Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sylvia Lindberg | -- | 4084 | 2024-01-09 10:03:34 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Lindberg, S.; Premkumar, A. Na+ Uptake and Accumulation under Salt Stress. Encyclopedia. Available online: https://encyclopedia.pub/entry/53593 (accessed on 07 February 2026).
Lindberg S, Premkumar A. Na+ Uptake and Accumulation under Salt Stress. Encyclopedia. Available at: https://encyclopedia.pub/entry/53593. Accessed February 07, 2026.
Lindberg, Sylvia, Albert Premkumar. "Na+ Uptake and Accumulation under Salt Stress" Encyclopedia, https://encyclopedia.pub/entry/53593 (accessed February 07, 2026).
Lindberg, S., & Premkumar, A. (2024, January 09). Na+ Uptake and Accumulation under Salt Stress. In Encyclopedia. https://encyclopedia.pub/entry/53593
Lindberg, Sylvia and Albert Premkumar. "Na+ Uptake and Accumulation under Salt Stress." Encyclopedia. Web. 09 January, 2024.
Copy Citation
High concentrations of sodium (Na+), chloride (Cl−), calcium (Ca2+), and sulphate (SO42−) are frequently found in saline soils. Crop plants cannot successfully develop and produce because salt stress impairs the uptake of Ca2+, potassium (K+), and water into plant cells. Different intracellular and extracellular ionic concentrations change with salinity, including those of Ca2+, K+, and protons. These cations serve as stress signaling molecules in addition to being essential for ionic homeostasis and nutrition. Maintaining an appropriate K+:Na+ ratio is one crucial plant mechanism for salt tolerance, which is a complicated trait.
cereals
chloride
cytosolic Ca2+, K+, Na+, pH
salt stress
1. Introduction
Soil salinity is harmful for plant growth and development as it inhibits the uptake of essential nutrients such as K+ and Ca2+ [1]. Salinity also causes reduced water uptake, changed metabolism, ionic imbalance, and toxicity [2]. In dry and warm areas, coastal areas, and where irrigation with saline water is common, salinity is often a serious problem. Sodium (Na+) and chloride (Cl−) are the most abundant elements in saline soils and cause most harmful effects. The Earth’s crust contains 3% sodium and the seas and oceans contain more than 5% [3]. Saline soils are sometimes alkaline, which also is harmful for plants [4].
Except for halophytes and C4 plants, Na+ is not an essential element for growth or reproduction [5]. On the other hand, chloride is an essential macronutrient for many higher plants, although a high chloride concentration might have a negative impact on plant growth [6][7]. Chloride has been underestimated as a toxic element, and in some plant species it is as harmful as sodium [8]. Most plant species, which are glycophytes, cannot survive in high-salinity soils, but halophytes grow even better under salinity. High salinity causes both osmotic stress and ion toxicity, which in turn also induce oxidative stress [9][10].
2. The Hydraulic Conductivity (Lp) Affects Ion Transport
The water flow through a plant decreases under salinity and affects the ion transport. Lu and Fricke [11] investigated the root hydraulic conductivity (Lp) in wheat under different NaCl concentrations and found that the Lp of cortex cells was differently affected by NaCl concentrations under day and night, and in the main axis of roots and lateral roots. The aquaporin-inhibitor hydrogen peroxide (H2O2) reduced Lp during the night, suggesting that these proteins were important for hydraulic conductivity. The authors suggested that the changes in root Lp in response to salt stress depended on altered activity of aquaporins in root and leaves. However, aquaporins facilitate diffusion of H2O2 through cellular membranes. Therefore, it cannot be excluded that the Lp decrease is a side effect of cell membrane damage by reactive oxygen species (ROS). Aquaporins play key roles in the hydraulic regulation of other types of abiotic stress too. They are intrinsic protein channels mainly in plasma membranes, ER, vacuoles, and plastids, facilitating the diffusion of water and small neutral molecules, and dissolved gases like CO2 and ammonia. Interestingly, aquaporins can be regulated by signaling intermediates, cytosolic Ca2+, pH, and reactive oxygen species (ROS) [12]. Genetically modified aquaporins could be future candidates for improving salt tolerance in plants.
3. Uptake of Na+ and Cl− at the Whole Plant Level
In most plant species, Na+ and Cl− can easily be absorbed by both the main root and lateral roots. The ions are transported through root hairs into the epidermis, cortex, endodermal cells, and into the parenchyma cells, layers of pericycle, and thereafter into the xylem for further passive transport by the transpiration stream in the shoot. Solutes and water can travel by the epidermis and cortex cells into the xylem in three ways: apoplastically by the cell walls and extracellular spaces, symplastically from cell to cell by cytoplasm and plasmodesmata, openings in the cell walls, and by a transmembrane pathway by plasma membranes and cell walls [13]. The endodermal cells form a central ring structure where the radial walls are thickened (Casparin strips) with hydrophobic suberin that prevents apoplastic ion transport. In the symplastic pathway, ions have to pass through one or several membranes.
Kronzucker and Britto [14] investigated which method plants used for the uptake of ions. The reports show that monocots, like wheat and rice, more often take up Na+ and Cl− by the apoplastic route. In rice, 50% of both Na+ and Cl− uptakes in the shoot were apoplastic [14][15]. In Arabidopsis and other dicots, some part of the ion uptake was significantly apoplastic. The solute permeability coefficients in Arabidopsis and rice were rather similar. By the symplastic pathway, the ions have to pass through several different channels or transporter proteins.
4. Ion Uptake across a Membrane
The driving force for the movement of an ion across a membrane depends on two components: one electrical and one chemical, depending on differences in charges and ion activities across the membrane [16].
Since the membrane potential difference across the plasma membrane is approximately −140 mV, a positive ion like Na+ can easily pass into the cytosol even at low concentrations [17].
5. Cellular Uptake of Na+
Na+ is transported into a plant cell by many different channels and transport proteins, such as nonselective cation channels, NSCCs, low-affinity cation transporter, LCT1, cation-chloride cotransporters, CCCs, high affinity-K+ transporters, HAKs, HKT1, HKT2, and the Shaker-type K channel, AKT1.
5.1. Cytosolic Uptake of Na+ in Wheat and Rice by NSCCs, CCCs, HKTs, and AKT
The cytosolic uptake of Na+ in wheat and rice under salinity is mainly mediated by nonselective ion channels, NSCCs, but also by transport proteins [3][14][18]. Monocots like wheat and rice use many different transporters. Davenport and Tester [19] showed that the NSCCs are the primary pathways for Na+ influx into wheat roots, as the influx is inhibited by Ca2+, a mechanism that is considered specific for that type of channel. However, later reports suggested that both K+ and Ca2+ may inhibit Na+ influx by NSCCs and also influx by LCT1 [14].
NSCCS are divided in depolarization-activated, hyperpolarization-activated, and voltage-insensitive channels. In barley, the voltage-insensitive NSCCs show both inward and outward currents [20]. NSCCs can also be characterized and named by their ligands and stimuli as cyclic nucleotide gated channels, CNGCs [21][22], and glutamate-like receptor proteins, GLRs [23].
Except for NSCCs, Na+ uptake can be mediated by cation-chloride cotransporters, CCCs, but other uptake mechanisms have been proposed as well. In wheat and rice, high-affinity K+ transporters, HAKs, HKT1, HKT2, low-affinity cation transporter, LCT1, and the Shaker-type K channel, AKT1, were shown to transport sodium [24][25], with references therein. In wheat, LCT1 was suggested to transport Ca2+ and Cd2+ [26]. In barley, HKTs were showed to improve salt tolerance, as overexpression caused increased tolerance [27]. The HvHKT1;1 in barley showed a higher selectivity of K+/Na+ and a high retention of K+ and Ca2+ in root cells under salinity [28].
More detailed information on Na+ transporters is available [14][23]. It can be concluded that, under salinity, Na+ is mainly transported into cereals by NSCCs, HKTs, and HAKs.
5.2. Cytosolic Na+ Uptake in Arabidopsis
Results from experiments with Arabidopsis, a dicot, suggest that NSCCs, such as CNGCs and GLRs [3][22][29][30] but also HKTs and HAKs, can transport sodium under salt stress [23]. The aquaporin, PIP2;1, was also shown to transport Na+ [31].
6. Long-Distance Translocation of Sodium
6.1. Long-Distance Translocation of Sodium in Rice by HKTs
Many reports show that HKT1 mediates Na+ translocation from roots to shoot in rice under salt stress. Cell-specific expression analysis by in situ PCR revealed that HKT1 was induced in the root epidermis, vascular cylinder, and shoot mesophyll cells of the sensitive rice, indicating transport of Na+ from root to shoot, where it is more damaging [32]. HKT1 induction also occurred in the shoot phloem, in the transition from phloem to mesophyll, and in the mesophyll cells of rice, suggesting a recirculating of K+ in the leaf. Horie et al. [33] showed that HKT1 mediates Na+ influx and transport, but not K+ influx and transport, and that HKT2 might transport both Na+ and K+. In the tolerant rice, an induction of OsHKT2 and OsVHA was obtained in the root epidermis, exodermis, and xylem tissue, which indicates a K+ and Na+ uptake and transport through the root xylem [32]. This cultivar could confer salt tolerance by decreasing the expression of HKT1 and increasing the expression of HKT2 in the shoot, leading to a low Na+/K+ concentration ratio, which is important for salt tolerance and ionic homeostasis [34].
Investigations show that HKT1 does not transport Na+ from root to shoot in all species. AtHKT1 in Arabidopsis can prevent xylem loading and translocation to the shoot [35], but in salt-sensitive rice, Na+ is translocated to the shoot by OsHKT1 [32]. OsHKT1;5 is suggested to recirculate Na+ by the phloem to the root tissue [36][37].
6.2. Long-Distance Translocation of Sodium in Barley by HKTs
Different transport mechanisms of HKT1;5 were demonstrated in the cultivated barley Hordeum vulgare and the halophyte ecotype Hordeum marinum, as the halophyte took up less Na+ into the shoot and higher K+ into the root, compared with Hordeum vulgare when subjected to 400 mM NaCl [38]. Thus, the halophyte maintained a higher K+/Na+ ratio, and also used less energy for salt tolerance than the cultivated ecotype. In several investigations, salt tolerance in the halophyte barley Hordeum maritimum was compared with salt tolerance in Hordeum vulgare.
6.3. Long-Distance Translocation of Sodium in Arabidopsis by HKTs
An investigation in Arabidopsis suggested that the high expression of AtHKT1;1 in the mature part of the Arabidopsis root stele decreased Na+ accumulation into the shoot and increased salt tolerance [39]. Moreover, other research on Arabidopsis indicated that AtHKT1;1 controls the root accumulation of Na+ and retrieves Na+ from xylem but does not mediate root influx or recirculation in the phloem [40].
6.4. The CCCs, Cation-Chloride Cotransporters
Loss-of-function experiments with CCCs showed that this protein can transport K+, Na+, and Cl− in symport [41]. CCCs are suggested to be involved in the long-distance translocation of Na+ [42]. It is uncertain if the CCCs retain Na+ and Cl− at the xylem parenchyma cells, where CCCs are expressed, or translocate these ions into the xylem.
7. Salt Tolerance
7.1. Plants Tolerate Salt Stress by Different Mechanisms
Salinity stress in plants causes osmotic stress, Na+ and Cl+ toxicity, and negative effects on ion homeostasis, as a high salt concentration can decrease the uptake of K+, Ca2+, and NO3 [25][43]. The osmotic stress is prior to the ionic stress and, in barley, more serious for the plant to combat than ionic stress, as it might be connected with the formation of ROS [44]. Osmotic stress depends on the fact that the osmotic potential is more negative outside the roots than inside under a high salt concentration, which makes water uptake more difficult.
Plants have developed several mechanisms to resist salinity [30][43]. The first minutes to days of osmotic stress cause a reduced water uptake and plant growth reduction. Plants can withstand the first phase by a reduction in cell expansion and closing their stomata; but under ionic stress, cytosolic Na+ and Cl− concentrations can be toxic and affect the metabolism to such a degree that after a long time may cause cell death.
As reported for barley, salt tolerance does not only depend on a low uptake of Na+, but also on the plant’s ability to retain K+ and Ca2+ [28][45]. Plant might have a higher selectivity for K+ over Na+, by accumulating a low amount of Na+ in root cells, and increase their K+ concentration at high external Na+, which would lead to K+/Na+ homeostasis [43]. Higher plants can also exclude Na+ and Cl− from the leaves, by preventing xylem loading, recirculate Na+ in the phloem, or transport these ions into the vacuoles to avoid toxic concentrations in the cytosol.
7.2. Halophytes and Glycophytes
Halophytes can survive much higher concentrations of NaCl, 100–200 mM Na+ and Cl−, than glycophytes, and the tissue concentration can be much higher, >500 mM [46]. Halophytes even grow better under high salt. With some exceptions, glycophytes and halophytes might possess similar tolerance mechanisms, but the reaction strength is higher or starts more rapidly in halophytes [46][47]. Halophytes usually transport larger amounts of Na+ and/or K+ into the vacuole than the glycophytes, to use as a cheap osmoticum. Some halophytes have salt glands which can extrude Na+ from the leaf cells [43].
High salt conditions can cause K+ deficiency both in glycophytes and halophytes [48]. One reason for this might be the competition between K+ and Na+ at the uptake sites [49][50], as these ions have the same positive charges. The ionic radius is smaller for K+, 98 pm, than for Na+, 133 pm, but the hydrated Na+ has a larger ionic radius than hydrated K+. The selectivity for the uptake of K+ over Na+ is different in different plants, usually Na+ can inhibit the uptake of K+, but the opposite is less common, at least if the K+ concentration is less than 75 mM [51]. Another reason is that the presence of a high Na+ concentration causes a depolarization of the plasma membrane leading to K+ efflux from the cells by GORK and NSCCS channels [52] (Figure 1).
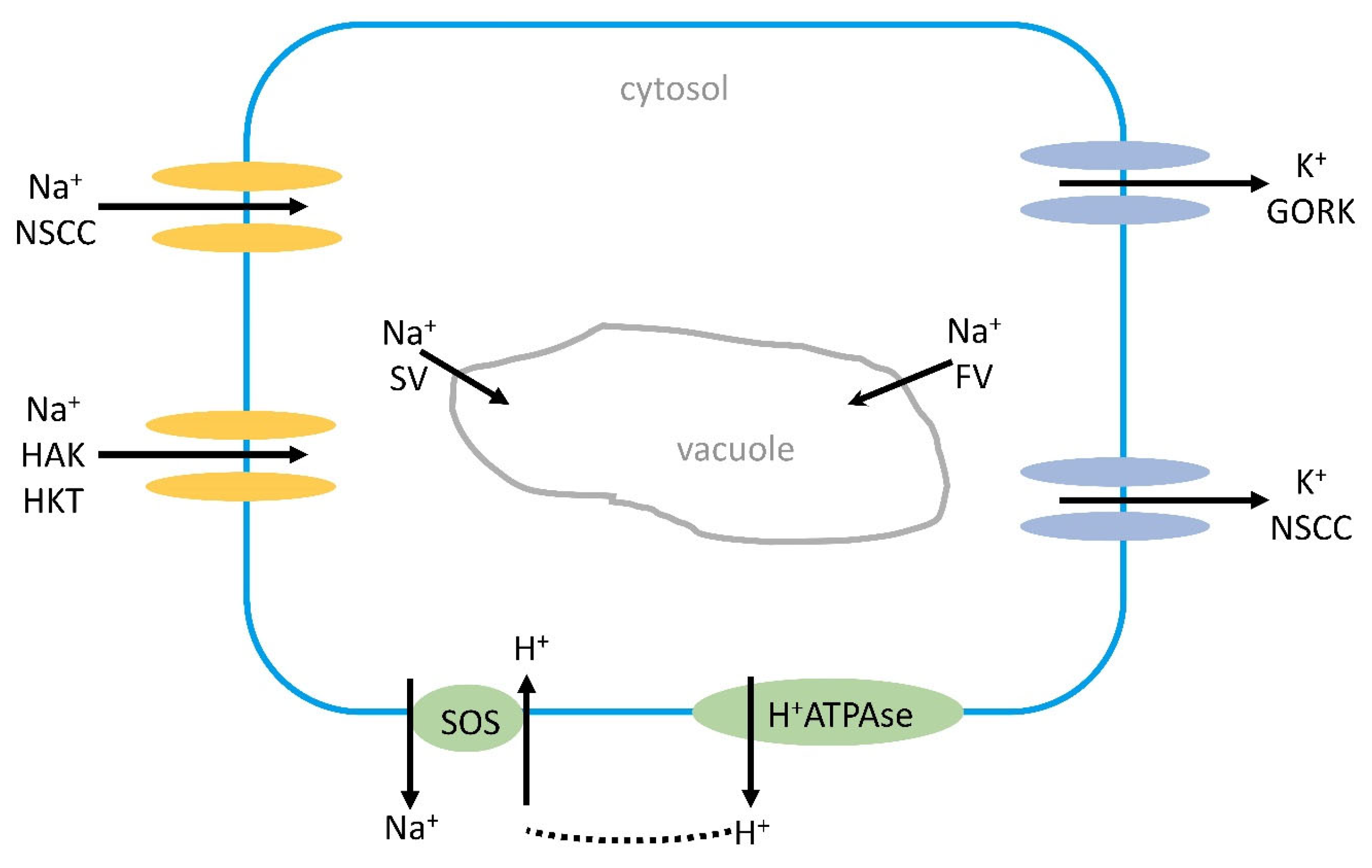
Figure 1. Na+ influx into a root cell or mesophyll cell in the leaf causes an efflux of K+ from the cell by GORKs or NSCCs by Na+-induced membrane depolarization. The depolarization might activate the H+ATPase in the plasma membrane, pumping out protons that can be used for the Na+/H+ antiporter (SOS1). Na+ can also leak into the vacuole by SVs or FVs. Under high salinity stress, most Na+ is transported via NSCCs and HKTs into cells, and also by HAKs. GORK, outwards-rectifying K+ channel; HAK, high-affinity K+ channel; NSCC, nonselective cation channel; SV, slow vacuolar channel; FV, fast vacuolar channel.
Depolarization also causes an influx of Ca2+ [53]. The depolarization is not always negative as it may lead to an activation of the H+ATPase, and the protons pumped out to apoplast can be used by the SOS1 antiporter. A more recent report suggests that GORK, an outward-rectifying K+ channel, may operate to switch off energy-consuming anabolic reactions and instead use the energy for stress adaptation [54]. The K+ efflux could be a signaling mechanism under salt stress; for instance, an increased H+ATPase activity and K+ influx from the vacuole to compensate for the K+ loss. The SKOR channel, STELAR K+-outward rectifier, might also be involved in K+ efflux induced by depolarization.
7.3. Regulation of Na+ Transport at the at Xylem/Parenchyma Cell Border
Not only is a low cytosolic Na+ in the plant cells important for tolerance, but also in the prevention of ion loading into the xylem at the parenchyma–xylem border for further transport to the shoot. It was shown that both the root and shoot of the halophyte quinoa had a higher K+ concentration than the root and shoot of the glycophyte pea, and under salinity this concentration was even higher [55]. Despite a higher concentration of Na+ in quinoa roots than in pea roots, the K+/Na+ ratio was higher in quinoa.
To explain these results, electrophysiological measurements of K+ and H+ fluxes from mechanically isolated root xylem of pea and quinoa were performed [56][57]. The addition of 20 mM NaCl and ABA to the xylem medium, mimicking the natural xylem solution [58], caused a strong K+ efflux from the stelar cells of pea but no efflux from quinoa [55], reflecting K+ retention in quinoa roots. In pea, K+ was translocated to the shoot to compensate for a lower uptake of K+ in the root. There was also a H+ efflux from the stelar tissue of both species, but the efflux was more pronounced in pea. ABA accumulates in the root under salt stress [59]. There it might stimulate the SOS protein [60]. The addition of 50 µM ABA induced a net H+ uptake into xylem-parenchyma cells of both species and a net K+ efflux, suggesting an ion exchanger at the xylem–parenchyma interface [61]. The results corroborate results showing a higher concentration of K+ in the shoots of quinoa than in pea under salt stress [55]. Quinoa keeps more K+ as osmotic regulation in the roots than pea does. The high concentration of Na+ found in the shoot of quinoa may depend on the fact that quinoa uses Na+ as osmoregulation in the shoot.
7.4. Different Barley Cultivars Differ in Salt Tolerance
Metabolomic and transcriptomic analyses of barley genotypes showed that the halophyte Hordeum marinum under salt stress used more Na+ and K+ ions for osmotic regulation and root tolerance than the cultivated Hordeum vulgare, and also increased the glycolysis and TCA cycle to obtain a high energy supply, necessary for shoot tolerance [62].
7.5. Tolerant Rice Cultivars Have Different Salt-Tolerance Mechanisms
In three rice cultivars showing different salt tolerance, the most sensitive cultivar, cv. VD20, accumulated less Na+ than the other two cultivars [63]. The most tolerant cv. AGPPS114 accumulated more Na+ and also contained higher concentrations of proline and glycine betaine as osmotic regulation than the other two cultivars. Moreover, VD20 showed a higher expression of the HKTs transporters, HKT1;4 and HKT1;5, than the other cultivars, as analyzed by real-time PCR. The authors reported that the tolerant cultivar showed a higher expression of the SOS1 and NHX1 than the sensitive cultivars, which might explain the salt tolerance of the former cultivar.
7.6. SOS1 Role in Salt Tolerance
A low concentration of Na+ in the cytosol is important for salt tolerance. Plants are able to transport Na+ from the cytosol into apoplasts by the Na+/H+ antiporter SOS1. The localization of SOS1 in the plasma membrane was revealed by confocal imaging of a SOS1–green fluorescent protein fusion in transgenic Arabidopsis [60]. Expression analyses showed that SOS1 was present in the plasma membranes of root tips and in parenchyma cells of the xylem/symport boundary. Thus, the SOS1 protein should have another function too: to reduce the transport of Na+ from xylem parenchyma cells into the xylem vessels, which would also be of importance for salt tolerance. Conflicting results were recently published from an investigation on the gene expression of SOS1 and Na+-flux measurements, which stated that it is only the SOS1 transporters in the outer root tissue that exclude Na+ from the root cytosol, but that SOS1 operating in the stele actively loads Na+ to the xylem transpiration stream [64].
Cytosolic Na+ concentration and fluxes in living cells can be analyzed by dual-wavelength fluorescence microscopy and the sodium-binding fluorescent dye SBFI, AM [65]. By the use of this technique, measurements on mesophyll protoplasts from Arabidopsis shoots showed that the sos1 mutant took up more Na+ into the cytosol than did the Wt protoplasts when the external solution contained 100 mM NaCl [66]. Moreover, the Arabidopsis nhx mutant, localized in tonoplasts and having a functional SOS1, also took up more Na+ into cytosol than the Wt, probably because both the Na+/H antiporters are involved in the efflux of Na+ from cytosol. The main function of NHX is believed to be a regulator of pH and intracellular homeostasis [67]. In Arabidopsis, there are four isoforms of NHX and nhx triple and quadruple knockouts showed reduced growth. A lack of any vacuolar-NHX activity resulted in reduced Na+ uptake and no K+ uptake, suggesting that these antiporters take up K+ but also some Na+. They also reported a Na+-uptake transporter, which was independent of proton transport.
7.7. Na+ and K+ Transport into the Vacuole
For a long time, it has been stressed that the Na+/H+ antiporter NHX in the tonoplast and in some endomembranes is important for salt tolerance, as it might transport Na+ out from the cytosol. However, the work in [68] reported that K+ concentration decreased when the Na+ concentration was increased in the vacuole. Moreover, coordinated transport by NHX and KEAs, K+-efflux antiporters, was reported to result in salt tolerance [69]. Thus, NHX might be more important for K+ transport into the vacuole and for pH regulation, even if NHX to a lesser degree also mediates the transport of Na+ [67]. Recent findings suggest that the CCX, a cation/Ca2+ exchanger, is suggested as a better candidate for salt tolerance than NHX, as it deceases high concentrations of Na+ in cytosol and reduces reactive oxygen species [70].
8. Measurements of Cytosolic Ion Changes in Different Species/Cultivars under Salinity
To compare the influx kinetics of Na+ species, D’Onofrio and coworkers (2005) [71], Kader and Lindberg (2005) [72] (Figure 2), and Sun et al. [55] used epifluorescence microscopy and the Na+-binding fluorescent dye SBFI, AM, the acetoxymethyl ester of the benzofuran isophtalate, SBFI. The fluorescent Na+ indicator CoroNA Green, AM, can also be used to measure Na+ concentrations in the cytosol. Dyes in AM-ester form can penetrate the plasma membrane and enter into the cytosol, where they are split by esterases into the Na+-binding fluorescent form. Ratiometric measurements at two excitation wavelengths make the result more reliable than one-wavelength measurement, as different dye concentrations, photobleaching, and the thickness of the cell show little effect [65][73].
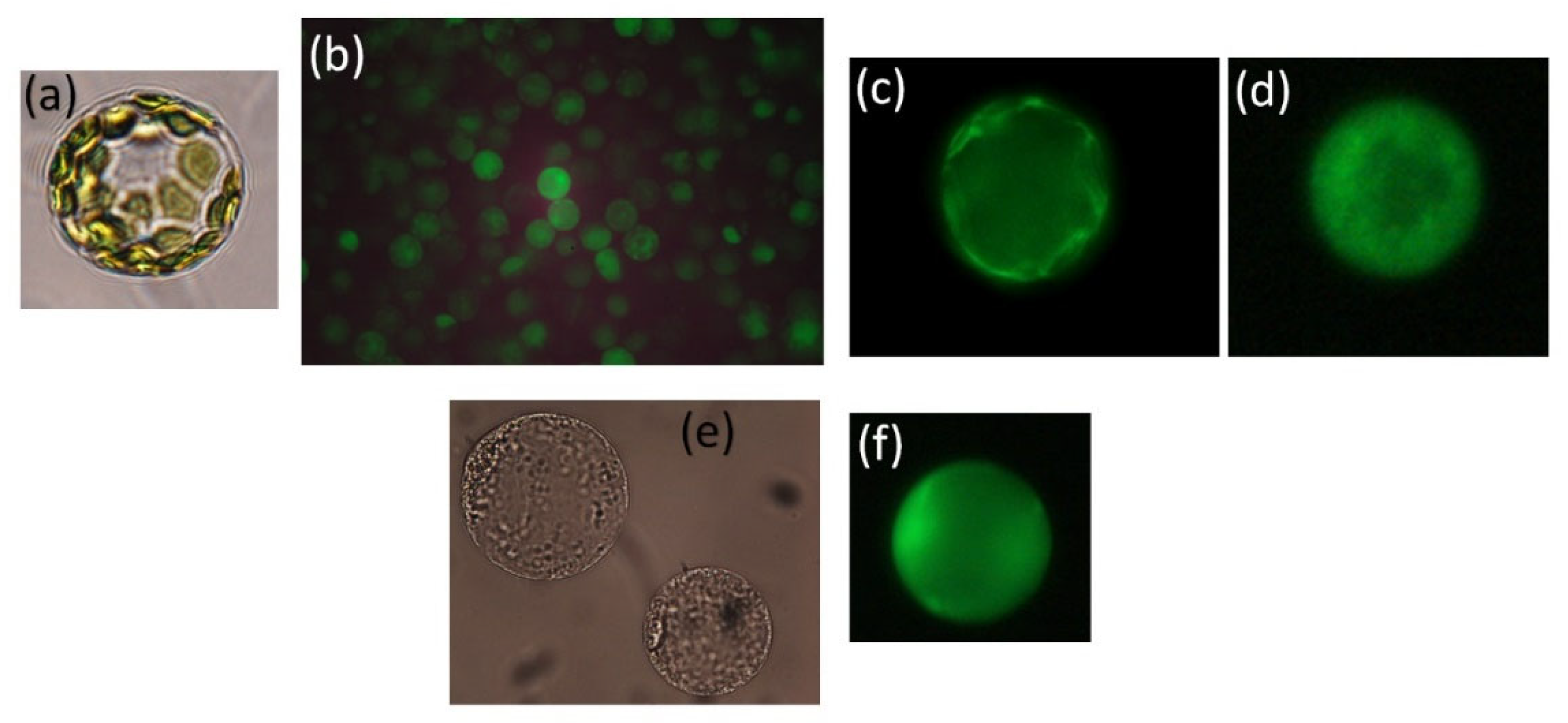
Figure 2. Protoplasts from rice mesophyll in transmitted light (a), and labelled with Fura 2 (b), labelled with SBFI (c), protoplasts from rice root labelled with SBFI (d), from wheat root in transmitted light (e), and labelled with SBFI (f). Fluorescence emission was measured at 530–550 nm.
8.1. Cytosolic Na+ Influx and Efflux from Salt-Tolerant and -Sensitive Species of Quince, Sugar Beet, and Wheat Differ
D’Onofrio et al. [71] showed that the amplitude and duration of Na+ influx into protoplasts from species that are highly salt tolerant, such as quince (Cydonia oblonga Mill), salt tolerant sugar beet (Beta vulgaris L.cv. Monohill), and less tolerant wheat (Triticum aestivum L. cv. Kadett) were in the order: wheat > sugar beet > quince. The quince protoplasts took up sodium only from a Ca2+ free buffer containing 200–400 mM NaCl. The Na+ influx was transient in quince, but in wheat and sugar beet it increased to a certain level and was then stable. As 1.0 mM external Ca2+ inhibited the influx, it is likely that Na+ influx at high salt was mediated by nonselective-cation channels [29][74]. Only the halophyte quince was able to carry out a rapid efflux of Na+ from the cytosol.
8.2. Cytosolic Na+ and pH Changes Are Different in the Halophyte Quinoa and the Glycophyte Pea
Different dynamics were obtained in the cytosolic influx of Na+ in the glycophyte pea, Pisum sativum, and the halophyte quinoa, Chenopodium quinoa [55]. The cytosolic Na+ concentration, [Na+cyt], was analyzed in mesophyll protoplasts after the cultivation of seedlings with and without 100 mM NaCl, and upon addition of NaCl to the protoplast medium.
The addition of 100 mM NaCl to control protoplasts of quinoa caused a transient [Na+ cyt] influx and the maximal concentration was obtained after 360 s, at the same time as for salt-adapted quince [55][71]; but when NaCl was added to salt-cultivated quinoa protoplasts, the maximal Na+ influx was obtained later (after 450–500 s) and was mainly transient [55].
The addition of NaCl (50 mM) to pea control protoplasts caused another reaction: the influx increased and was then stable at the same Na+ level. In addition, NaCl addition to pea protoplasts from seedlings pretreated with salinity showed a gradual increase for a long time.
The different reaction obtained in salinity-cultivated quinoa suggests an adaptation mechanism and might depend on a rapid activation of the H+-ATPase, which was absent in pea, as the Na+ influx in pea increased with time [75]. Quinoa grows optimally at 100–200 mM NaCl and is tolerant not only to salt stress but also to drought and frost, and thus is suitable for cultivation in areas where no other plants survive [76].
8.3. Cytosolic Na+ Influx and Efflux in Tolerant and Sensitive Rice
The same differences in Na+-influx dynamics were also obtained when comparing the influx in tolerant rice cv. Pokkali with sensitive rice cv. BRRIDhan29 [72].
Pharmacological analysis indicated that NSCCs were the main pathways for Na+ influx in the tolerant rice cultivar, but that both NSCCs and high-affinity K+ channels HKTs contributed to influx in the sensitive one. The transient influx of Na+ suggests that tolerant rice has a mechanism for fast extrusion of Na+ that protects the cytosol from ion toxicity. This rice cultivar also has less PM permeability to Na+ compared to salt-sensitive rice [77]. Inhibitor analysis suggested that tolerant rice transported Na+ from the cytosol into the vacuole by the Na+/H+ antiporter NHX in the tonoplast, but the sensitive cultivar transported Na+ out of the protoplast by the Na+/H+ antiporter SOS1 in the plasma membrane [78][79][80][81].
Expression analyses by real-time RT-PCR of the OsVHA genes, which encode the H+-ATPase in the tonoplast demonstrated that the OsVHA transcripts were induced immediately after Na+ stress in the tolerant rice cultivar, but in the sensitive one, the expression of OsVHA was low and delayed 6 h. The tonoplast H+-ATPase is supposed to be important for salinity tolerance as it builds up an electrochemical gradient for the transport of cations into the vacuole [33][82]. These findings confirmed the results from protoplast experiments that showed a fast efflux of sodium from the cytosol of the tolerant rice, but little sodium efflux from the sensitive rice [32][72].
References
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673.
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043.
- Maathuis, F.J.M. Sodium in plants: Perception, signaling and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858.
- Fang, S.; Hou, X.; Liang, X. Response mechanisms of plants under saline-alkali stress. Front. Plant Sci. 2021, 12, 667458.
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258.
- Colmeneros-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peindo-Torrubia, P.; Rosales, M.A. Chloride as a beneficial macronutrient in higher plants: New Roles and regulation. Int. J. Mol. Sci. 2019, 20, 4686.
- Franco-Navarro, J.D.; Rosales, M.A.; Cubero-Font, P.; Calvo, P.; Álvarez, R.; Diaz-Espejo, A.; Colmenero-Flores, J.M. Chloride as a macronutrient increases water-use efficiency by anatomically driven reduced stomatal conductance and increased mesophyll diffusion to CO2. Plant J. 2019, 99, 815–831.
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentration of Na+ and Cl− ions in soil solution has simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459.
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71.
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integrat. Plant Biol. 2018, 60, 796–804.
- Lu, Y.; Fricke, W. Changes in root hydraulic conductivity in wheat (Triticum aestivum L.) in response to salt stress and day/night can best be explained through altered activity of aquaporins. Plant Cell Environ. 2023, 46, 747–763.
- Maurel, C.; Boursiac, Y.; Luu, D.-T.; Santoni, V.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Res. 2015, 95, 1321–1358.
- Flowers, T.; Yeo, A. The driving force for water and solute movement. In Plant Solute Transport; Yeo, A., Flowers, T., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 1992; pp. 29–45.
- Kronzucker, H.J.; Britto, D.T. Sodium transport in plants: A critical review. New Phytol. 2011, 189, 54–81.
- Shi, Y.; Wang, Y.; Flowers, T.J.; Gong, H. Silicon decreases chloride transport in rice (Oryza sativa L.). J. Plant Physiol. 2013, 170, 847–853.
- Amtmann, A.; Sanders, D. Mechanisms of Na+ uptake by plant roots. Adv. Bot. Res. 1999, 29, 75–112.
- Zhang, J.L.; Flowers, T.J.; Wang, S.M. Mechanisms of sodium uptake by roots of higher plants. Plant Soil 2010, 326, 45–60.
- Haro, R.; Banuelos, M.A.; Rodriguez-Navarro, A. High affinity uptake of sodium in land plants. Plant Cell Physiol. 2010, 51, 68–79.
- Davenport, R.J.; Tester, M. A weakly voltage-dependent, nonselective cation channel mediates toxic sodium influx in wheat. Plant Physiol. 2000, 12, 823–834.
- Demidschik, V.; Maathuis, F.J.M. Physiological roles of nonselective cation channels in plants: From salt stress to signaling and development. New Phytol. 2007, 175, 387–404.
- Leng, Q.; Mercier, R.W.; Hua, B.; Fromm, H.; Berkowitz, G.A. Electrophysiological analysis of cloned cyclic nucleotide-gated ion channels. Plant Physiol. 2002, 128, 400–410.
- Keisham, M.; Mukherjee, S.; Bhatla, S. Mechanisms of sodium transport in plants—Progresses and challenges. Int. J. Mol. Sci. 2018, 19, 647.
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanism of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017.
- Golldack, D.; Su, H.; Quigley, F.; Kamasani, U.R.; Munoz-Garay, C.; Balderas, E.; Popova, O.V.; Bennett, J.; Hans, J.; Bohnert, H.J.; et al. Characterization of an HKT-type transporter in rice as a general alkali cation transporter. Plant J. 2002, 31, 529–542.
- Tester, M.; Davenport, R.J. Na+ transport and Na+ tolerance in higher plants. Ann. Bot. 2003, 91, 503–527.
- Greger, M.; Ahmad, H.; Kabir, A.H.; Landberg, T.; Maity, P.J.; Lindberg, S. Silicate reduces cadmium uptake into cells of wheat. Environ. Poll. 2016, 211, 90–97.
- Mian, A.; Oomen, R.J.; Isayenkov, S.; Sentenac, H.; Maathuis, F.J.; Véry, A.A. Over-expression of a Na+ and K+ permeable HKT transporter in barley improves salt tolerance. Plant J. 2011, 68, 468–479.
- Han, Y.; Yin, S.; Huang, l.; Wu, X.; Zeng, J.; Liu, X.; Qiu, L.; Munns, R.; Chen, Z.-H.; Zhang, G. A sodium transporter HvHKT1 confers salt tolerance in barley via regulating tissue cell ion homeostasis. Plant Cell Physiol. 2018, 59, 1976–1989.
- Essah, P.A.; Davenport, R.; Tester, M. Sodium influx and accumulation in Arabidopsis. Plant Physiol. 2003, 133, 307–318.
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80.
- Byrt, C.S.; Zhao, M.; Kourghi, M.; Bose, J.; Henderson, S.W.; Qiu, J.; Gilliham, M.; Schultz, C.; Schwarz, M.; Ramesh, S.A.; et al. Non-selective cation channel activity of aquaporin AtPIP2;1 regulated by Ca2+ and pH. Plant Cell Environ. 2017, 40, 802–815.
- Kader, M.A.; Seidel, T.; Golldack, D.; Lindberg, S. Expressions of OsHKT1, OsHKT2 and OsVHA are differently regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. J. Exp. Bot. 2006, 57, 4257–4268.
- Horie, T.; Yoshida, K.; Nakayama, H.; Yamada, K.; Oiki, S.; Shinmyo, A. Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J. 2001, 27, 129–138.
- Rubio, F.; Nieves-Cordones, M.; Horie, T.; Shabala, S. Doing ‘business as usual’ comes with a cost: Evaluating energy cost of maintaining plant intracellular K+ homeostasis under saline conditions. New Phytol. 2020, 225, 1097–1104.
- Berthomieu, P.; Cone, G.; Nublat, A.; Brackenbury, W.J.; Lambert, C.; Savio, C.; Uozumi, N.; Oiki, S.; Yamada, K.; Cellier, F.; et al. Functional analysis of AtHKT1 in Arabidopsis shows that Na recirculation by the phloem is crucial for salt tolerance. EMBO J. 2003, 22, 2004–2014.
- Ren, H.; Gao, J.P.; Li, L.G. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat. Gen. 2005, 37, 1141–1146.
- Brini, F.; Masmoudi, K. Ion transporters and abiotic stress tolerant plants. ISRN Mol. Biol. 2012, 2012, 927436.
- Huang, L.; Kuang, L.; Wu, L.; Wu, D.; Zhang, G. Comparison in functions of HKT1;5 transporters between Hordeum marinum and Hordeum vulgare in responses to salt stress. Plant Growth Reg. 2019, 89, 309–319.
- Møller, I.S.; Gilliham, M.; Jha, D.; Mayo, G.M.; Roy, S.J.; Coates, J.C.; Haseloff, J.; Tester, M. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell 2009, 21, 2163–2178.
- Davenport, R.; Munoz-Mayer, A.; Jha, D.; Essah, P.A.; Rus, A.; Tester, M. The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ. 2007, 30, 497–507.
- Colmeneros-Flores, J.M.; Martinez, G.; Gamba, G.; Vazquez, N.; Iglesias, D.J.; Brumos, J.; Talon, M. Identification and functional characterization of cation-chloride cotransporters in plants. Plant J. 2007, 50, 278–292.
- Henderson, S.W.; Wege, S.; Gilliham, M. Plant cation-chloride cotransporters (CCC): Evolutionary origin and functional insights. Int. J. Mol. Sci. 2018, 19, 492.
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biol. 2008, 59, 651–681.
- Isayenkov, S.V.; Dabravolski, S.A.; Pan, T.; Shabala, S. Phylogenetic diversity and physiological roles of plant monovalent cation/H+ antiporters. Front. Plant Sci. 2020, 11, 573564.
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665.
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2014, 115, 419–431.
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963.
- Wang, C.M.; Zhang, J.L.; Liu, X.S.; Li, Z.; Wu, G.Q.; Cai, J.Y.; Wang, S.M. Puccinellia tenuiflora maintains a low Na+ level under salinity by limiting unidirectional Na+ influx resulting in a high selectivity for K+ over Na+. Plant Cell Environ. 2009, 32, 486–496.
- Schachtman, D.P.; Schroeder, J.I. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 1994, 370, 655–658.
- Schachtman, D.P.; Liu, W.H. Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci. 1999, 4, 281–287.
- Wang, S.M.; Zhang, J.L.; Flowers, T.J. Low-affinity Na+ uptake in the halophyte Suaeda maritima. Plant Physiol. 2007, 145, 559–571.
- Zepeda-Jazo, I.; Shabala, S.; Chen, Z.; Pottosin, I.I. Na+-K+ transport. in roots under salt stress. Plant Signal. Behav. 2008, 3, 401–403.
- Roberts, S.K.; Tester, M. Permeation of Ca2+ and monovalent cations through an outwardly rectifying channel in maize root stelar cells. J. Exp. Bot. 1997, 48, 839–846.
- Adem, G.D.; Chen, G.; Shabala, L.; Chen, Z.H. GORK channel: A master switch of plant metabolism. Trends Plant Sci. 2020, 25, 434–445.
- Sun, Y.; Lindberg, S.; Shabala, L.; Morgan, S.; Shabala, S.; Jacobsen, S.E. A comparative analysis of cytosolic Na+ changes under salinity between halophyte quinoa (Chenopodium quinoa) and glycophyte pea (Pisum sativum). Environ. Exp. Bot. 2017, 141, 154–160.
- Shabala, S.; Shabala, L.; Cuin, T.A.; Pang, J.; Percey, W.; Chen, Z.; Wegner, L.H. Xylem ionic relations and salinity tolerance in barley. Plant J. 2010, 61, 839–853.
- Shabala, S. Signaling by potassium: Another second messenger to add to the list? J. Exp. Bot. 2017, 68, 4003–4007.
- Shabala, S.; Hariadi, Y.; Jacobsen, S.E. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J. Plant Physiol. 2013, 170, 906–914.
- Jia, W.; Wang, Y.; Zhang, S.; Zhang, J. Salt-stress-induced ABA accumulation is more sensitively triggered in roots than in shoots. J. Exp. Bot. 2002, 53, 2201–2206.
- Shi, H.Z.; Quintero, F.J.; Pardo, J.M.; Zhu, J.K. The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 2002, 14, 465–477.
- Zhu, M.; Zhou, M.; Shabala, L.; Shabala, S. Physiological and molecular mechanisms mediating xylem Na+ loading in the context of salinity stress tolerance. Plant Cell Environ. 2016, 40, 1009–1020.
- Huang, L.; Kuang, L.; Li, X.; Wu, L.; Wu, D.; Zhang, G. Metabolomics and transcriptomic analyses reveal the reason why Hordeum marinum has higher salt tolerance than Hordeum vulgare. Env. Exp. Bot. 2018, 156, 48–61.
- Ngoc, N.T.; Tri, P.N.; Le Hong, T.; Quoc, C.D. Biomolecular evaluation of three contrasting rice cultivars (Oryza sativa L.) in salt stress response at seedling stage. Plant Sci. Today 2022, 9, 491–503.
- Foster, K.J.; Miklavcic, S.J. A comprehensive biophysical model of ion and water transport in plant roots. II. Clarifying the rules of SOS1 in the salt-stress response Arabibopsis. Front. Plant Sci. 2019, 10, 1121.
- Halperin, S.J.; Lynch, J.P. Effect of salinity on cytosolic Na+ and K+ in root hairs of Arabidopsis thaliana: In vivo measurement using the fluorescent dyes SBFI and PBFI. J. Exp. Bot. 2003, 54, 2035–2043.
- Morgan, S.H.; Kader, M.A.; Lindberg, S. Cytosolic sodium influx in mesophyll protoplasts of Arabidopsis thaliana, Wt, sos1;1, and nhx1 differs and induces different calcium changes. Plants 2022, 11, 3439.
- Bassil, E.B.; Zhang, S.; Gong, H.; Tajima, H.; Blumwald, E. Cation specificity of vacuolar NHX type Cation/H+ antiporters. Plant Physiol. 2019, 179, 616–629.
- Chen, Z.; Wu, Y.; Di, L.; Shen, Y.; Wang, G. AtCCX1 transports Na+ and K+ in Pitch pastoris. Afr. J. Biotechnol. 2011, 10, 9743–9750.
- Zhu, X.; Pan, T.; Zhang, X.; Fan, L.; Quintero, F.J.; Zhao, H.; Su, X.; Li, X.; Villalta, I.; Mendoza, I.; et al. K+ efflux antiporters 4, 5, and 6 mediate pH and K+ homeostasis in endomembrane compartments. Plant Physiol. 2018, 178, 1657–1678.
- Yang, J.; Li, W.; Guo, X.; Chen, P.; Cheng, Y.; Mao, K.; Ma, F. Cation/Ca2+ exchanger1 (MdCCX1), a plasma membrane-localized Na+ transporter, enhances plant salt tolerance by inhibiting excessive accumulation of Na+ and reactive oxygen species. Front. Plant Sci. 2021, 12, 746189.
- D’Onofrio, C.; Kader, A.; Lindberg, S. Uptake of sodium in quince, wheat and sugar beet protoplasts determined by the fluorescent sodium-binding benzofuran isophthalate dye. J. Plant Physiol. 2005, 162, 421–428.
- Kader, A.; Lindberg, S. Uptake of sodium in protoplasts of salt-sensitive and salt-tolerant cultivars of rice, Oryza sativa L. J. Exp. Bot. 2005, 56, 3149–3158.
- Negulescu, P.A.; Harootunian, A.; Tsien, R.Y.; Machen, T.E. Fluorescence measurement of cytosolic free Na concentration influx and efflux in gastri cells. Cell Regul. 1990, 1, 259–268.
- Demidschik, V.; Tester, M. Sodium fluxes through nonselective cation channels in plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol. 2002, 128, 379–387.
- Bose, J.; Rodrigo-Moreno, A.; Lai, D.; Xie, Y.; Shen, W.; Shabala, S. Rapid regulation of plasma membrane H+ATPase activity is essential to salinity tolerance in two halophyte species, Atriplex lentiformis and Chenopodium quinoa. Ann. Bot. 2015, 115, 481–494.
- Ruiz, K.B.; Biondi, S.; Martínez, E.A.; Orsini, F.; Antognoni, F.; Jacobsen, S.E. Quinoa—A model crop for understanding salt tolerance mechanisms in halophytes. Plant Biosyst. 2016, 150, 357–371.
- Anil, V.S.; Krishnamurthy, H.; Mathew, M. Limiting cytosolic Na confers salt tolerance in rice cell in culture: A two-photon microscopy study of SBFI-loaded cells. Physiol. Plant. 2007, 129, 607–621.
- Blumwald, E.; Poole, R.J. The Na+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris. Plant Physiol. 1985, 78, 163–167.
- Hassidim, M.; Braun, Y.; Lerner, H.R.; Reinhold, L. Na+/H+ and K+/H+ antiport in root membrane vesicles isolated from the halophyte Atriplex and the glycophyte cotton. Plant Physiol. 1990, 94, 1795–1801.
- Zhu, J.K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445.
- Mahi, H.E.; Pérez-Hormaec, H.J.; Luca, A.D.; Villalta, I.; Espartero, J.; Gámez-Arjona, F.; Fernández, J.L.; Bundó, M.; Mendoza, I.; Mieulet, D.; et al. A critical role of sodium flux via the plasma membrane Na+/H+ exchanger SOS1 in the salt tolerance of rice. Plant Physiol. 2018, 180, 1046–1065.
- Sze, H.; Schumacher, K.; Muller, M.; Padmanaban, S.; Taiz, L. A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+ATPase. Trends Plant Sci. 2002, 7, 157–161.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revision:
1 time
(View History)
Update Date:
09 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




