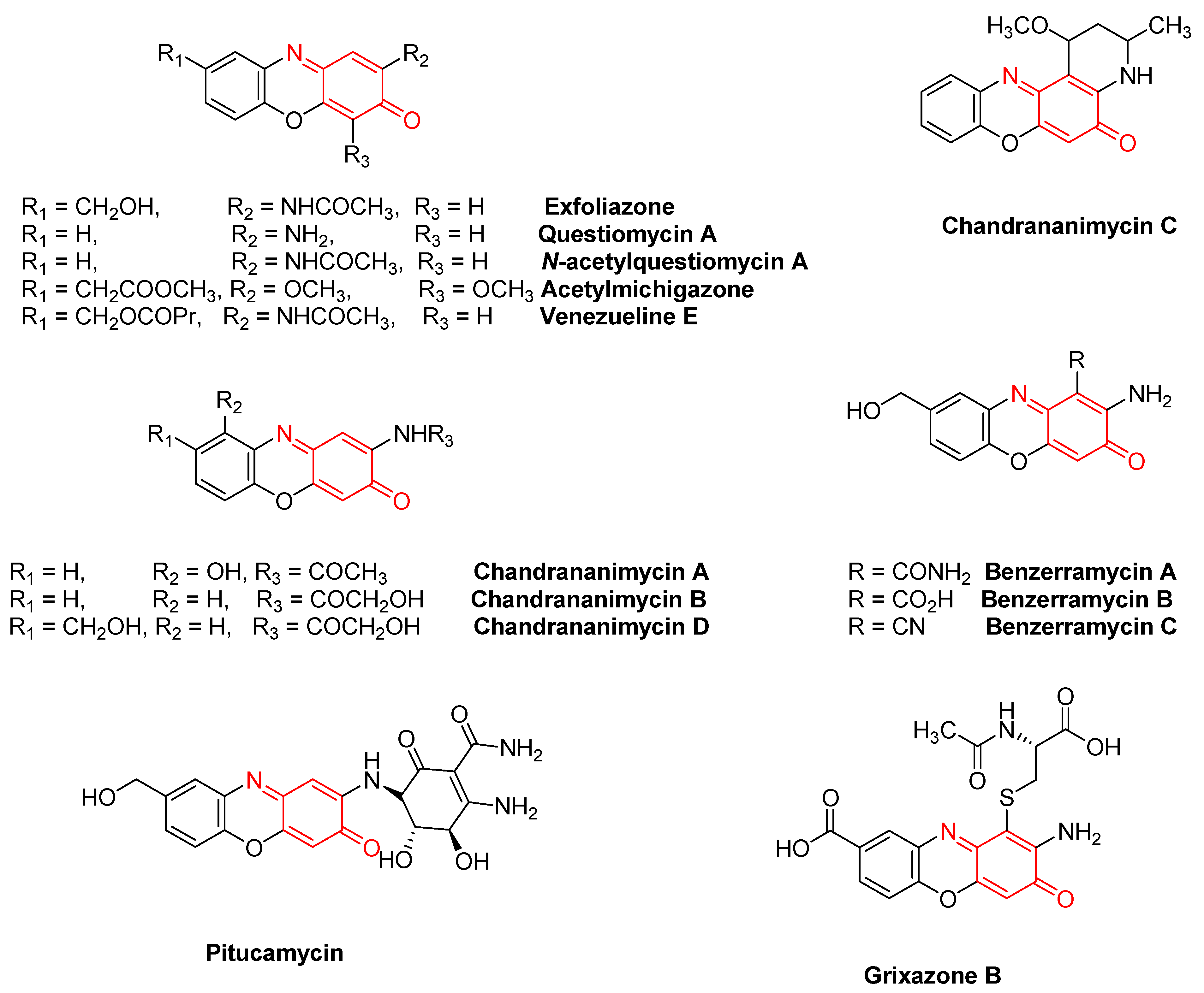
Figure 2. Some of the representative examples of quinoneimines in natural products.
Quinoneimine dyes are based on the structure of the fictional compound para-quinone-di-imine 2, from which the name of the dye class originates. There are several subgroups of quinoneimine dyes, such as the azines 3, the oxazines 4, and the thiazines 5 (Figure 3).
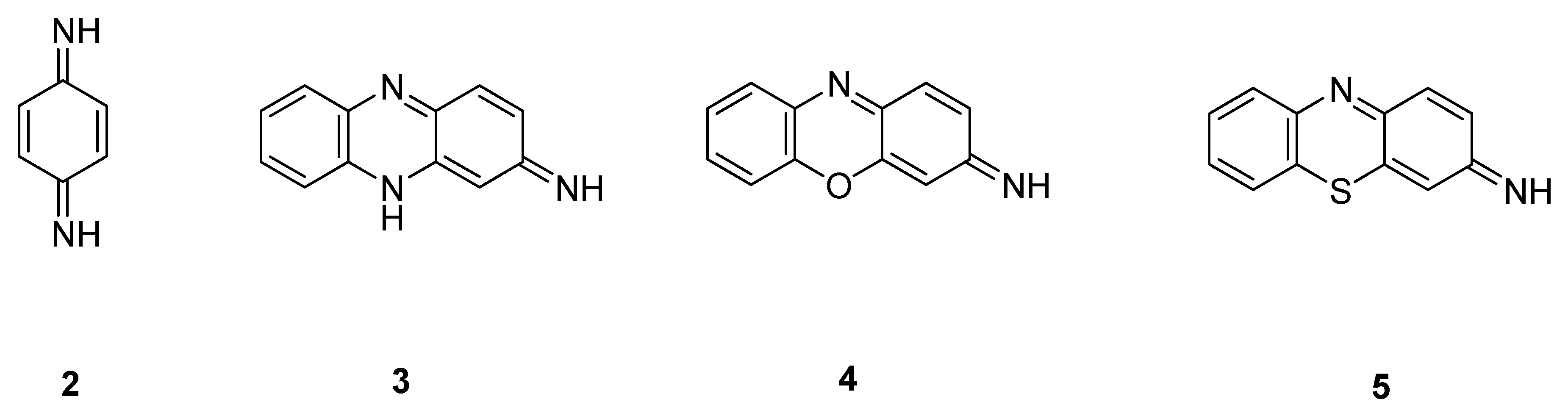
Figure 3. Some subgroups of quinoneimine dyes.
Quinoneimine dyes are commonly used in colour photography and in the production of pencils, as well as for dyeing paper and fur. In addition, they are used as chemical indicators [35].
Some commonly used and important quinoneimine dyes are neutral red, safranin O, Nile blue, Nile red, Meldola’s blue, gallocyanin, gallamine blue, celestine blue B, and the methylene blue homologues (Figure 4).
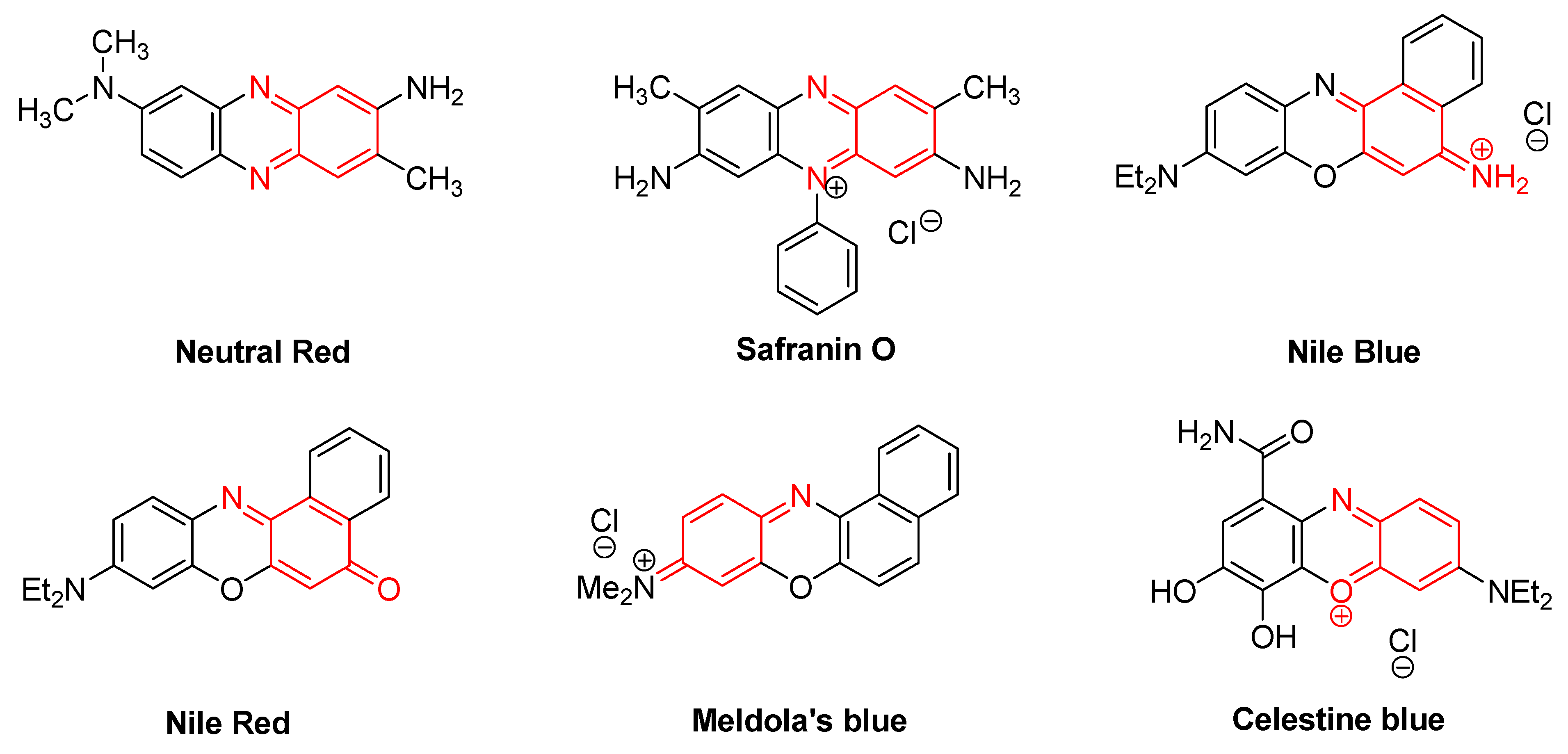
Figure 4. Some of the common quinoneimine dyes.
In microbiology, neutral red is used in the MacConkey agar to differentiate bacteria for lactose fermentation [36]. It also acts as a pH indicator, changing from red to yellow between pH 6.8 and 8.0.
Safranin is the classic counterstain in both Gram stains and endospore stains. It is also used as redox indicator in analytical chemistry.
Nile blue and Nile red are fluorescent dyes [37]. They have reasonably high fluorescence quantum yields in nonpolar solvents and they fluoresce at reasonably long wavelengths [38].
Meldola’s blue dye is used mainly as a pigment in textiles, paper, and paints. It has also been used in electrochemical experiments involving DNA, wherein the dye mediates electron transport [39].
Celestine blue dye is used with iron-aluminium complexes as a substitute for haematoxylin in H–E (haematoxylin–eosin) staining because of its resistance to low-pH solutions. It has been used as a new electroactive indicator in DNA biosensors and is also applicable to HOCl detection in living cells and to assaying the chlorinating activity of myeloperoxidase [40].
4. Biological Activity of N-Phenylquinoneimine Scaffolds
Quinoneimines are key abiotic and biological components that intercalate with DNA [11]. Quinoneimines and diimines are of interest in chemistry, and the former moieties have been proposed as intermediates in a number of biological processes [41]. Their diverse biological activities and synthetic applications have attracted the synthetic community to synthesise these important alkaloids [42][43][44][45]. Imai S. et al. [10] have reported exfoliazone (Figure 2), a phenoxazine antibiotic showing antifungal activity against V. ceratosperma. Pitucamycin and Chandrananimycin D have been found to exhibit antiproliferative activities against a number of cell lines and only a weak cytotoxicity [33].
Chandrananimycins A–C (Figure 2), isolated from Actinomadura sp. Isolate M048, have been reported to have high biological activity against Staphylococus aureus, Bacillus subtilis, and Streptomyces viridochromogenes [31]. They have also exhibited antialgal activity against the microalgae Chlorella vulgaris, Chlorella sorokiniana, and Scenedesmus suspicatus and antifungal activity against Mucor miehei and Candida albicans. Compounds such as Chandrananimycins A–C, containing the phenoxazine-3-one chromophore, are frequently encountered as metabolites of microorganisms. They are yellow-to-orange-coloured compounds and exhibit antibacterial [46], antifungal [10], phytotoxic [47], and anticancer activities. In addition, some are also known to show potent cell-growth-stimulating activity [9]. Due to their DNA intercalation, these complex phenoxazinone derivatives have shown pronounced antimicrobial [48], antitumor [49], and anticancer potency [50], with some of them also exhibiting anticoccidial activity [51]. Table 1 summarises the quinoneimine derivatives occurring as natural products and their biological activities.
Table 1. Naturally occurring quinoneimines and their biological activities.
| Quineimine Derivatives as Natural Products | Biological Activities (References) |
|---|---|
| Exfoliazone | Antibiotic, antifungal, antitumor, growth-promoting activities [9][10] |
| Questiomycin A, N-acetylquestiomycin A, and Acetylmichigazone | Growth stimulatory and inhibitory effects [9] |
| Venezuelines A–G | Cytotoxic and antitumor activities [30] |
| Chandrananimycins A–D | Antibacterial, antifungal, antialgal, phytotoxic, and anticancer activities [31][33] |
| Actinomycins | Antibacterial, antitumor, and anticancer activities [52][53][54][55] |
| Pitucamycin | Antiproliferative and cytotoxic activities [33] |
| Grixazone B | Antimicrobial [33] |
| Benzerramycin A–C | Antiproliferative [32] |
| Cinnabarin and Cinnabarinic acid | Antibacterial, antimicrobial [34] |
5. Significance of Quinoneimine-Based Drugs
Exfoliazone (Figure 2) is an antibiotic that is active against Valsa ceratosperma, the causative fungus of the apple canker disease [10].
The actinomycins are a family of chromopeptide antitumor antibiotics isolated from various Streptomyces strains [50]. Actinomycins C3 and D have found clinical application as anticancer drugs, particularly in therapy for Wilm’s tumor [52] and soft tissue sarcomas [53] in children, and are still of interest in molecular biology [50].
Actinomycin D (Figure 5) has also been proposed as a therapeutic agent for AIDS, because of its potency as an inhibitor of HIV-1 minus-strand transfer [54]. It is a chemotherapy medication used to treat a number of types of cancer. This includes rhabdomyosarcoma, Ewing’s sarcoma, trophoblastic neoplasm, testicular cancer, and certain types of ovarian cancer [55].
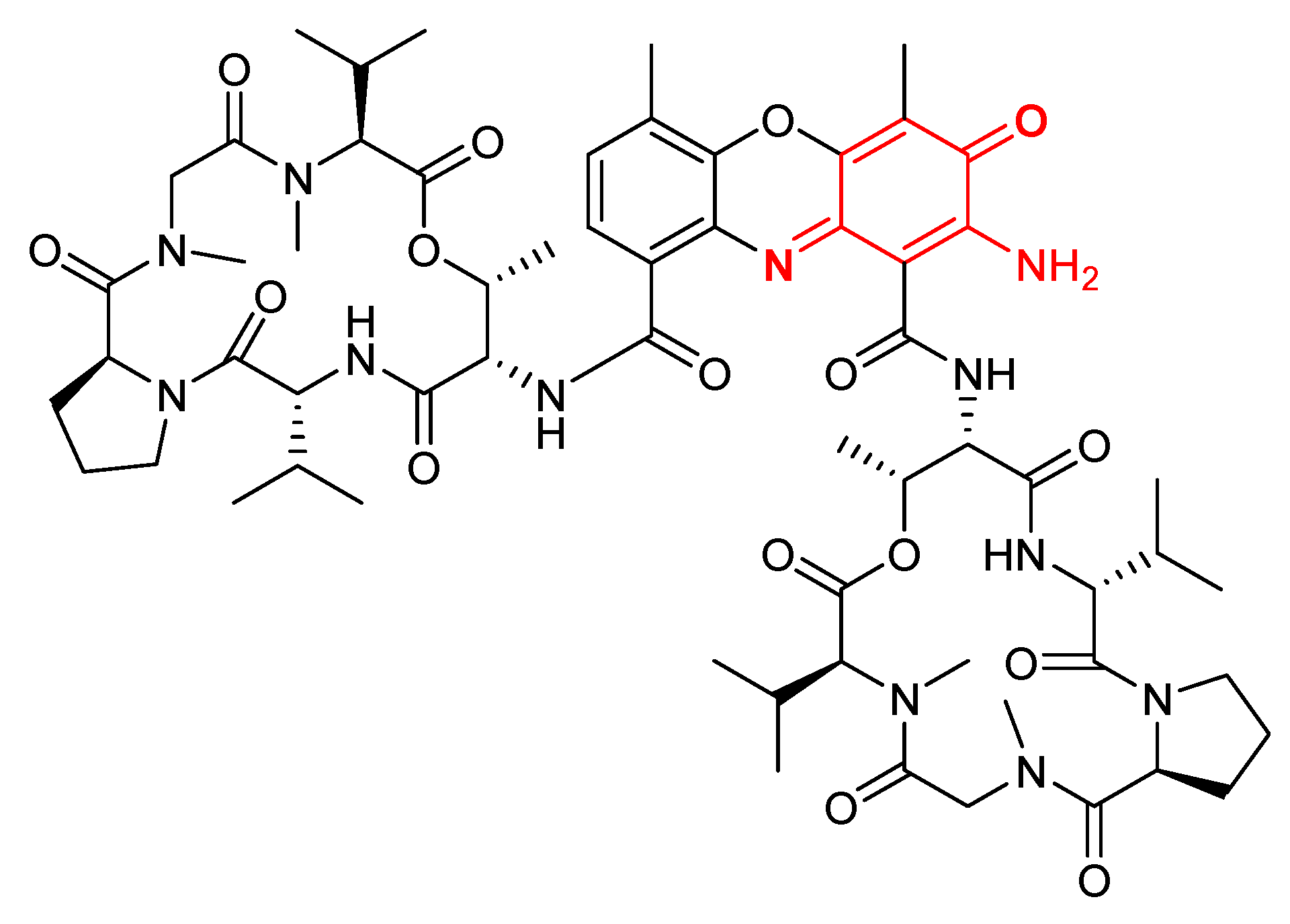
Figure 5. Structure of Actinomycin D.
Some chemicals and drugs have been reported to form reactive quinone and quinoneimine metabolites [56]. Quinoneimines are found as highly redox-active molecules and electrophiles, with both properties being crucial for their reactivity in biological systems. They are highly reactive organic chemicals and comprise a class of toxicological intermediates [57][58] that interact alone by generating reactive oxygen species (ROS) in biological systems to promote inflammatory reactions and reactive immune cells, oxidise DNA, and induce toxicity. They can be responsible for effects in vivo, including immunotoxicity, cytotoxicity, and carcinogenesis [57]. Quinoneimines reduce the oxygen to reactive oxygen species, acting as prooxidants, and, as electrophiles, they form covalent bonds with tissue nucleophiles.
Important drug molecules that lead to the formation of quinoneimine reactive metabolites include Lumiracoxib (non-steroidal anti-inflammatory), Diclofenac (non-steroidal anti-inflammatory), Paracetamol (antipyretic), Amodiaquine (antimalarial), Gefitinib (kinase inhibitor), and Eriotinib (kinase inhibitor) (Figure 6).
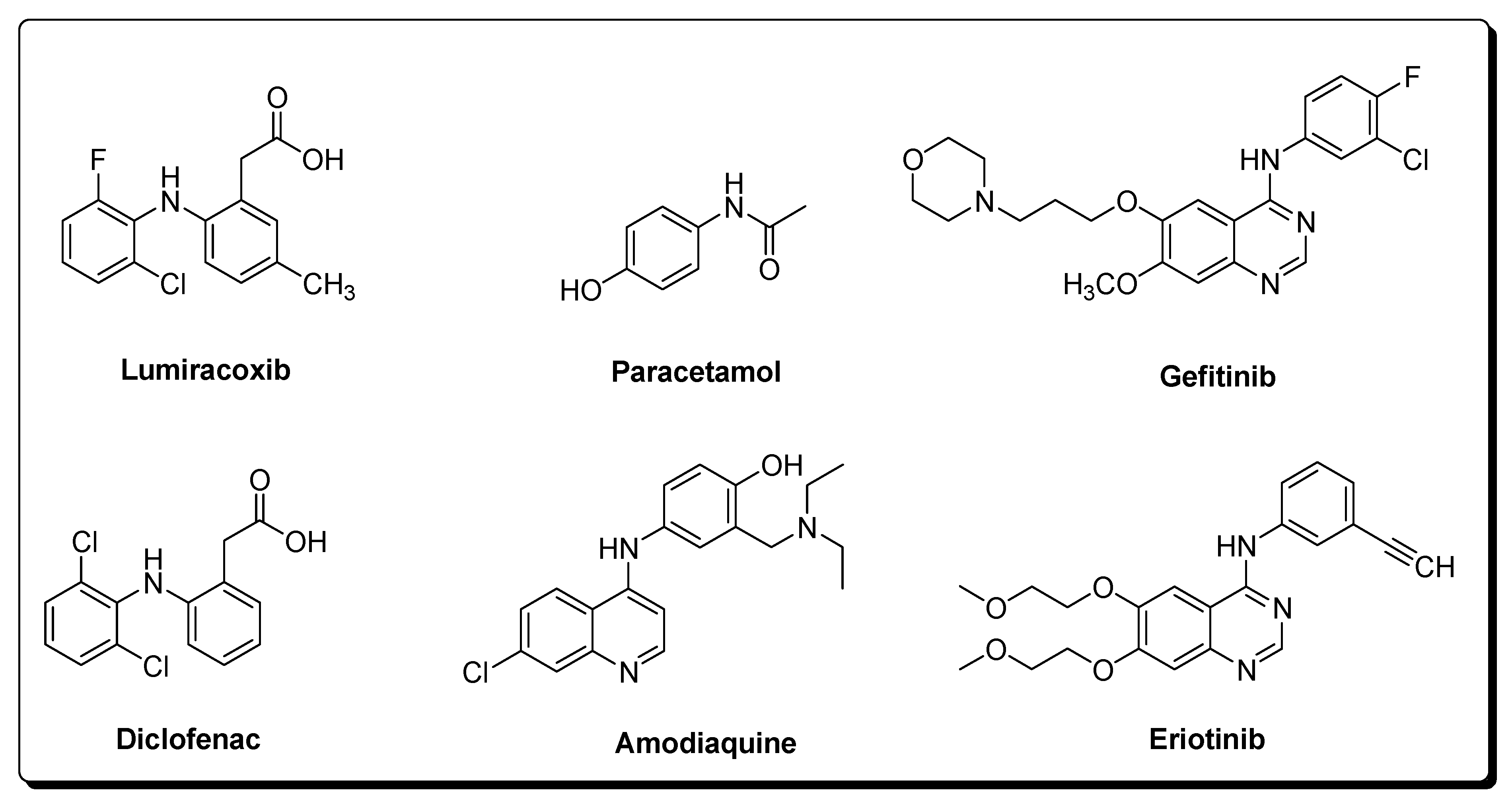
Figure 6. Some drugs that form quinoneimine reactive metabolites [56].
Paracetamol is widely used as an over-the-counter remedy to treat fever and pain. N-acetyl-p-aminophenol (APAP), the active ingredient in Paracetamol, is metabolised via 3 pathways: glucuronidation, sulfation, and glutathione conjugation. Glucuronidation and sulfation produce nontoxic metabolites for excretion. N-acetyl-p-benzoquinoneimine (NAPQI) is a toxic intermediate produced via cytochrome P450 2E1 (CYP 2E1; the main metabolising agent) and cytochrome P450 3A4 (CYP3A4) metabolism. NAPQI is then conjugated by glutathione (GSH) to form a nontoxic metabolite for excretion (Scheme 1) [56].
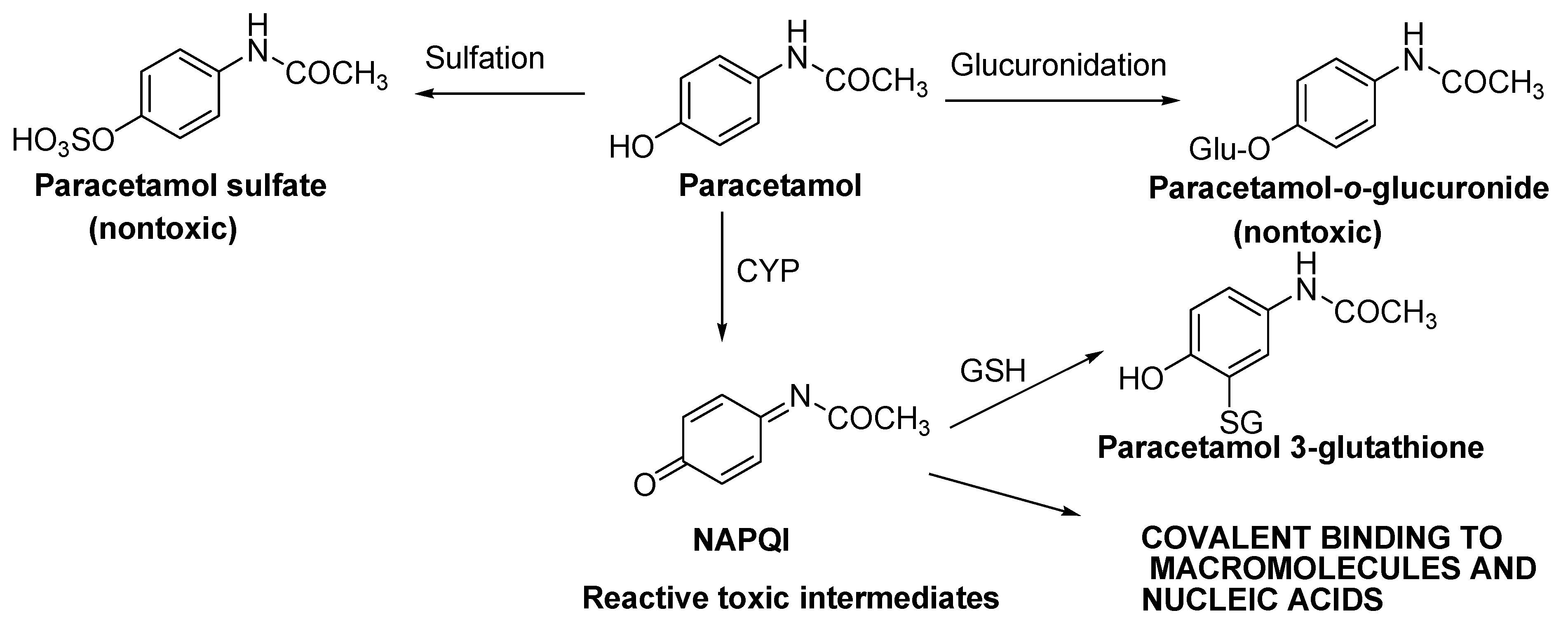
Scheme 1. Paracetamol bioactivation to reactive species (quinoneimine) [56].
6. Synthesis of Quinoneimines
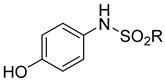
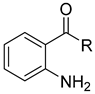
The common method of preparing N-phenylquinoneimines is through the oxidation of the corresponding hydroxydiphenylamine by using various oxidising agents (Table 2).
Table 2. Various oxidising agents used in the preparation of quinoneimines.
| Substrate | Oxidising Agent | Solvent | Temp (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| 6 | HgO | Benzene | Reflux | 1 | 78 | [59] |
| 6 | Ag2CO3 on Celite | Toluene | rt | 30 min | 99 | [60] |
 |
Pb(OAc)4 | Acetic acid | rt | 1 | 58 | [61] |
| 6 | Hypochlorite | Heptane | rt | 1 | 99 | [62] |
| 6 | Hydrogen peroxide | Toluene | 35 | 25 min | 99 | [63] |
| 6 | Activated carbon catalyst | Methanol | 50 | 1 | 90 | [64] |
 |
2-iodoxybenzoic acid | Dimethyl sulfoxide | rt | 35 min | 84–98 | [20] |
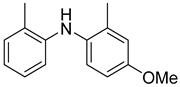 |
Dess–Martin periodinane | Dichloromethane | rt | 8 | 56 | [65] |
| 6 | K2Cr2O7 | Acetone | 40 | 45 min | 97 | [66] |
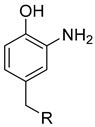 |
K3[Fe(CN)6] | Phosphate buffer | rt | 30 min | n.r. | [67] |
Other oxidising agents used include iodoxybenzene and iodosylbenzene [68][69]. The use of Ag2CO3 on Celite for the oxidation of 4-hydroxydiphenylamine 6 is shown in Scheme 2. The Ag2CO3 adsorbed onto Celite, also known as Fétizon’s reagent, is a solid-supported oxidising agent (and so is easily removed after the reaction); it provides the iminoquinones in an excellent yield and is preferred to other methods [60][70]. The method uses two equivalents of Ag2CO3 and, at room temperature, 99% conversion to product 1 is observed in 24 h.
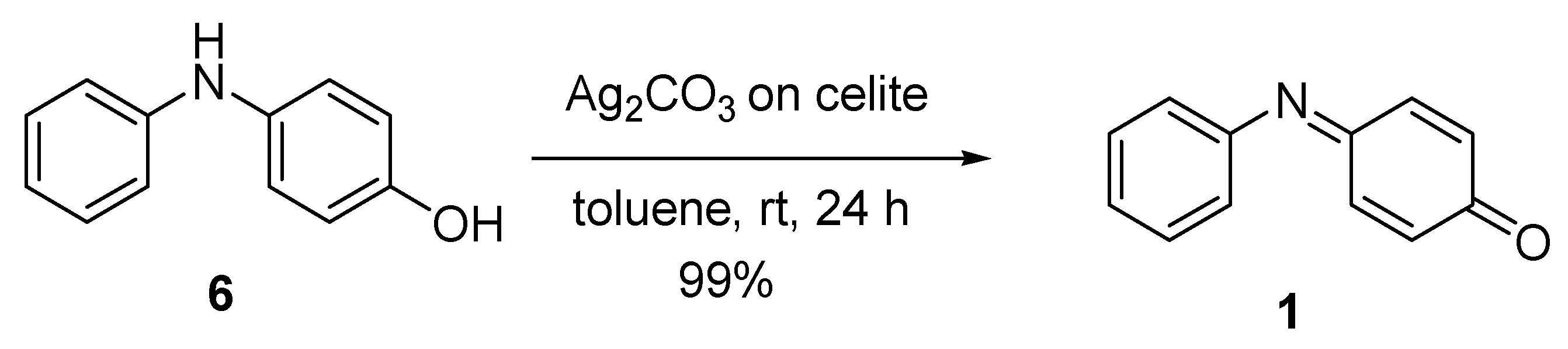
Scheme 2. Preparation of N-phenylquinoneimine 1 [60].
Most of these methods have disadvantages, such as the use of solvents and toxic reagents, a robust play environment, and low efficiency due to polymerisation, hydrolysis, and dimerisation. Electrochemical methods, on the other hand, are known as suitable, moderate, economical, fast, and easy methods that have been used for the synthesis of new quinoneimine derivatives [71].
References
- Sunderhaus, J.D.; Martin, S.F. Applications of multicomponent reactions to the synthesis of diverse heterocyclic scaffolds. Chem. Eur. J. 2009, 15, 1300–1308.
- Burke, M.D.; Schreiber, S.L. A Planning Strategy for Diversity-Oriented Synthesis. Angew. Chem. Int. Ed. 2004, 43, 46–58.
- Spring, D.R. Diversity-oriented synthesis; a challenge for synthetic chemists. Org. Biomol. Chem. 2003, 1, 3867–3870.
- Garcia-Castro, M.; Zimmermann, S.; Sankar, M.G.; Kumar, K. Scaffold Diversity Synthesis and Its Application in Probe and Drug Discovery. Angew. Chem. Int. Ed. 2016, 55, 7586–7605.
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89.
- Adachi, M.; Murata, Y.; Nakamura, S. The Relationship between the Structures and Absorption Spectra of Cyan Color Indoaniline Dyes. J. Org. Chem. 1993, 58, 5238–5244.
- Tao, W.; Barra, M. Inhibition of quinone-imine dye deamination by complexation with para-sulfonated calixarenes. J. Org. Chem. 2001, 66, 2158–2160.
- Barra, M.; Tan, A.; Wong, S. Hydroxide-catalysed decomposition of benzoquinone-imine dyes. Dye. Pigment. 2004, 61, 63–67.
- Imai, S.; Noguchi, T.; Seto, H. Studies on cell growth stimulating substances of low molecular weight: Part 2. Exfoliazone and lavanducyanin, potent growth-promoting substances of rat liver cell line, RLN-8, produced by streptomyces exfoliatus and streptomyces aeriouvifer. J. Antibiot. 1993, 46, 1232–1238.
- Imai, S.; Shimazu, A.; Furihata, K.; Furihata, K.; Hayakawa, Y.; Seto, H. Isolation and Structure of a New Phenoxazine Antibiotic, Exfoliazone, Produced by Streptomyces Exfoliatus. J. Antibiot. 1990, 43, 1606–1607.
- Bolognese, A.; Correale, G.; Manfra, M.; Lavecchia, A.; Mazzoni, O.; Novellino, E.; Barone, V.; Pani, A.; Tramontano, E.; La Colla, P.; et al. Antitumor agents. 1. Synthesis, biological evaluation, and molecular modeling of 5H-pyridophenoxazin-5-one, a compound with potent antiproliferative activity. J. Med. Chem. 2002, 45, 5205–5216.
- Cortés-Salazar, F.; Beggah, S.; van der Meer, J.R.; Girault, H.H. Electrochemical As(III) whole-cell based biochip sensor. Biosens. Bioelectron. 2013, 47, 237–242.
- Bhattacharya, S.; Boone, S.R.; Pierpont, C.G. Stereoselective oxidation of a coordinated phenoxazinylate radical with molecular oxygen. J. Am. Chem. Soc. 1990, 112, 4561–4562.
- Bhattacharya, S.; Pierpont, C.G. Semiquinone Imine Complexes of Ruthenium. Coordination and Oxidation of the 1-Hydroxy-2, 4, 6, 8-Tetra-tert-Butylphenoxazinyl Radical. Inorg. Chem. 1992, 31, 2020–2029.
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Del. Rev. 2008, 60, 1307–1315.
- Stephanopoulos, N.; Carrico, Z.M.; Francis, M.B. Nanoscale integration of sensitizing chromophores and porphyrins with bacteriophage MS2. Angew. Chem. Int. Ed. 2009, 48, 9498–9502.
- Yadav, A.; Mathur, P. p-Quinoneimine as an intermediate in the oxidative coupling of 2-amino-5-methylphenol to 4a,7-dimethyldihydro-2-aminophenoxazinone catalyzed by a monomeric copper(II) complex. Catal. Commun. 2014, 55, 1–5.
- Kuznetsov, V.; Gorohovsky, S.; Levy, A.; Meir, S.; Shkoulev, V.; Menashe, N.; Greenwald, M.; Aizikovich, A.; Ofer, D.; Byk, G.; et al. Approaches for introducing high molecular diversity in scaffolds: Fast parallel synthesis of highly substituted 1H-quinolin-4-one libraries. Mol. Divers. 2004, 8, 437–448.
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur containing scaffolds in drugs: Synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216.
- Chandrasekar, S.; Sekar, G. An efficient synthesis of iminoquinones by a chemoselective domino ortho-hydroxylation/oxidation/imidation sequence of 2-aminoaryl ketones. Org. Biomol. Chem. 2016, 14, 3053–3060.
- Adesina, A.D. Molecular Diversity from N-phenylquinoneimine. Ph.D. Thesis, Newcastle University, Newcastle upon Tyne, UK, 2020. Available online: https://theses.ncl.ac.uk/jspui/handle/10443/5040 (accessed on 17 December 2020).
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614.
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129.
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661.
- Waltenberger, B.; Mocan, A.; Šmejkal, K.; Heiss, E.H.; Atanasov, A.G. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules 2016, 21, 807.
- Tintore, M.; Vidal-Jordana, A.; Sastre-Garriga, J. Treatment of multiple sclerosis—Success from bench to bedside. Nat. Rev. Neurol. 2019, 15, 53–58.
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216.
- Vittum, P.W.; Brown, G.H. Indoaniline dyes. I. Some phenol blue derivatives with substituents in the phenol ring. J. Am. Chem. Soc. 1946, 68, 2235–2239.
- Josephy, P.D.; Mason, R.P.; Eling, T. Chemical structure of the adducts formed by the oxidation of benzidine in the presence of phenols. Carcinogenesis 1982, 3, 1227–1230.
- Ren, J.; Liu, D.; Tian, L.; Wei, Y.; Proksch, P.; Zeng, J.; Lin, W. Venezuelines A–G, new phenoxazine-based alkaloids and aminophenols from Streptomyces venezuelae and the regulation of gene target Nur77. Bioorg. Med. Chem. Lett. 2013, 23, 301–304.
- Maskey, R.P.; Li, F.C.; Qin, S.; Fiebig, H.H.; Laatsch, H. Chandrananimycins A-C: Production of novel anticancer antibiotics from a marine Actinomadura sp. isolate M048 by variation of medium composition and growth conditions. J. Antibiot. 2003, 56, 622–629.
- Gomes, P.B.; Nett, M.; Dahse, H.M.; Sattler, I.; Martin, K.; Hertweck, C. Bezerramycins A–C, antiproliferative phenoxazinones from Streptomyces griseus featuring carboxy, carboxamide or nitrile substituents. Wiley Online Libr. 2010, 2010, 231–235.
- Gomes, P.B.; Nett, M.; Dahse, H.-M.; Hertweck, C. Pitucamycin: Structural merger of a phenoxazinone with an epoxyquinone antibiotic. J. Nat. Prod. 2010, 73, 1461–1464.
- Gripenberg, J. Fungus pigments VIII: The structure of cinnabarin and cinnabarinic acid. Acta Chem. Scand. 1958, 12, 603–610.
- Stepanov, B.I. Vvedenie v khimiiu I tekhnologiiu organicheskikh krasitelei. In The Great Soviet Encyclopedia, 3rd ed.; Prokhorov, A.M., Ed.; Soviet Encyclopedia: Moscow, Russia, 1971.
- Supriatin, Y.; Sumirat, V.A.; Herdiani, M. Growth Analysis of Escherichia coli and Salmonella typhi on MacConkey Agar Modification. J. Phys. Conf. Ser. 2021, 1764, 012207.
- Wichmann, C.F.; Liesch, J.M.; Schwartz, R.E. L-671,329, A New Antifungal Agent II. Structure Determination. J. Antibiot. 1989, 42, 168–173.
- Jose, J.; Burgess, K. Benzophenoxazine-based fluorescent dyes for labeling biomolecules. Tetrahedron 2006, 62, 11021–11037.
- Kerman, K.; Oezkan, D.; Kara, P.; Karadeniz, H.; Oezkan, Z.; Erdem, A.; Jelen, F.; Oezsoez, M. Electrochemical detection of specific DNA sequences from PCR amplicons on carbon and mercury electrodes using Meldola’s blue as an intercalator. Turk. J. Chem. 2004, 28, 523–533. Available online: https://www.acarindex.com/turkish-journal-of-chemistry/electrochemical-detection-of-specific-dna-sequences-from-pcr-amplicons-on-carbon-and-mercury-electrodes-using-meldola39s-blue-as-an-intercalator-783586 (accessed on 25 January 2022).
- Reut, V.E.; Kozlov, S.O.; Kudryavtsev, I.V.; Grudinina, N.A.; Kostevich, V.A.; Gorbunov, N.P.; Grigorieva, D.V.; Kalvinkovskaya, J.A.; Bushuk, S.B.; Varfolomeeva, E.Y.; et al. New Application of the Commercially Available Dye Celestine Blue B as a Sensitive and Selective Fluorescent “Turn-On” Probe for Endogenous Detection of HOCl and Reactive Halogenated Species. Antioxidants 2022, 11, 1719–1733.
- Swenton, J.S.; Shih, C.; Chen, C.P.; Chou, C.T. Preparation of quinone imine ketals via intramolecular condensation of amino-substituted quinone monoketals. Anodic oxidation chemistry of trifluoroacetamide derivatives of 1,4-dimethoxybenzenes and 4-methoxyphenols. J. Org. Chem. 1990, 55, 2019–2026.
- Aubart, K.M.; Heathcock, C.H. A Biomimetic Approach to the Discorhabdin Alkaloids: Total Syntheses of Discorhabdins C and E and Dethiadiscorhabdin D. J. Org. Chem. 1999, 64, 16–22.
- Koutentis, P.A.; Loizou, G.; Lo Re, D. Synthesis of Triazafluoranthenones via Silver(I)-Mediated Nonoxidative and Oxidative Intramolecular Palladium-Catalyzed Cyclizations. J. Org. Chem. 2011, 76, 5793–5802.
- Inoue, K.; Ishikawa, Y.; Nishiyama, S. Synthesis of tetrahydropyrroloiminoquinone alkaloids based on electrochemically generated hypervalent iodine oxidative cyclization. Org. Lett. 2010, 12, 436–439.
- Nair, V.; Rajesh, C.; Dhanya, R.; Rath, N.P. Lewis-acid promoted annulation of p-quinoneimines by allylsilanes: A facile entry into benzofused heterocycles. Org. Lett. 2002, 4, 953–955.
- Eggert, C. Laccase-catalyzed formation of cinnabarinic acid is responsible for antibacterial activity of Pycnoporus cinnabarinus. Microbiol. Res. 1997, 152, 315–318.
- Kinjo, J.; Yokomizo, K.; Awata, Y.; Shibata, M.; Nohara, T.; Teramine, T.; Takahashi, K. Structures of phytotoxins, AV-toxins C, D and E, produced by zonate leaf spot fungus of mulberry. Tetrahedron Lett. 1987, 28, 3697–3698.
- Marsh, J.P., Jr.; Goodman, L. Synthesis of possible actinomycin D precursors. Can. J. Chem. 1966, 44, 799–806.
- Almeida, R.G.; Valença, W.O.; Rosa, L.G.; de Simone, C.A.; de Castro, S.L.; Barbosa, J.M.C.; Pinheiro, D.P.; Paier, C.R.K.; de Carvalho, G.G.C.; Pessoa, C.; et al. Synthesis of quinone imine and sulphur-containing compounds with antitumor and trypanocidal activities: Redox and biological implications. RSC Med. Chem. 2020, 11, 1145–1160.
- Lackner, H.; Bahner, I.; Shigematsu, N.; Pannell, L.K.; Mauger, A.B. Structures of five components of the actinomycin Z complex from Streptomyces fradiae, two of which contain 4-chlorothreonine. J. Nat. Prod. 2000, 63, 352–356.
- Westley, J.W.; Liu, C.M.; Blount, J.F.; Todaro, L.; Sello, L.H.; Troupe, N. Isolation and characterization of three novel polyether antibiotics and three novel actinomycins as cometabolites of the same Streptomyces sp. X-14873, ATCC 31679. J. Antibiot. 1986, 39, 1704–1711.
- Green, D.M. Wilm’s tumour. Eur. J. Cancer 1997, 33, 409–418.
- Womer, R.B. Soft tissue sarcomas. Eur. J. Cancer 1997, 33, 2230–2234.
- Guo, J.; Wu, T.; Bess, J.; Henderson, L.E.; Levin, J.G. Actinomycin D Inhibits Human Immunodeficiency Virus Type 1 Minus-Strand Transfer in In Vitro and Endogenous Reverse Transcriptase Assays. J. Virol. 1998, 72, 6716–6724.
- Dactinomycin. The American Society of Health-System Pharmacists. Available online: https://www.drugs.com/monograph/dactinomycin.html (accessed on 1 April 2007).
- Klopčič, I.; Dolenc, M.S. Chemicals and Drugs Forming Reactive Quinone and Quinone Imine Metabolites. Chem. Res. Toxicol. 2019, 32, 1–34.
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160.
- Porubek, D.; Rundgren, M.; Larsson, R.; Albano, E.; Ross, D.; Nelson, S.D.; Moldéus, P. Quinone Imines as Biological Reactive Intermediates. In Biological Reactive Intermediates III: Mechanisms of Action in Animal Models and Human Disease; Kocsis, J.J., Jollow, D.J., Witmer, C.M., Nelson, J.O., Snyder, R., Eds.; Springer: Boston, MA, USA, 1986; pp. 631–644.
- Burmistrov, K.S.; Burmistrov, S.I. Redox potentials of N-arylquinonimines. Ukr. Khimicheskii Zhurnal 1978, 44, 832–835.
- Baragona, F.; Lomberget, T.; Duchamp, C.; Henriques, N.; Lo Piccolo, E.; Diana, P.; Montalbano, A.; Barret, R. Synthesis of 5-substituted 2,3-dihydrobenzofurans in a one-pot oxidation/cyclization reaction. Tetrahedron 2011, 67, 8731–8739.
- Adams, R.; Looker, J.H. Quinone Imides. IV. p-Quinone Monosulfonimides. J. Am. Chem. Soc. 1951, 73, 1145–1149.
- Fields, D.L., Jr.; Lodaya, J.S. Preparation of Quinoneimines from Hydroxyphenylamines Using Hypochlorite as Oxidizing Agent. WO9952860A1, 21 October 1999.
- Fields, D.L., Jr.; Lohr, R. Preparation of Quinoneimines from Hydroxyphenylamines Using Hydrogen Peroxide and a Catalyst. WO9952859A1, 21 October 1999.
- Fields, D.L., Jr.; Stern, M.K.; Lodaya, J.S. Preparation of N-Phenylbenzoquinoneimines via Oxidation of Hydroxydiphenylamines Using a Modified Activated Carbon Catalyst. WO9936395A2, 22 July 1999.
- Ma, H.; Wu, S.; Sun, Q.; Li, H.; Chen, Y.; Zhao, W.; Ma, B.; Guo, Q.; Lei, Z.; Yan, J. A new method for the synthesis of iminoquinones via DMP-mediated oxidative reaction. Synthesis 2010, 19, 3295–3300.
- Cottman, K.S. Preparation of a N-Substituted Phenylenediamine. U.S. Patent 4968843A, 6 November 1990.
- Capehart, S.L.; ElSohly, A.M.; Obermeyer, A.C.; Francis, M.B. Bioconjugation of Gold Nanoparticles through the Oxidative Coupling of ortho-Aminophenols and Anilines. Bioconjugate Chem. 2014, 25, 1888–1892.
- Barret, R.; Daudon, M. Synthesis of Quinone-Imines with Iodoxybenzene. Synth. Commun. 1990, 20, 1543–1549.
- Barret, R.; Daudon, M. Preparation of quinone-imide ketals from amides with hypervalent organo-iodine compounds. Tetrahedron Lett. 1991, 32, 2133–2134.
- Balogh, V.; Fetizon, M.; Golfier, M. Oxidations with Silver Carbonate/Celite. V. Oxidations of Phenols and Related Compounds. J. Org. Chem. 1971, 36, 1339–1341.
- Mehrdadian, M.; Khazalpour, S.; Amani, A. Electrochemical synthesis of new quinone-imines with assisted of 4-ethynylaniline and para-toluidine as nucleophile. Electrochim. Acta 2022, 427, 140849.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
10 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




