
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Amer Chabili | -- | 3036 | 2024-01-09 11:16:10 | | | |
| 2 | Sirius Huang | Meta information modification | 3036 | 2024-01-10 02:18:00 | | |
Video Upload Options
Significant progress has been achieved in the use of biostimulants in sustainable agricultural practices. These new products can improve plant growth, nutrient uptake, crop yield and quality, stress adaptation and soil fertility, while reducing agriculture’s environmental footprint. Although it is an emerging market, the biostimulant sector is very promising, hence the increasing attention of the scientific community and agro-industry stakeholders in finding new sources of plant biostimulants. Pro- and eucaryotic microalgae have gained prominence and can be exploited as biostimulants due to their ability to produce high-value-added metabolites. Several works revealed the potential of microalgae- and cyanobacteria-based biostimulants (MCBs) as plant growth promoters and stress alleviators, as well as encouraging results pointing out that their use can address current and future agricultural challenges.
1. Introduction
2. Microalgae-Based Biostimulants: From Phototrophic Microorganisms to Biostimulants
2.1. Selection of Microalgae Strains with High Biostimulant Potential
2.2. Microalgae Cultivation and Biomass Production
| Green Microalgae Strains | Biomass Growth Medium | Application Methods/Crop Plants or Seeds | Biostimulant Effects | Reference |
|---|---|---|---|---|
| Scenedesmus obliquus | Pretreated brewery wastewater | Centrifuged, ultrasonicated, and enzyme hydrolyzed biomass applied on watercress seeds (Lepidium sativum), mung bean (Vigna radiata), and cucumber (Cucumis sativus L.) |
|
[30] |
| Tetradesmus obliquus; Chlorella protothecoides | Piggery wastewater | Fresh microalgal biomass applied on cucumber (Cucumis sativus), barley (Hordeum vulgare), wheat (Triticum aestivum), soybean (Glycine max), watercress (Nasturium officinale), and tomato (Lycopersicon esculentum) |
|
[31] |
| Chlorella vulgaris UAL-1; Chlorella sp. UAL-2; Chlorella vulgaris UAL-3; Chlamydopodium fusiforme UAL-4 |
Secondary-treated urban wastewater supplemented with concentrate |
Aqueous extracts applied on watercress (Lepidium sativum L.), soybean (Glycine max L.), cucumber (Cucumis sativus L.), and wheat (Triticum aestivum L.) seeds |
|
[32] |
| Chlorella vulgaris (13–1); Scenedesmus obliquus (B2-2) |
Untreated municipal wastewater |
Algal biomass (intact and broken cells) and culture supernatant applied on tomato (Solanum lycopersicum) and barely (Hordeum vulgare) seeds |
|
[33] |
2.3. Extraction and Application Methods of Microalgae- and Cyanobacteria-Based Biostimulants
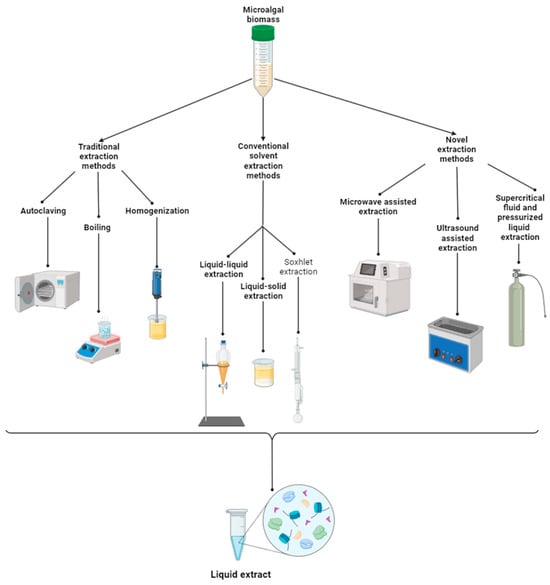
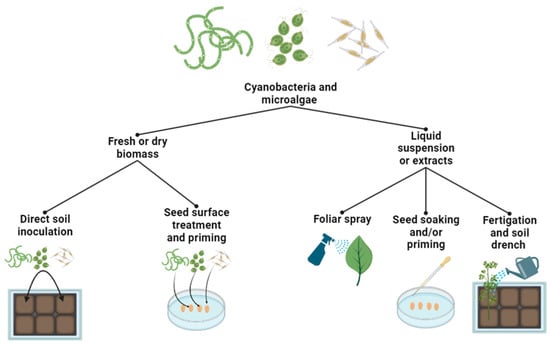
2.4. Mechanisms and Modes of Action of Microalgae- and Cyanobacteria-Based Biostimulants
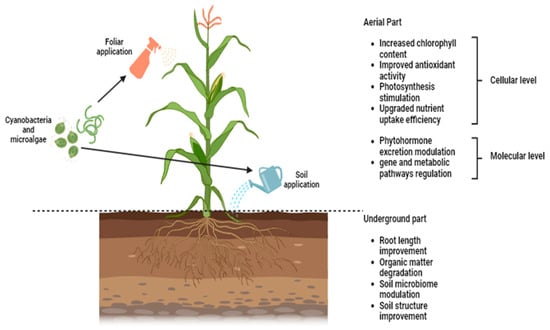
References
- FAO. The future of food and agriculture—Alternative pathways to 2050|Global Perspectives Studies|Food and Agriculture Organization of the United Nations. In Food and Agriculture Organization; FAO: Rome, Italy, 2018; p. 224. ISBN 978-92-5-130158-6.
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2014, 31, 1–17.
- González-Pérez, B.K.; Rivas-Castillo, A.M.; Valdez-Calderón, A.; Gayosso-Morales, M.A. Microalgae as biostimulants: A new approach in agriculture. World J. Microbiol. Biotechnol. 2021, 38, 4.
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Microbiota and Biofertilizers, Ecofriendly Tools for Reclamation of Degraded Soil Environs; Springer: Berlin/Heidelberg, Germany, 2022; Volume 2, ISBN 9783030610098.
- Calabi-Floody, M.; Medina, J.; Rumpel, C.; Condron, L.M.; Hernandez, M.; Dumont, M.; Mora, M.d.L. Smart Fertilizers as a Strategy for Sustainable Agriculture, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 147.
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Soil microalgae and cyanobacteria: The biotechnological potential in the maintenance of soil fertility and health. Crit. Rev. Biotechnol. 2019, 39, 981–998.
- Bomfim, C.A.; Coelho, L.G.F.; do Vale, H.M.M.; de Carvalho Mendes, I.; Megías, M.; Ollero, F.J.; dos Reis Junior, F.B. Brief history of biofertilizers in Brazil: From conventional approaches to new biotechnological solutions. Braz. J. Microbiol. 2021, 52, 2215–2232.
- Yousfi, S.; Marín, J.; Parra, L.; Lloret, J.; Mauri, P.V. A Rhizogenic Biostimulant Effect on Soil Fertility and Roots Growth of Turfgrass. Agronomy 2021, 11, 573.
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a Biostimulant on the Heat Tolerance Associated with Photosynthetic Capacity, Membrane Thermostability, and Polyphenol Production of Perennial Ryegrass. Crop Sci. 2007, 47, 261–267.
- European Commission. Regulation of the European Parliament and of the Council Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, 2019, 114.
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14.
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049.
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192.
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213.
- Du Jardin, P.; Xu, L.; Geelen, D. Agricultural Functions and Action Mechanisms of Plant Biostimulants (PBs): An Introduction. Chem. Biol. Plant Biostimul. 2020, 1, 3–30.
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front. Plant Sci. 2018, 871, 01782.
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5.
- Kapoore, R.V.; Wood, E.E.; Llewellyn, C.A. Algae biostimulants: A critical look at microalgal biostimulants for sustainable agricultural practices. Biotechnol. Adv. 2021, 49, 107754.
- Cadoret, J.-P.; Bernard, O. La Production de Biocarburant Lipidique Avec Des Microalgues: Promesses et Défis. J. Soc. Biol. 2008, 202, 201–211.
- Issa, A.A.; Hemida Abd-Alla, M.; Ohyam, T. Nitrogen Fixing Cyanobacteria: Future Prospect. Adv. Biol. Ecol. Nitrogen Fixat. 2014, 1, 24–48.
- Sankaran, R.; Show, P.L.; Nagarajan, D.; Chang, J.S. Exploitation and Biorefinery of Microalgae; Elsevier B.V.: Amsterdam, The Netherlands, 2018; ISBN 9780444639929.
- Singh, S. A review on possible elicitor molecules of cyanobacteria: Their role in improving plant growth and providing tolerance against biotic or abiotic stress. J. Appl. Microbiol. 2014, 117, 1221–1244.
- Górka, B.; Korzeniowska, K.; Lipok, J.; Wieczorek, P.P. The Biomass of Algae and Algal Extracts in Agricultural Production. Algae Biomass Charact. Appl. 2018, 8, 103–114.
- Márquez-Rocha, F.J.; Palma-Ramírez, D.; García-Alamilla, P.; López-Hernández, J.F.; Santiago-Morales, I.S.; Flores-Vela, A.I. Microalgae Cultivation for Secondary Metabolite Production. Microalgae Physiol. Appl. 2020, 11, 193–204.
- Vuppaladadiyam, A.K.; Prinsen, P.; Raheem, A.; Luque, R.; Zhao, M. Microalgae cultivation and metabolites production: A comprehensive review. Biofuels Bioprod. Biorefin. 2018, 8, 743.
- Liang, Y.; Kashdan, T.; Sterner, C.; Dombrowski, L.; Petrick, I.; Kröger, M.; Höfer, R. Algal Biorefineries; Elsevier B.V.: Amsterdam, The Netherlands, 2015; ISBN 9780444634535.
- Branco-Vieira, M.; Mata, T.; Martins, A.; Freitas, M.; Caetano, N. Economic analysis of microalgae biodiesel production in a small-scale facility. Energy Rep. 2020, 6, 325–332.
- Ummalyma, S.B.; Sahoo, D.; Pandey, A. Microalgal Biorefineries for Industrial Products. In Microalgae Cultivation for Biofuels Production; Academic Press: Cambridge, MA, USA, 2020; pp. 187–195.
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of Wastewaters by Microalgae and the Potential Applications of the Produced Biomass—A Review. Water 2020, 13, 27.
- Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Acién, F.G.; Gouveia, L. Biostimulant Potential of Scenedesmus obliquus Grown in Brewery Wastewater. Molecules 2020, 25, 664.
- Ferreira, A.; Melkonyan, L.; Carapinha, S.; Ribeiro, B.; Figueiredo, D.; Avetisova, G.; Gouveia, L. Biostimulant and biopesticide potential of microalgae growing in piggery wastewater. Environ. Adv. 2021, 4, 100062.
- Morillas-España, A.; Lafarga, T.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; González-López, C. Microalgae based wastewater treatment coupled to the production of high value agricultural products: Current needs and challenges. Chemosphere 2022, 291, 132968.
- Alling, T.; Funk, C.; Gentili, F.G. Nordic microalgae produce biostimulant for the germination of tomato and barley seeds. Sci. Rep. 2023, 13, 3509.
- Nwuche, C.O.; Ekpo, D.C.; Eze, C.N.; Aoyagi, H.; Ogbonna, J.C. Use of Palm Oil Mill Effluent as Medium for Cultivation of Chlorella sorokiniana. Br. Biotechnol. J. 2014, 4, 305–316.
- Selmani, N.; Mirghani, M.E.S.; Alam, Z. Study the Growth of Microalgae in Palm Oil Mill Effluent Waste Water. IOP Conf. Ser. Earth Environ. Sci. 2013, 16, 12–16.
- Priya, H.; Prasanna, R.; Ramakrishnan, B.; Bidyarani, N.; Babu, S.; Thapa, S.; Renuka, N. Influence of cyanobacterial inoculation on the culturable microbiome and growth of rice. Microbiol. Res. 2015, 171, 78–89.
- Ferreira, A.; Figueiredo, D.; Ferreira, F.; Marujo, A.; Bastos, C.R.; Martin-Atanes, G.; Ribeiro, B.; Štěrbová, K.; Marques-Dos-Santos, C.; Acién, F.G.; et al. From piggery wastewater to wheat using microalgae towards zero waste. Algal Res. 2023, 72.
- Álvarez-González, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Casas, M.E.; Matamoros, V.; Gonzalez-Flo, E.; Díez-Montero, R. The potential of wastewater grown microalgae for agricultural purposes: Contaminants of emerging concern, heavy metals and pathogens assessment. Environ. Pollut. 2023, 324, 121399.
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20.
- Li, P.; Xu, G.; Li, S.-P.; Wang, Y.-T.; Fan, T.-P.; Zhao, Q.-S.; Zhang, Q.-W. Optimizing Ultraperformance Liquid Chromatographic Analysis of 10 Diterpenoid Compounds in Salvia miltiorrhiza Using Central Composite Design. J. Agric. Food Chem. 2008, 56, 1164–1171.
- Du, G.; Zhao, H.; Song, Y.; Zhang, Q.; Wang, Y. Rapid simultaneous determination of isoflavones in Radix puerariae using high-performance liquid chromatography-triple quadrupole mass spectrometry with novel shell-type column. J. Sep. Sci. 2011, 34, 2576–2585.
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591.
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176.
- Kim, G.M.; Kim, Y.K. Drying Techniques of Microalgal Biomass: A Review. Appl. Chem. Eng. 2022, 33, 145–150.
- Wiltshire, K.H.; Boersma, M.; Möller, A.; Buhtz, H. Extraction of pigments and fatty acids from the green alga Scenedesmus obliquus (Chlorophyceae). Aquat. Ecol. 2000, 34, 119–126.
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; Ahn, C.Y.; Oh, H.M. Comparison of Several Methods for Effective Lipid Extraction from Micro-algae. Bioresour. Technol. 2010, 101, S75–S77.
- Navarro-López, E.; Gallardo-Rodríguez, J.J.; Cerón-García, M.d.C.; Gallego-López, I.; Acién-Fernández, F.G.; Molina-Grima, E. Extraction of phytostimulant molecules from Scenedesmus almeriensis using different extractor systems. J. Appl. Phycol. 2023, 35, 701–711.
- Gitau, M.M.; Farkas, A.; Balla, B.; Ördög, V.; Futó, Z.; Maróti, G. Strain-Specific Biostimulant Effects of Chlorella and Chlamydomonas Green Microalgae on Medicago truncatula. Plants 2021, 10, 1060.
- Bayona-Morcillo, P.J.; Plaza, B.M.; Gómez-Serrano, C.; Rojas, E.; Jiménez-Becker, S. Effect of the foliar application of cyanobacterial hydrolysate (Arthrospira platensis) on the growth of Petunia x hybrida under salinity conditions. J. Appl. Phycol. 2020, 32, 4003–4011.
- Refaay, D.A.; El-Marzoki, E.M.; Abdel-Hamid, M.I.; Haroun, S.A. Effect of foliar application with Chlorella vulgaris, Tetradesmus dimorphus, and Arthrospira platensis as biostimulants for common bean. J. Appl. Phycol. 2021, 33, 3807–3815.
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of microalgal extracts as biostimulants through seed treatment and foliar spray for tomato cultivation. Ind. Crop. Prod. 2020, 151, 112453.
- La Bella, E.; Baglieri, A.; Rovetto, E.I.; Stevanato, P.; Puglisi, I. Foliar Spray Application of Chlorella vulgaris Extract: Effect on the Growth of Lettuce Seedlings. Agronomy 2021, 11, 308.
- Puglisi, I.; Barone, V.; Fragalà, F.; Stevanato, P.; Baglieri, A.; Vitale, A. Effect of Microalgal Extracts from Chlorella vulgaris and Scenedesmus quadricauda on Germination of Beta vulgaris Seeds. Plants 2020, 9, 675.
- Rupawalla, Z.; Shaw, L.; Ross, I.L.; Schmidt, S.; Hankamer, B.; Wolf, J. Germination screen for microalgae-generated plant growth biostimulants. Algal Res. 2022, 66, 102784.
- Jafarlou, M.B.; Pilehvar, B.; Modarresi, M.; Mohammadi, M. Performance of Algae Extracts Priming for Enhancing Seed Germination Indices and Salt Tolerance in Calotropis procera (Aiton) W.T. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 493–502.
- Van Do, T.C.; Tran, D.T.; Le, T.G.; Nguyen, Q.T. Characterization of Endogenous Auxins and Gibberellins Produced by Chlorella sorokiniana TH01 under Phototrophic and Mixtrophic Cultivation Modes toward Applications in Microalgal Biorefinery and Crop Research. J. Chem. 2020, 2020, 4910621.
- Suchithra, M.; Muniswami, D.M.; Sri, M.S.; Usha, R.; Rasheeq, A.A.; Preethi, B.A.; Dineshkumar, R. Effectiveness of green microalgae as biostimulants and biofertilizer through foliar spray and soil drench method for tomato cultivation. S. Afr. J. Bot. 2021, 146, 740–750.
- El-Eslamboly, A.A.S.A.; El-Wanis, M.M.A.; Amin, A.W. Algal application as a biological control method of root-knot nematode Meloidogyne incognita on cucumber under protected culture conditions and its impact on yield and fruit quality. Egypt. J. Biol. Pest Control. 2019, 29, 18.
- Chanda, M.; Benhima, R.; Elmernissi, N.; Kasmi, Y.; Karim, L.; Sbabou, L.; Youssef, Z.; ElArroussi, H. Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci. Rep. 2020, 10, 2820.
- Ergun, O.; Dasgan, H.; Isık, O. Effects of microalgae Chlorella vulgaris on hydroponically grown lettuce. Acta Hortic. 2020, 1273, 169–175.
- Dasgan, H.Y.; Kacmaz, S.; Arpaci, B.B.; Ikiz, B.; Gruda, N.S. Biofertilizers Improve the Leaf Quality of Hydroponically Grown Baby Spinach (Spinacia oleracea L.). Agronomy 2023, 13, 575.
- Santini, G.; Rodolfi, L.; Biondi, N.; Sampietro, G.; Tredici, M.R. Effects of cyanobacterial-based biostimulants on plant growth and development: A case study on basil (Ocimum basilicum L.). J. Appl. Phycol. 2022, 34, 2063–2073.
- Stirk, W.A.; Bálint, P.; Vambe, M.; Kulkarni, M.G.; van Staden, J.; Ördög, V. Effect of storage on plant biostimulant and bioactive properties of freeze-dried Chlorella vulgaris biomass. J. Appl. Phycol. 2021, 33, 3797–3806.
- Kumar, S.; Korra, T.; Singh, U.B.; Singh, S.; Bisen, K. Microalgal Based Biostimulants as Alleviator of Biotic and Abiotic Stresses in Crop Plants. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–216.
- Kumari, M.; Swarupa, P.; Kesari, K.K.; Kumar, A. Microbial Inoculants as Plant Biostimulants: A Review on Risk Status. Life 2023, 13, 12.
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162.
- Bhupenchandra, I.; Devi, S.H.; Basumatary, A.; Dutta, S.; Singh, L.K.; Kalita, P.; Bora, S.S.; Saikia, A.; Sharma, P.; Bhagowati, S.; et al. Biostimulants: Potential and Prospects in Agriculture. Int. Res. J. Pure Appl. Chem. 2020, 21, 20–35.
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273.
- Ramakrishnan, B.; Maddela, N.R.; Venkateswarlu, K.; Megharaj, M. Potential of microalgae and cyanobacteria to improve soil health and agricultural productivity: A critical view. Environ. Sci. Adv. 2023, 2, 586–611.
- Tinte, M.M.; Masike, K.; Steenkamp, P.A.; Huyser, J.; van der Hooft, J.J.J.; Tugizimana, F. Computational Metabolomics Tools Reveal Metabolic Reconfigurations Underlying the Effects of Biostimulant Seaweed Extracts on Maize Plants under Drought Stress Conditions. Metabolites 2022, 12, 487.




