Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Aravin Prince Periyasamy | -- | 6210 | 2024-01-08 19:47:14 | | | |
| 2 | Peter Tang | Meta information modification | 6210 | 2024-01-09 06:38:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Periyasamy, A.P. Remediation of Textile-Dye-Containing Wastewater. Encyclopedia. Available online: https://encyclopedia.pub/entry/53570 (accessed on 03 February 2026).
Periyasamy AP. Remediation of Textile-Dye-Containing Wastewater. Encyclopedia. Available at: https://encyclopedia.pub/entry/53570. Accessed February 03, 2026.
Periyasamy, Aravin Prince. "Remediation of Textile-Dye-Containing Wastewater" Encyclopedia, https://encyclopedia.pub/entry/53570 (accessed February 03, 2026).
Periyasamy, A.P. (2024, January 08). Remediation of Textile-Dye-Containing Wastewater. In Encyclopedia. https://encyclopedia.pub/entry/53570
Periyasamy, Aravin Prince. "Remediation of Textile-Dye-Containing Wastewater." Encyclopedia. Web. 08 January, 2024.
Copy Citation
Water makes up most of the Earth, although just 0.3% is usable for people and animals. The huge oceans, icecaps, and other non-potable water resources make up the remaining 99.7%. Water quality has declined due to pollution from population growth, industry, unplanned urbanization, and poor water management. The textile industry has significant global importance, although it also stands as a major contributor to wastewater generation, leading to water depletion and ecotoxicity. This issue arises from the extensive utilization of harmful chemicals, notably dyes.
effluents
ecotoxicity
dyes
carcinogenicity
textile wastewater
sustainability
1. Introduction
Currently, ecosystems are primarily experiencing harm due to the exhaustion of natural resources and the deterioration of the environment resulting from industrial expansion and environmental emergencies [1][2]. Water pollution is a significant environmental issue posing significant risks to water, the primary life-sustaining element on Earth, emphasizing its crucial role in supporting life [3][4]. Pollution is primarily caused by the insufficient potable water supply and the harmful exposure to various chemicals and pathogens in the polluted water and food chain. Water pollution is largely defined by two main problems: the lack of safe drinking water and the dangerous exposure to various chemicals and pathogens found in contaminated water and the food chain [5][6]. Water pollution is characterized by the overabundance of harmful substances in water bodies, resulting from both natural and human activities [7][8].
The textile industry is a significant contributor to water pollution [9], and it is also responsible for approximately 20% of global water pollution [10], as the second largest polluter after the oil industry [10]. In comparison to other industrial sectors, the textile industry is known to have the highest water and chemical consumption, with over 8000 species being utilized [11][12][13]. The wastewater generated by this industry is often characterized by a significant amount of unfixed colors and dyeing auxiliaries [14][15][16][17]. Approximately 800,000 tons of dyes are produced annually, with 10–15% of this quantity being lost to the environment [18]. Over 10,000 distinct synthetic dye varieties have been introduced, with 70% of them belonging to the azo type. In general, dyes are classified into various types such as direct, reactive, basic, acidic, disperse, vat, sulfur, metal complex, and mordant dyes [10].
Dyes are a class of organic compounds that possess the ability to impart color to a diverse range of substrates [19][20]. Frequently, these compounds are recognized for their ionization properties and notable water solubility, leading to their facile dissemination into both the surroundings and human physiology [21]. The intricate aromatic structures of these substances pose a challenge for biodegradation and render them inert, thereby rendering their elimination a more arduous and laborious task. In contrast to metal ions, dyes can be classified in various ways. The most prevalent classification method is based on the charge exhibited upon dissolution, which leads to the formation of three distinct groups: anionic (inclusive of reactive, acid, and direct dyes), cationic (encompassing all basic dyes), and non-ionic (comprising disperse dyes). Dyes can be categorized into acid and base based on the various associated groups that dictate the hue of the color. Acid dyes are anionic chemicals containing acid moieties in their molecular structure such as sulfonic SO32− and carboxylic -COO¯, while base dyes are cationic ones presenting quaternary amine groups -NH4+ [22]. Another systematic method of classification is the color index, which is related to the chemical structure of the dye substance; however, due to the complexity of nomenclature from the chemical structure, the classification based on color application is the most preferable [13]. With respect to chemical structure, a variety of groups such as azo, diazo, anthraquinone, nitro, diphenylmethane and triphenylmethane, indigoid and thionindigoid, anthraquinoid, xanthene, phthaleins and metal complex dyes are known (Figure 1). Meanwhile, the mode of application and substrate-based scale classifies them into reactive, acid, base, vat, direct, solvent, disperse, and azoic dyes [23][24].
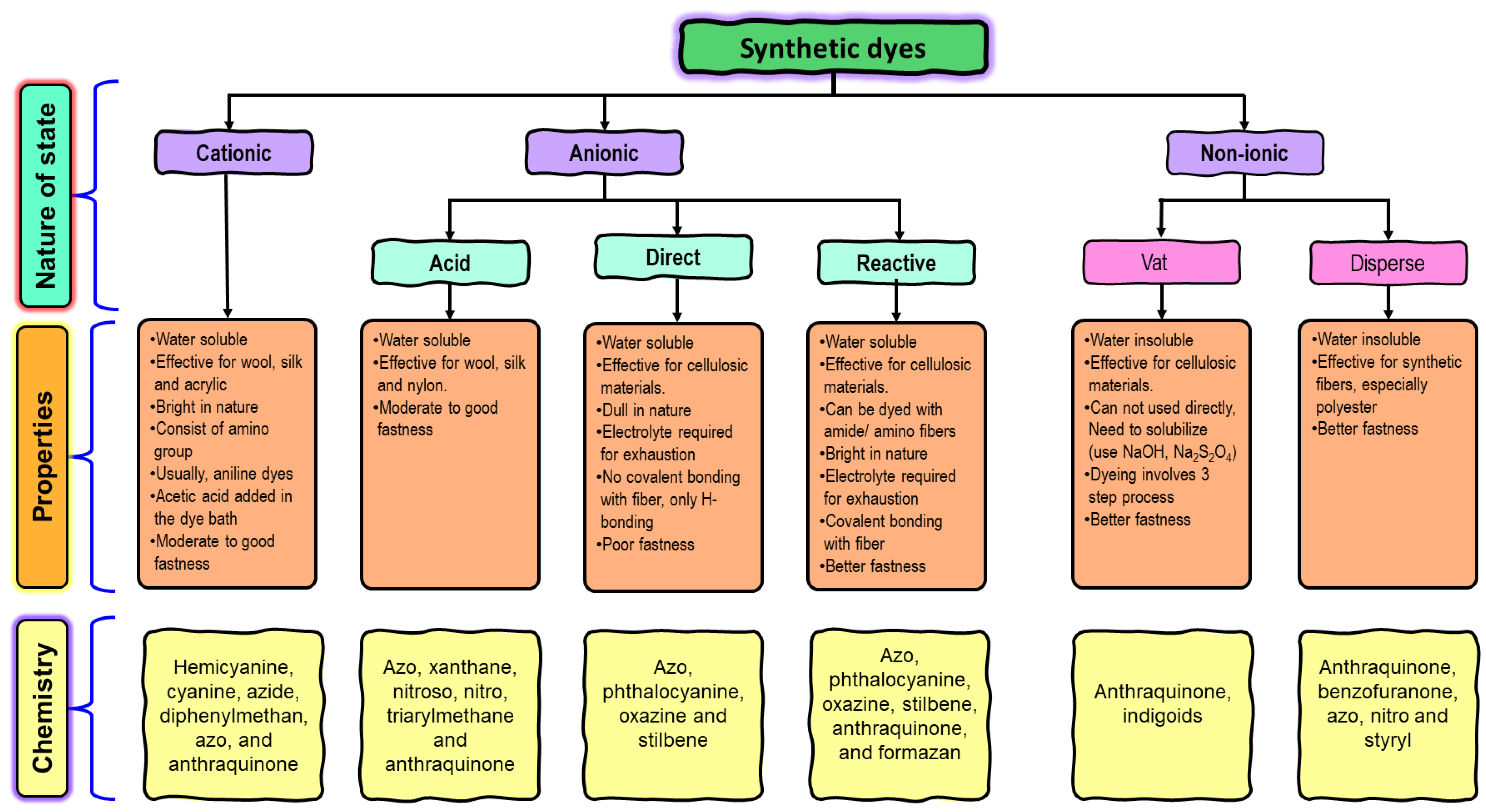
Figure 1. Classification of dyes used in the textile industry.
The classification of colored substances can be divided into two categories: natural and synthetic. Synthetic substances have become the predominant choice in the market due to their vast array of available colors and cost-effectiveness, as noted in [21]. The utilization of synthetic dyes, which are derived from benzene and its derivatives, has supplanted the use of conventional natural dyes, leading to the development of over 10,000 dyes with varying chemical structures and characteristics [25]. The compounds exhibit intricate conjugated architectures that pose challenges in terms of their elimination [25]. Certain dyes, such as azo dyes, possess a high degree of toxicity and carcinogenicity because of their toxic metabolites and aromatic amine byproducts. The removal of anionic and non-ionic dyes through conventional techniques poses a challenge due to their high water solubility and resistance to degradation of non-ionized fused aromatic rings, respectively. In the interim, it has been observed that biological techniques are not entirely effective in the complete elimination of reactive and acidic dyes [7]. In general, azo dyes exhibit a high susceptibility to degradation at their azo N=N linkage, leading to the formation of hazardous aromatic byproducts during treatment. Conversely, other categories of dyes are characterized by a low degradability, which limits the range of viable treatment options. Prior to discharge into the aquatic environment, it is imperative to subject the wastewater generated by the textile sector to appropriate treatment measures.
2. Common Treatment Methods for Textile Dyes
The primary approaches utilized in the treatment of wastewater may be categorized into three distinct groups: biological, chemical, and physical. One example of physical techniques is the utilization of membrane technology. On the other hand, chemical methods encompass processes such as oxidation, coagulation, and photochemical oxidation. Additionally, biological approaches include the implementation of anaerobic/aerobic sequential processes [26]. Oxidation is a chemical process that encompasses several techniques, including bleaching, chlorination, and ozonation. These techniques include the utilization of specific chemicals such as hydrogen peroxide, permanganate, chlorine, chlorine dioxide, and ozone (O3), respectively [13][24][27][28].
3. Effluent from the Textile Industry: Human and Environmental Issues
The effluents discharged by the textile industry in their untreated state consist of a wide array of organic contaminants, including unfixed colors, acids, alkalis, and notably, very poisonous dyes [29]. The textile business employs many categories of dyes, with azo dyes being the predominant group utilized, accounting for over 60% of the industry’s usage [30]. Azo dyes are characterized by their structural composition, which includes one or more azo groups. The discharge of unfixed azo dyes into wastewater is attributed to the inefficiency of textile dyeing processes, accounting for a range of 10–50% [31][32][33]. Certain textile manufacturing facilities employ wastewater treatment methods to break down the released free azo dyes in order to mitigate their impact on the environment. Conversely, there are other industries that release untreated industrial effluents straight into water sources, hence presenting significant ecotoxicological risks and causing harmful effects on organisms (see Figure 2). Farmers in various Asian nations, such as India, Bangladesh, Vietnam, and Indonesia, have historically employed the practice of irrigating their agricultural lands with untreated industrial effluents present in wastewater [34][35]. This practice has had detrimental effects on both soil quality and crop germination rates. Furthermore, the presence of toxic chemicals in these effluents has had a significant adverse impact on agricultural productivity, which in turn has had a notable influence on the gross domestic product (GDP) of these countries [36]. The introduction of azo dyes into water bodies has been seen to have detrimental effects on light penetration, hence negatively impacting the growth and productivity of algae and aquatic plants [37]. Additionally, the presence of these colors has been found to hinder the formation of dissolved oxygen (DO) in the water. Moreover, the ingestion of dyes by fish and other creatures can lead to the metabolic conversion of these substances into hazardous intermediates inside their systems, so exerting detrimental effects on the well-being of both the fish and their predators [38]. Azo dyes present in industrial effluents can potentially come into contact with humans and other mammals through two primary routes: oral consumption and direct skin contact [39]. The intestinal microflora present in the human gastrointestinal tract is responsible for the conversion of azo dyes into amino acids that possess toxic properties. These toxic amino acids have detrimental effects on numerous tissues inside the human body [29][40].
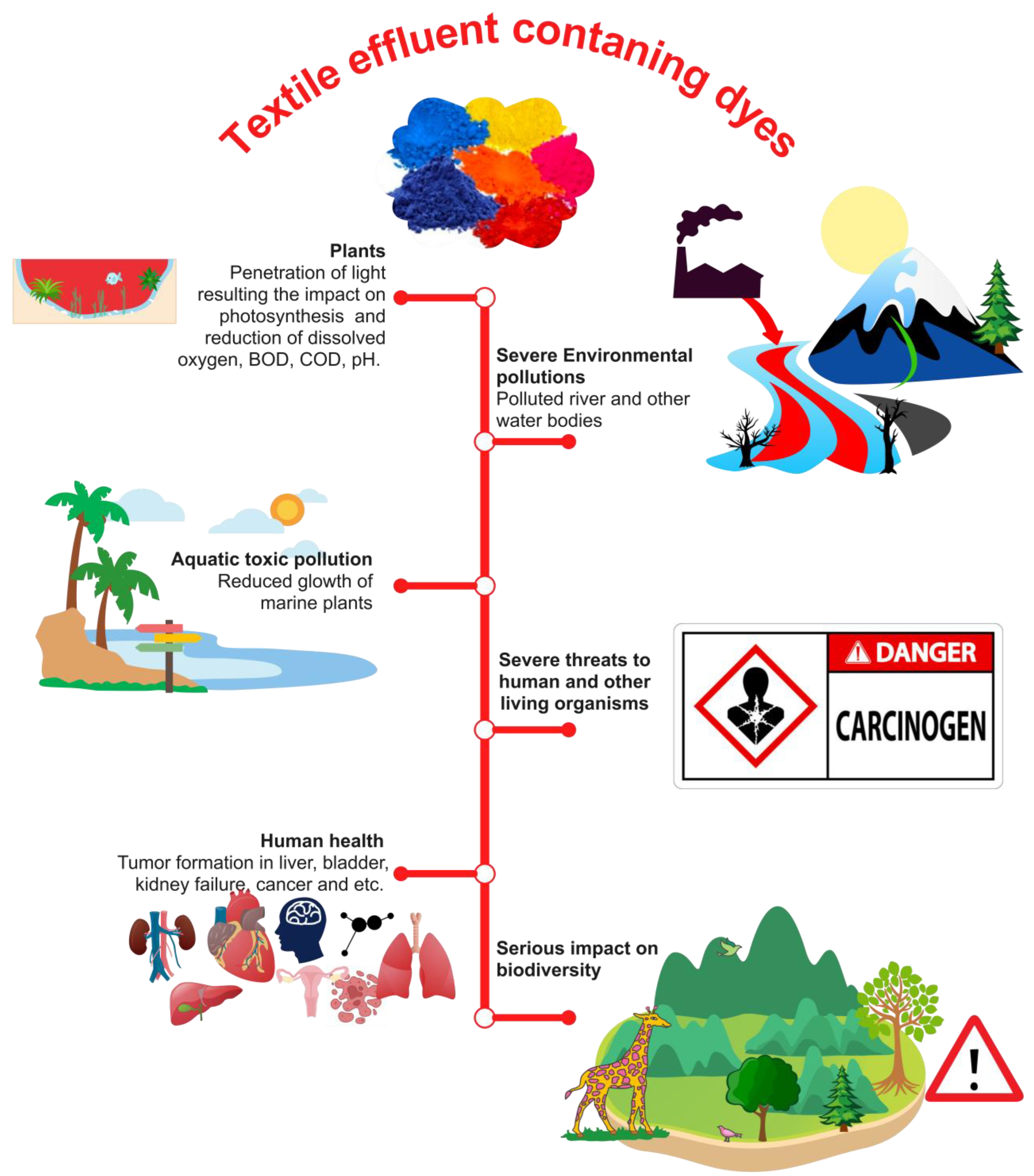
Figure 2. Influence of dye-containing textile wastewater on the environment and health hazardous.
4. Sustainable Wastewater Treatment for the Remediation
4.1. Bioadsorbents in Wastewater Treatment
The utilization of conventional chemical coagulation methods results in the generation of sludge, which is then disposed of in landfills. This practice has been found to contribute to the emission of harmful components, including gases that have the potential to contribute to global warming. Additionally, the disposal of this sludge in landfills has risks such as landfill leaching and contamination of groundwater [41]. The introduction section of this entry discusses the environmental threat posed by textile effluent containing high levels of color, BOD, COD, TDS, and TSS. Biological treatment is preferred over chemical treatment for sustainable treatment. Generally, the presence of complex groups in dyes, along with the recalcitrance of organic pollutants and their low degradability, restrict the efficacy of biological treatment methods [42]. Therefore, on this occasion, bioadsorbents play a significant role in the dye and heavy metal removal. The exploitation of domestic and agricultural wastes as adsorbents has emerged as a convenient alternative. Numerous adsorbents derived from biomass wastes have been created and utilized as very effective agents for the removal of various pollutants from water and wastewater. These waste materials have been used either in their original form or following suitable modifications. Various agricultural and food waste materials, such as Azolla [43], banana peel [44][45][46][47], cabbage waste [48], chitosan [49][50][51][52], citrus peel [53][54], Citrus limonum leaves [55], corn cob [56], orange peel [57][58], peanut hull [59], rice husk [60][61], sawdust [62], and sugar cane bagasse [63] have demonstrated successful utilization as adsorbents for the purpose of eliminating diverse types of contaminants.
Adsorption generally convert the pollutants from a liquid to a solid phase. This technique has several advantages, including simple, cost-effectiveness, convenience of operation, non-toxicity, and reactive surface atoms. Bioadsorbents are frequently employed for the treatment of textile effluent water owing to their economical, eco-friendly, locally accessible, sustainable, efficient, renewable, and readily disposable characteristics. They surpass commercially available activated carbon in terms of quality, rendering the latter’s high cost unjustifiable. Inexpensive sorbents possess a notable ability to absorb certain dyes, particularly reactive dyes, leading to the accumulation of significant amounts of hydroxylates in wastewater as a result of inadequate fixation of the dyestuff. Adsorption is advantageous over alternative approaches due to its simplicity, cost-effectiveness, ease of operation, non-toxic nature, presence of reactive surface atoms, and large surface area [64]. Currently, a global revolution is underway advocating for the recycling of organic wastes from agriculture, forests, and industries into economically viable products [64]. Some of the commonly used bio adsorbents and their nature of activity in treating textile effluent water are explained below.
The peel of Citrus limetta has been shown to be a cost-effective adsorbent for the removal of various colors [53]. Every year, a significant proportion of citrus fruit (~40% to 60%) is discarded in landfills. Research indicates that the global citrus processing industry generates a substantial amount of trash, estimated at approximately 120 million tons [54], creating serious ecological issue. As an example, orange peels are employed for the removal of 1-naphthyl amine dye from wastewater generated by the textile industry. The findings of the study indicated that the adsorption capacity of the peel waste had a positive correlation with the concentration of dye ions. Additionally, it was observed that the percentage of dye ion removal also rose as the original dye ion concentration increased. Furthermore, the utilization of orange peels in the preparation of activated carbon has proven to be effective as an adsorbent for the removal of MB [65]. Banana fiber is an economically accessible and abundantly available material, owing to its substantial cultivation and extensive presence as a crop, with a global count over 25 billion banana or plantain trees [66]. Banana powder has demonstrated promising potential as a biosorbent for the removal of MB dye. This is attributed to the presence of many functional groups on the surface of banana particles, as well as their uneven morphology [67]. Another study found that banana peel is particularly successful in removing reactive dyes, with 90% of the dyes being removed in 5 min [68]. The utilization of ash derived from banana stem as a potential bio adsorbent for dye removal has promising results. This is evidenced by its ability to achieve a 95% removal efficiency for MB dye [69]. The effectiveness of banana stem ash may be attributed to its diverse array of components and functional groups, as well as its rough and porous surface characteristics. Recent research provides further evidence supporting the removal of 91% of color from the Banana stem [70]. Some of the resent studies confirms that the waste extraction from coffee waste shows promising adsorbents for the dyes [71].
Coconut coir dust refers to a lightweight, porous particle that is separated from the husk during the process of fiber extraction. The weight of coir dust accounts for approximately 35% of the total weight of coconut husk. Coconut coir is comprised of cellulose, lignin, pectin, and hemicellulose. The presence of hydroxyl groups in cellulose and lignin facilitates the adsorption of dyes [72]. Bio chars produced from coconut coir have enhanced dye adsorption capabilities due to their significantly higher specific surface area [73]. The research focuses on investigating the efficacy of coconut shell-activated carbon as a means of removing direct yellow DY-12 dyes. The study demonstrates that the adsorption process is particularly effective under acidic pH conditions. The findings of the study indicate that the process of adsorption exhibits heterogeneity, characterized by the formation of many layers. Furthermore, the adsorption process was seen to be endothermic in nature and occurred spontaneously [64]. Once tea has been prepared, the residual leaves are classified as waste, similar to other forms of biomass. The abundant availability of this waste has led to the increased interest in utilizing discarded tea leaves as an adsorbent [74]. Given the abundance and easy accessibility of this trash, its conversion into an adsorbent is economically viable, offering the added benefit of waste management. The utilization of raw tea waste, as well as its chemically and magnetically modified forms, in conjunction with activated carbon, has been widely employed for the remediation of water contaminated with dyes. In this study, a batch scale reactor was utilized to manufacture and apply tea powder for the purpose of removing MB from an aqueous solution. The effectiveness of adsorption was seen to improve with longer contact time, higher solution pH values, and increasing dose of waste black tea powder [75]. The residual tea waste possesses a significant calorific value, making it suitable for utilization in steam generation within the textile sector following appropriate saturation [76].
The different form of chitosan (i.e., nanoparticles, derivatives, nanofilms, and nanofibers) is employed as a bio adsorbent. This application aims to substitute activated carbon in the pre-treatment of textile effluent, with a specific focus on the removal of metal ions, particularly chromium, as well as colors. The ability to repeatedly utilize these bio adsorbents with diluted NaOH while maintaining the same level of efficacy is noted, rendering it an intriguing aspect [77]. Cactus juice and aloe vera juice were employed as flocculants for the treatment of textile effluent [78]. The color removal efficiency achieved above 85%. Furthermore, the removal efficiencies for total solids, suspended particles, and dissolved solids were found to be 90% [79]. The efficacy of water chestnut peel in the removal of cationic RhB shows promising results [80].
Various plant-based waste materials and biomasses have been found to have significant efficacy in the adsorption and retention of dyes. The primary constituents of plant leaves encompass cellulose, hemicellulose, pectins, and lignin, and additionally it contains many functional groups, such as carboxyl, hydroxyl, carbonyl, amino, and nitro, which can interact with the functional groups of the dyes [81]. The adsorption of Acid Orange 52 (AO-52) dye using Paulownia tomentosa Steud leaves biomass showed promising results [82]. In a separate investigation, the adsorption of Acid Red 27 (AR-27), an anionic dye, was examined utilizing hyacinth leaves [83]. Basic Red 46 (BR-46) dye exhibited strong affinity towards pine tree leaf-based adsorbents [84]. Ashoka leaf powder exhibited interactive behavior towards rhodamine B (RhB), Malachite Green, and Brilliant Green dyes [85]. A novel lignocellulosic biosorbent material, obtained from fully developed leaves of the sour cherry plant (Prunus cerasus L.), has remarkable efficacy in the removal of Methylene Blue and crystal violet dyes [86]. The coffee waste demonstrates a characteristic three-dimensional carbon structure, with a rough surface and a porous system that allows it to function as a promising adsorbent for the removal of anionic CR and RB5 dyes from aqueous solutions [87]. The experimental findings indicate that the utilization of powdered lemon leaves resulted in the removal of Malachite Green up to a maximum efficiency of 82.21%. The highest sorption capacity (qmax) of lemon leaf powders is 8.08 mg/g [88]. In another study, the cationic amino modified banana leaves show the excellent sorption for Congo Red (CR) dyes [89].
4.2. Dye Removal by Biological Methods
Although it is true that certain microorganisms can degrade auxochromes and chromophores found in dyes, hence facilitating the removal of organic materials from textile waste, it is worth noting that some of these microorganisms are also capable of mineralizing colors into carbon dioxide and water (see Figure 3). The rationality of color removal in biological processes, even conventional ones, has not been empirically shown Figure 3. The rate of removal is contingent upon several factors, including the concentration of O2, the ratio of organic load to microorganism load and dye load, and the temperature range [90][91].
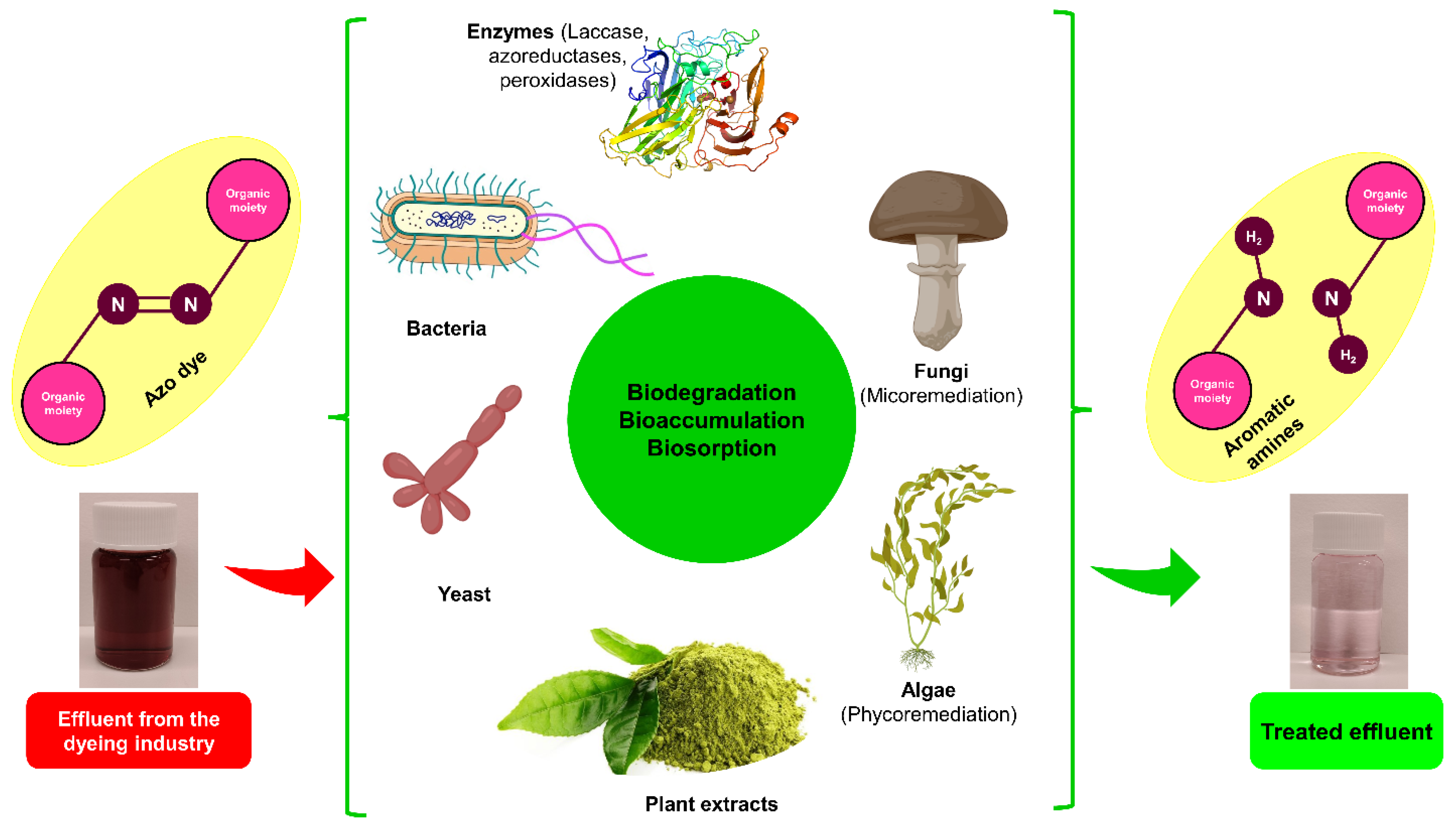
Figure 3. Treatment of textile wastewater by biological and biosorption methods.
4.3. Membrane Separation
Membrane separation technology is commonly employed for the treatment of effluents generated by textile dyeing processes. During the filtering process, the micropores included in the membrane filter effectively separate the organic compounds from the effluent by utilizing selective membrane permeability. The classification of this phenomenon encompasses four distinct categories, namely ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RO), and forward osmosis. The process of separation may be effectively achieved by the utilization of UF, which has shown great potential as a technique. The elimination of dissolved compounds occurs at a reduced transmembrane pressure through the utilization of UF. The utilization of polyelectrolyte complexes, in conjunction with cellulose acetate and inert polymers, is applied in the production of UF membranes that exhibit the capacity to efficiently regulate flow. The normal range for pore size is between 0.001 and 0.02 μm. NF is an intermediate technique between reverse osmosis and ultrafiltration, characterized by the use of membranes with nanometer-scale pores (0.5–10 nm) and operating at pressures of 5–40 bar. NF is a very sophisticated membrane-based technique that demonstrates remarkable efficacy in the removal of heavy metals [92][93][94]. The membranes of NF possess a thin outer layer that is typically non-porous, operating at the nanoscale, and exhibiting a high level of permeability [95]. One of the primary benefits of NF is its reduced energy consumption, which leads to a higher efficiency in the removal of contaminants [96]. Presently, several textile industries employ RO as a means of treating their effluent. RO is categorized as a membrane-based technique. The RO membranes effectively capture suspended particles through their small pores, hence mitigating fouling. The pre-treatment procedure plays a crucial role in the regulation of turbidity levels and fouling tendencies [95]. Figure 4 illustrates the classification of membrane filtering techniques together with their respective advantages and disadvantages.
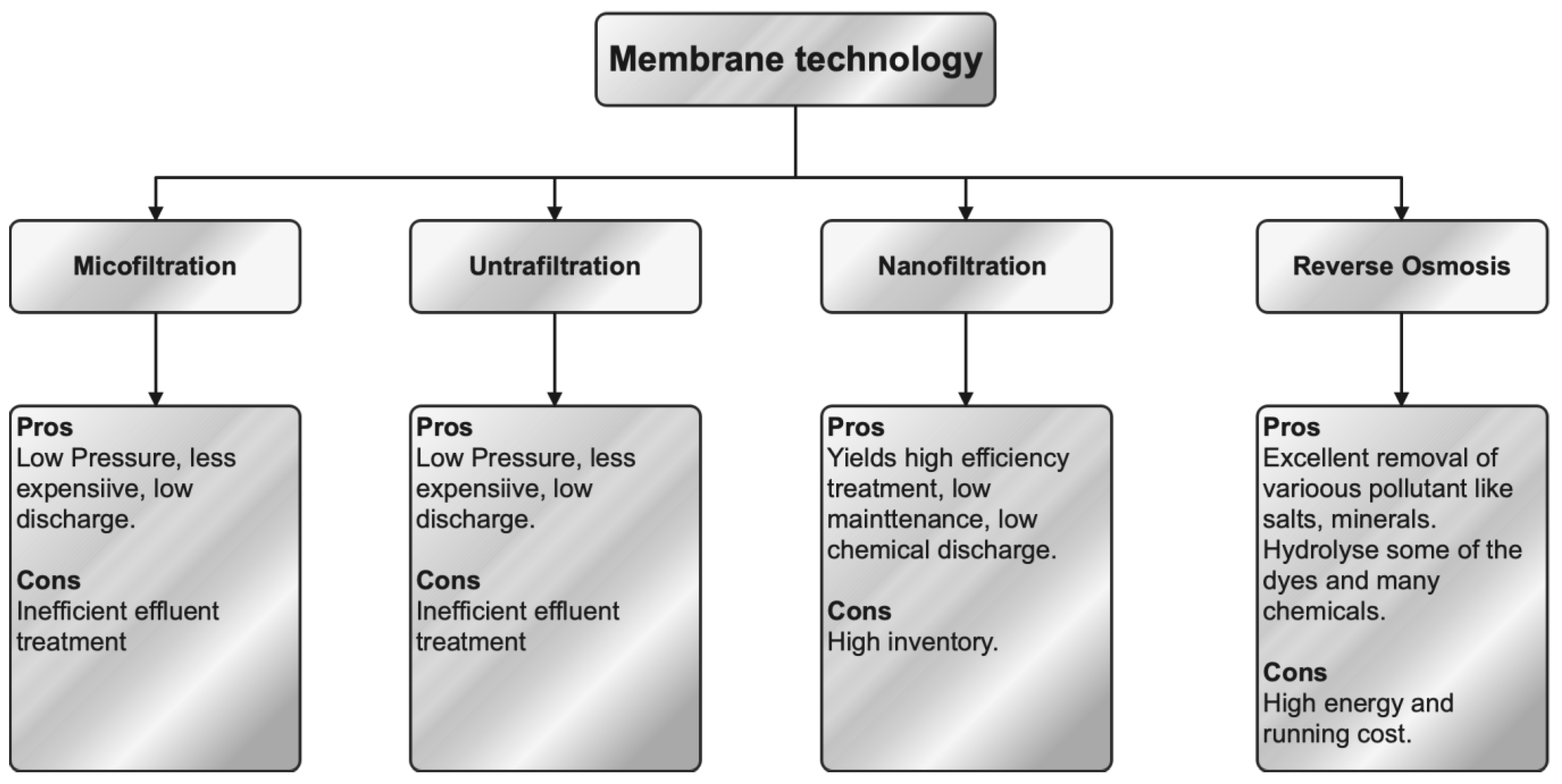
Figure 4. Classification, advantages, and disadvantages of membrane technology.
4.4. Other Techniques
4.4.1. Granular Activated Carbon (GAC)
Carbon is a non-metallic element that is abundantly present in nature and finds extensive use across many applications in daily human existence. Graphite has a wide range of applications, including as a source of fuel, lubrication, material for pencils, electrodes, and as a means of water filtration [97]. Activated carbon refers to a kind of carbon that has been specifically engineered to possess small, low-volume holes and a significantly increased surface area. This enhanced surface area facilitates the process of adsorption or chemical reactions, hence enabling the purification of both liquids and gases. GAC refers to a specific form of carbon that is capable of being retained in a 50-mesh sieve [98][99][100]. This type of carbon can be obtained from various sources by different extraction procedures and with varying degrees of activation. The substance is offered in many forms, including granules, powder, and pellets. Activated carbon is commonly derived from many sources such as coconut shell, hard and soft wood, peat, olive pits, lignite, and bituminous coal using chemical or steam-based processes. The activated carbon has a surface area of 500 m2/g, indicating its porous characteristics. Various studies have been conducted utilizing a range of biomass materials such as bagasse, coal, rice husk, coconut husk, nutshell, lemongrass, sawdust, cocoa shells, grape peels, and cassava peels. These biomass materials have been subjected to activation processes involving ZnCl2, phosphoric acid, microwave assistance, microwave assistance combined with KOH activation, and steam pressure. The objective of these studies is to investigate the efficacy of these activated biomass materials in the removal of dye from effluent water [98][99][100][101].
4.4.2. The Advanced Oxidation Process (AOP)
The AOP is mostly observed in the field of water purification, but more recently, it has been employed for the remediation of textile effluents. Hydroxyl or sulphate radicals are liberated in sufficient amounts to facilitate the elimination of both organic and inorganic substances, pollutants, and to enhance the water’s biodegradability. In comparison to chlorine and ozone, these substances exhibit superior performance in terms of water decontamination and disinfection. Various categories utilize the hydroxyl radical. Various methods have been employed in the field of environmental remediation, including UV-based processes, ozone treatment, Fenton reactions, and the utilization of sulphate radicals, among others, additionally UV has advantages for disinfection properties. The AOP is well recognized as a prominent technique for the treatment of industrial wastewater, owing to the considerable oxidative potential shown by ozone and the resulting generation of hydroxyl radicals (OH) [102]. Extensive research has been conducted on the application of ozone-based AOPs in both simulated and actual environmental circumstances. The use of auxiliary agents in the dye and their impact on dye degradation, as well as the influence of different salts on the process of ozonation, were investigated through the application of the AOP [103]. The AOP has gained significant popularity in the field of leachate treatment and water reuse [104]. There exist several forms of AOPs, including ozone, ozone/hydrogen peroxide, ozone/UV, UV/TiO2, UV/hydrogen peroxide, Fenton reactions, Photo-Fenton reactions, ultrasonic irradiation, heat/persulfate, UV/persulfate, Fe(II)/persulfate, and OH-/persulfate [105].
4.4.3. Color Removal by Fenton Oxidation
The Fenton oxidation method is a very promising technique for the treatment of textile wastewater due to its cost-effectiveness and ease of implementation [106]. The major objective of Fenton oxidation is the decolorization of the effluent, although it also possesses the ability to degrade organic pollutants. Hydrogen peroxide can be employed as an oxidizing agent, either in the presence or absence of a catalyst. Notable catalysts that can be utilized include ferrous salts, Al3+, and Cu2+ [107]. The efficacy of Fenton’s reagent has been demonstrated in the treatment of many types of industrial effluent as well as a wide range of dyes. The Fenton process demonstrates a high level of effectiveness in removing color, with an efficiency of 98% achieved at a pH of 3. Similarly, the Fenton process exhibits a significant capability for removing COD, with an efficiency of 85% achieved at a pH of 3 [107][108]. The most efficient decolorization of effluent for all dyestuffs occurs at a pH value of 3, within the range of 2.5–4. The utilization of this reduced value is attributed to the substantial production of OH [108]. When H2O2 and (Fe2+) are combined under these specific pH conditions, hydroxide ions (OH−) are generated by a complicated series of interconnected reactions [108][109].
4.4.4. Color Removal by Peroxide (H2O2)
Hydrogen peroxide has a high degree of efficiency and contains the OH− radical, which is accountable for both the chemical breakdown and mineralization of organic molecules, and is generated by the reaction involving another oxidant, H2O2. Furthermore, treatment of halogenated substances results in the generation of non-hazardous halide ions and non-toxic molecules, including carbon dioxide (CO2) and H2O [110]. A notable observation is that the efficiency of H2O2 addition in a recirculated photoreactor is significantly higher when performed in a single-step manner, as opposed to multiple-step addition [110]. Due to its short lifespan, the generation of OH− occurs in situ by the reaction induced by UV irradiation, as follows,
H2O2 + UV = 2 OH−
The breakdown of organic pollutants is facilitated by the OH− radical through four primary routes, radical addition, hydrogen abstraction, electron transfer, and radical combination [111][112]. The application of H2O2-UV results in the degradation of the chromophore configuration of the dye, leading to its decomposition in normal environmental circumstances. This process generates O2, which may be effectively utilized for aerobic treatment [111]. The effectiveness of wastewater decolorization is enhanced in an acidic environment [111][112]. H2O2 is utilized in the oxidation of alkali, resulting in the formation of O2 and H2O. This process generates accessible hydrogen peroxide for the hydroxyl radical (OH−). The reduction in radical production leads to a decrease in decolorization efficiency [111].
4.4.5. Ozonation
Ozonation is considered as an environmentally sustainable method for treating wastewater owing to its lack of residue production and absence of chlorinated byproducts, which are known to be toxic. This process effectively oxidizes color, odor, and bacteria without generating any detrimental substances [113][114][115]. The decomposition of organic compounds, detergents, and phenols into smaller molecular components is aided by the process of oxidation, which may be achieved using commercially available sodium hypochlorite. As a potential alternative, the implementation of ozonation might be regarded as a feasible solution to supplant the utilization of hypochlorite [116][117][118][119][120]. Typically, the process is conducted at alkaline conditions, characterized by a pH greater than 9, as the degradation of ozone in water is enhanced under such circumstances. The process of oxidizing inorganic compounds and dissolved organic molecules with ozone involves two distinct processes. The direct reaction of ozone molecules exhibits a higher degree of selectivity, characterized by a relatively slow reaction rate. This reaction is particularly advantageous in acidic conditions. The indirect response exhibited by free radicals, including OH− and HOO, is characterized by reduced selectivity and a preference for basic conditions. Another noteworthy characteristic is that the reactivity of the dye is enhanced when it possesses an electron-donating group at its ortho and para locations, as opposed to an electron-withdrawing group [30][121][122]. The ozonation process is impeded by the presence of salts, such as NaCl or Na2SO4. However, it is worth noting that the presence of NaCl is more undesirable compared to Na2SO4. This is since Na2SO4 generates sulfate or peroxysulfate radicals, which might somewhat facilitate the ozonation process [114]. The use of an ozonation membrane biological reactor offers a means to enhance the removal of harmful substances such as pesticides, while simultaneously reducing the reliance on traditional coagulation methods. This innovative approach also lowers the need for additional biological treatment, resulting in a simplified operational procedure [123][124].
4.4.6. Photocatalytic Oxidation
The photocatalytic process is often regarded as the predominant method for treating textile effluent water due to its distinct advantage of optical absorption, a characteristic not shared by other AOPs. Photocatalysts could be stimulated by radiation, resulting in the generation of exceptionally reactive photo-induced charge carriers (free radicals, notably hydroxyl radicals (OH·) that engage in chemical reactions with contaminants [125][126][127]. These free radicals facilitate the oxidation of organic molecules, leading to their full conversion into non-toxic chemicals, such as carbon dioxide CO2 and H2O by the absorption of photons. The mitigation of pollutants carried out under ambient temperature and pressure conditions has the potential to successfully address the issue of excessive energy consumption associated with conventional approaches, such as electrochemical technology. The systematic exploration of photocatalysis research started in the early 1970s.
4.4.7. The Sequencing Batch Reactor (SBR)
The implementation of the Sequencing Batch Reactor (SBR) was undertaken with the objective of mitigating the presence of nitrogen and phosphorus in piggery waste, as well as facilitating the biodegradation of sulfonated azo and diazo reactive colors found in textile effluent [128][129][130]. The study has presented findings on the effectiveness of SBR in the elimination of azo dye. It has been proposed that the inclusion of aerobic bacteria capable of degrading amines in the SBR system might enhance the complete mineralization of reactive azo dye under anaerobic conditions [129][131].
4.5. Treatment of Dyes Using Hybrid Technologies
In recent times, there has been a growing interest in hybridized techniques. The importance of a process that combines elements from a blend process measure may be described as “synergistic” and “combinatorial” in nature. These solutions are characterized by their efficiency since they include the utilization of a single container to execute several tasks. The technique shown in Figure 5 is an integrated or blended approach for the treatment of dye waste. Furthermore, it is important to clearly articulate and acknowledge significant modifications in the advantages of hybrid methodologies.
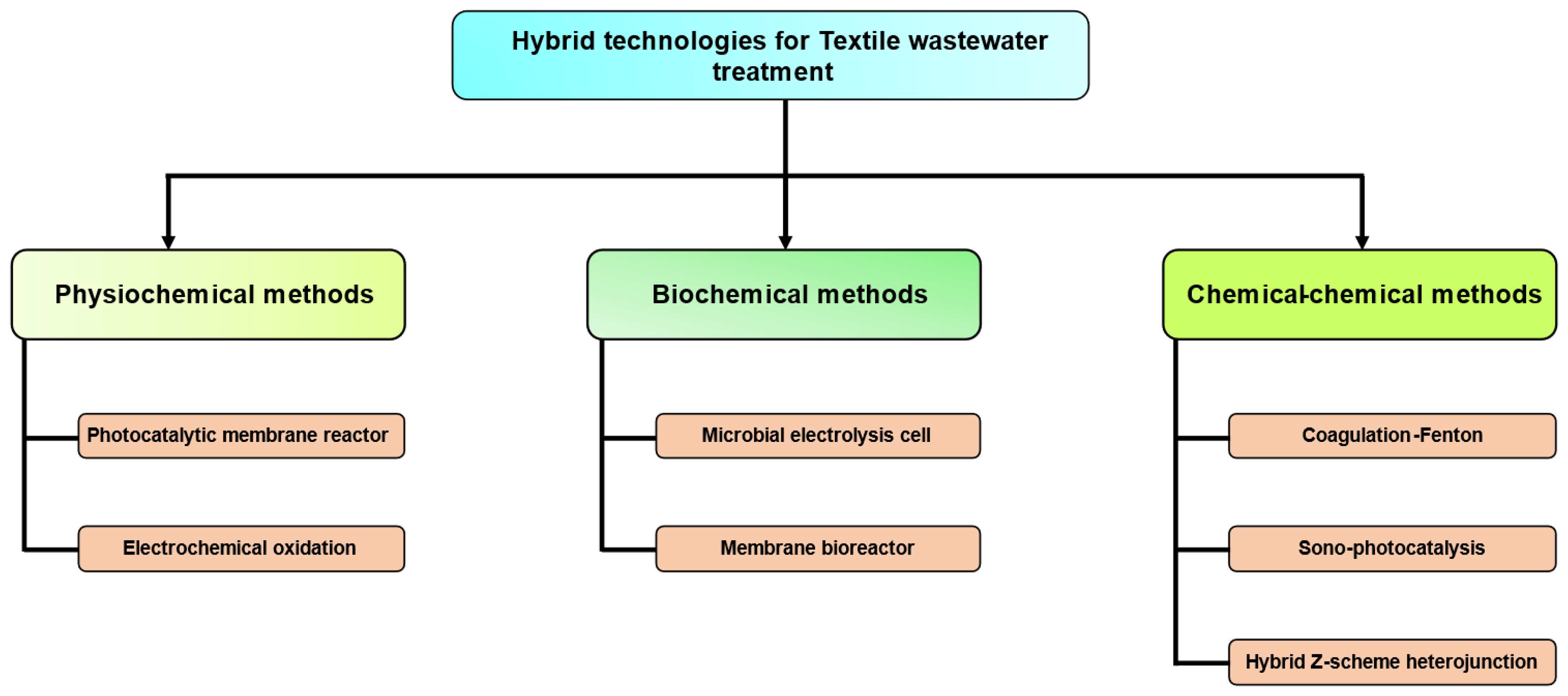
Figure 5. Treatment of dyes using hybrid technologies.
4.6. Sustainable Sludge Management
Sludge refers to the leftover, semi-solid substance that remains after the treatment of wastewater generated from textile processes. During the physical-chemical treatment process, the release of heavy metal concentration results in the formation of a chemical sludge. In contrast, impoverished soils might experience an additional advantage through the application of nutrient-rich biological sludge, which contains nitrogen and phosphorus, as well as useful organic matter. The primary issue is in the expeditious and forceful way sludge contaminates water sources. However, it is worth noting that certain locations within developing nations continue to dispose of sludge in inappropriate and environmentally unfriendly ways, such as through land disposal or by releasing it into the sea. To attain sustainable development, it is imperative to employ efficient recycling methods and utilize waste materials properly, rather than resorting to burning or landfilling, which can result in the deposition of hazardous substances such as heavy metals. Practicing prudent management is crucial when it comes to the disposal of sludge, notwithstanding the inherent challenges involved. Various elements play a significant role in the management of sludge, including local and national geographical considerations, agronomic issues, economic aspects, and stakeholder perception [132][133].
Methods of Sludge Treatment
Anaerobic digestion refers to the process of decomposing sludge in an atmosphere devoid of oxygen. The significant characteristics of anaerobic digestion are the reduction in mass, formation of methane, and enhancement of dewatering qualities in the fermented sludge [132][133][134][135][136][137]. A higher level of investment in the digesting chamber is associated with a slower pace of deterioration. To enhance the biodegradability of sludge, several pre-treatment methods may be employed. These include thermal pre-treatment, enzymatic treatment, ozonation, chemical solubilization by acidification or alkaline hydrolysis, as well as mechanical sludge disintegration and ultrasonic pre-treatment [138][139]. The hypothesis posits that the process of anaerobic digestion of textile waste leads to the generation of biogas, as evidenced by the works [140][141][142]. This relationship is depicted in Figure 6.
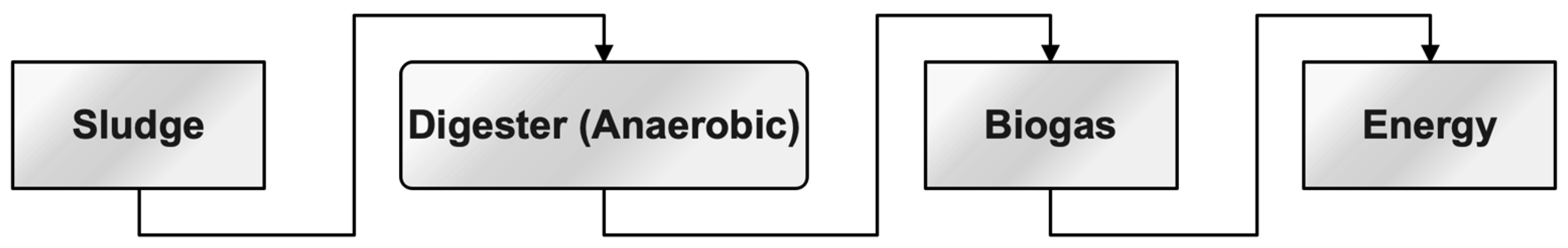
Figure 6. The sequence process for energy conversion from the sludge.
Aerobic digestion refers to the utilization of microorganisms within an oxygen-rich environment to facilitate the oxidation and decomposition of organic matter sludge. Aerobic sludge digestion is a procedure employed to decrease the levels of organic and inorganic constituents, as well as the overall volume, of sludge. The process under consideration exhibits temperature sensitivity and is susceptible to the presence of heavy metals, among other factors. However, it is noteworthy that despite its significant energy requirements, this process does not generate byproducts such as methane [143][144][145]. The pace of anaerobic digestion is constrained by the hydrolysis of organic materials in sludge, which subsequently leads to an increase in biogas output. This process serves as a pre-treatment technique that enhances the dewatering characteristics of the digested sludge [146]. The process of stabilizing sludge solids involves the application of several chemical treatments to the sludge in diverse manners. The application of polyelectrolytes as a conditioning agent for sludge dewatering operations has gained popularity due to its ability to enhance process yields [146][147][148].
4.7. Roadmap towards ZLD: Focus on Recovery and Reuse
Due to heightened environmental consciousness, escalating expenses related to wastewater treatment, and challenges pertaining to its disposal, there has been a perceptible shift in the public’s perspective on wastewater. Presently, zero liquid discharge (ZLD) is emerging as a prospective preventative measure that plays a significant role in safeguarding the environment against the adverse impacts of industrial activity. The ZLD method in the textile industry focuses on achieving the goal of eliminating any disposal of liquid waste resulting from various waste-generating processes [149][150]. It is transitioning from being perceived as a nuisance that is conveniently ignored to being recognized as a potential avenue for the reclamation of precious resources. This phenomenon is manifesting as a direct consequence of the confluence of these variables. Figure 7 illustrates a graphical depiction of the fundamental factors that drive ZLD, along with its numerous beneficial results.
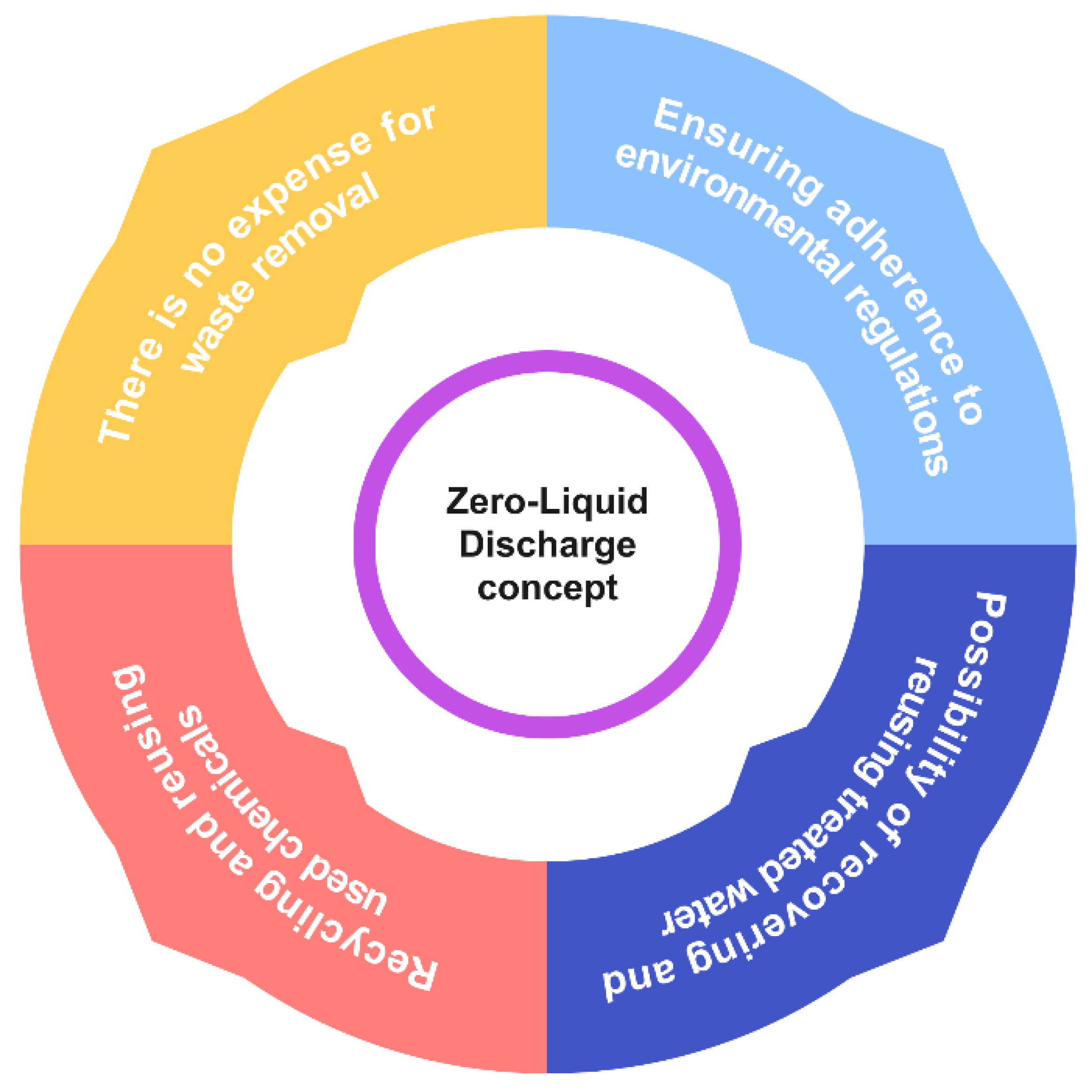
Figure 7. Benefits of the ZLD concept.
The practice of reusing and recycling wastewater has the potential to not only mitigate the demand for freshwater resources, but also facilitate the reduction in waste and surplus resources. The textile-processing sector is known for its significant use of colorants, chemicals, and other additives. Consequently, it has significant promise for several approaches to intense chemical recovery and water recycling. The process of recycling has become essential to the industrial sector due to the imposition of constraints on accessible water sources and regulations managing wastewater. Prior to commencing the treatment procedure, all potential avenues for recovery and recycling have been thoroughly explored and utilized to their fullest extent.
4.8. The Need for Technoeconomic Analysis
The discharge of effluent originating from the textile sector is resulting in substantial contamination due to the extensive use of colorants and hazardous chemicals, which are the primary contributors to water pollution and the escalating ecological risks. The escalating expenses associated with dye treatment facilities and the management of waste effluents are generating heightened public concern for environmental sustainability. The effective management and recovery of dyes and other chemical substances can contribute to the attainment of environmental sustainability and foster economic advancement. Furthermore, the substantial consumption of water underscores the imperative to engage in wastewater recycling. This has the potential to reduce the release of harmful compounds and create an atmosphere that is both safe and conducive to good health. The wastewaters under consideration consist of colors that include harmful and hazardous substances, such as pesticides, surfactants, and heavy metals. The cost of wastewater treatment often varies based on factors such as the specific treatment procedure employed, the spatial demands of the equipment, and the catalysts necessary for effectively removing contaminants. Furthermore, the operating expenses of the system encompass both personnel costs and maintenance expenditures. However, the prevailing price is contingent upon the region’s authoritative needs, the availability of feedstock, and the labor force [151][152]. However, it should be noted that relying only on a single treatment procedure may not be a viable approach for effectively degrading extremely contaminated dyes. This is mostly due to the development of intermediates during the treatment, which afterwards necessitate an additional treatment step. Consequently, this supplementary treatment incurs additional costs, thereby impacting the overall feasibility of the process. Hence, the implementation of a plan that combines several approaches will be crucial in addressing efficiency. However, these procedures include the integration of two treatment approaches and offer greater advantages in terms of pollutant degradation.
4.9. Life Cycle Assessment (LCA) in WWTPs
A life cycle assessment (LCA) is a comprehensive approach used to evaluate the total environmental impact of a product or process across its entire life cycle, from raw material extraction to disposal [153][154][155][156]. The primary goal of the LCA is to assess the whole range of environmental impacts resulting from the operation of the textile wastewater treatment facility. It is often essential to define the precise limits of the LCA investigation. The “Gate-to-Gate” methodology is a frequently used strategy in which different stages of the process are designated as gates. For example, we may classify the industry that emits pollutants as the first gate, and the release of cleaned effluent as the second gate. Furthermore, the analysis may also include the use of treated effluent in the textile sector. Moreover, it is crucial to establish a precise demarcation of the extent of the LCA studies carried out on wastewater treatment facilities (WWTPs). This encompasses delineating the parameters of the study, establishing the operational components, summarizing the LCA approach utilized, showcasing the life cycle inventory (LCI) information, and acknowledging the constraints, presumptions, and uncertainties linked to the examination.
LCA serves as a beneficial quantitative ecological assessment approach for examining various prospective functioning scenarios in the context of crucial water area planning. One of the advantages of LCA is in its ability to detect and quantify the impacts and influences of different process sequences, as well as assess the environmental effects of treatment technologies. LCA also facilitates the examination of pollution connections and aids in the achievement of effluent-free product creation. The LCA methodology was employed to conduct a comparative analysis of synthetic colors and natural colors, as well as synthetic finishes and biobased finishes, from an environmental perspective. Additionally, the study investigated the potential impacts of these materials on WWTPs. LCA offers decision makers and policy makers a consistent and transparent means to understand and interpret the ecological performance data of WWTPs in the textile sector. Hence, the utilization of LCA may facilitate the identification of research and development goals and provide guidance for enhancing innovation by mitigating challenges related to waste disposal and the discharge of hazardous chemicals. Figure 8 depicts the typical LCA framework on the WWTPs for the textile industry.
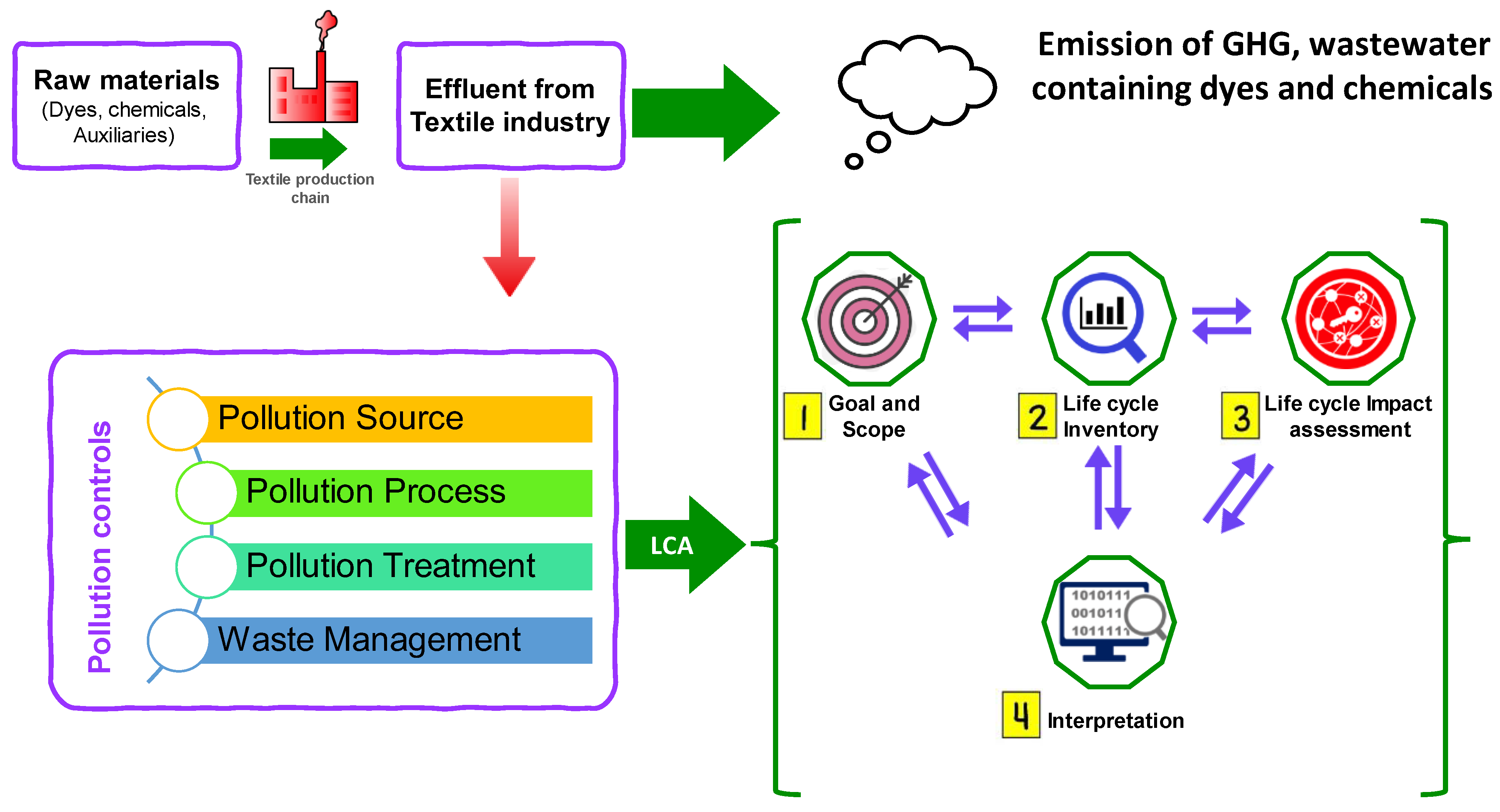
Figure 8. Life cycle assessment (LCA) framework for the wastewater treatment plants.
References
- Rayhan Sarker, M.; Mithun Ali, S.; Kumar Paul, S.; Haque Munim, Z. Measuring Sustainability Performance Using an Integrated Model. Measurement 2021, 184, 109931.
- Szilagyi, A.; Mocan, M.; Verniquet, A.; Churican, A.; Rochat, D. Eco-Innovation, a Business Approach towards Sustainable Processes, Products and Services. Procedia Soc. Behav. Sci. 2018, 238, 475–484.
- Crystal Gordon, Water: The Prime Essence Of Life. 2019. Available online: Https://Hubpages.Com/Education/Water-Is-the-Essence-of-Life-Itself (accessed on 20 September 2023).
- Ajmal, M.; Siddiq, M.; Aktas, N.; Sahiner, N. Magnetic Co–Fe Bimetallic Nanoparticle Containing Modifiable Microgels for the Removal of Heavy Metal Ions, Organic Dyes and Herbicides from Aqueous Media. RSC Adv. 2015, 5, 43873–43884.
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136.
- Gahlot, R.; Taki, K.; Kumar, M. Efficacy of Nanoclays as the Potential Adsorbent for Dyes and Metal Removal from the Wastewater: A Review. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100339.
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent Advances in New Generation Dye Removal Technologies: Novel Search for Approaches to Reprocess Wastewater. RSC Adv. 2015, 5, 30801–30818.
- Owa, F.D. Water Pollution: Sources, Effects, Control and Management. Mediterr. J. Soc. Sci. 2013, 4, 65–68.
- Ahmed, M.J.; Dhedan, S.K. Equilibrium Isotherms and Kinetics Modeling of Methylene Blue Adsorption on Agricultural Wastes-Based Activated Carbons. Fluid. Phase Equilib. 2012, 317, 9–14.
- Mohamed, A. Hassaan; Ahmed El Nemr Health and Environmental Impacts of Dyes: Mini Review. Am. J. Environ. Sci. Eng. 2017, 1, 64–67.
- Ahmad, A.A.; Hameed, B.H.; Aziz, N. Adsorption of Direct Dye on Palm Ash: Kinetic and Equilibrium Modeling. J. Hazard. Mater. 2007, 141, 70–76.
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological Methods for Textile Dye Removal from Wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876.
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water Air Soil. Pollut. 2018, 229, 225.
- Shukla, S.R. Pollution Abatement and Waste Minimisation in Textile Dyeing. In Environmental Aspects of Textile Dyeing; Christie, R.M., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2007; pp. 116–148. ISBN 9781845691158.
- Reddy, N.; Chen, L.; Zhang, Y.; Yang, Y. Reducing Environmental Pollution of the Textile Industry Using Keratin as Alternative Sizing Agent to Poly(Vinyl Alcohol). J. Clean. Prod. 2014, 65, 561–567.
- Hasanbeigi, A.; Price, L. A Technical Review of Emerging Technologies for Energy and Water Efficiency and Pollution Reduction in the Textile Industry. J. Clean. Prod. 2015, 95, 30–44.
- Periyasamy, A.P.; Tehrani-Bagha, A. A Review on Microplastic Emission from Textile Materials and Its Reduction Techniques. Polym. Degrad. Stab. 2022, 199, 109901.
- Klepacz-Smółka, A.; Sójka-Ledakowicz, J.; Paździor, K.; Ledakowicz, S. Application of Anoxic Fixed Film and Aerobic CSTR Bioreactor in Treatment of Nanofiltration Concentrate of Real Textile Wastewater. Chem. Pap. 2010, 64, 230–236.
- Karunakaran, G.; Periyasamy, A.P.; Militký, J. Color and Design for Textiles. In Fibrous Structures and Their Impact on Textile Design; Springer Nature: Singapore, 2023; pp. 119–148.
- Militký, J.; Venkataraman, M.; Periyasamy, A.P. (Eds.) Fibrous Structures and Their Impact on Textile Design; Springer Nature: Singapore, 2023; ISBN 978-981-19-4826-8.
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Adsorption of Dyes Using Different Types of Clay: A Review. Appl. Water Sci. 2017, 7, 543–568.
- Chisvert, A.; Miralles, P.; Salvador, A. Hair Dyes in Cosmetics. In Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2018; pp. 159–173.
- Singh, K. Rajani Srivastava an Overview of Textile Dyes and Their Removaltechniques: Indian Perspective. Polution Res. Pap. 2017, 36, 790–797.
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of Dyes in Textile Effluent: A Critical Review on Current Treatment Technologies with a Proposed Alternative. Bioresour. Technol. 2001, 77, 247–255.
- Mohd Adnan, M.A.; Muhd Julkapli, N.; Amir, M.N.I.; Maamor, A. Effect on Different TiO2 Photocatalyst Supports on Photodecolorization of Synthetic Dyes: A Review. Int. J. Environ. Sci. Technol. 2019, 16, 547–566.
- Samarghandi, M.R.; Hadi, M.; Moayedi, S.; Barjesteh, A.F. Hadi Wo-Parameter Isotherms of Methyl Orange Sorption by Pinecone Derived Activated Carbon. Iran. J. Environ. Health Sci. Eng. 2009, 6, 285–294.
- Raval, N.P.; Shah, P.U.; Shah, N.K. Malachite Green “a Cationic Dye” and Its Removal from Aqueous Solution by Adsorption. Appl. Water Sci. 2017, 7, 3407–3445.
- Roy, S.; Darabdhara, J.; Ahmaruzzaman, M. Recent Advances of Copper- BTC Metal-Organic Frameworks for Efficient Degradation of Organic Dye-Polluted Wastewater: Synthesis, Mechanistic Insights and Future Outlook. J. Hazard. Mater. Lett. 2024, 5, 100094.
- Periyasamy, A.P. Microfiber Emissions from Functionalized Textiles: Potential Threat for Human Health and Environmental Risks. Toxics 2023, 11, 406.
- Periyasamy, A.P.; Militky, J. Sustainability in Textile Dyeing: Recent Developments. In Sustainability in the Textile and Apparel Industries; Springer Nature: Cham, Switzerland, 2020; pp. 37–79.
- Sachidhanandham, A.; Periyasamy, A.P. Environmentally Friendly Wastewater Treatment Methods for the Textile Industry. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Springer International Publishing: Cham, Switzerland, 2021; pp. 2269–2307.
- Periyasamy, A.P.; Rwahwire, S.; Zhao, Y. Environmental Friendly Textile Processing. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–38.
- Periyasamy, A.P.; Ramamoorthy, S.K.; Lavate, S.S. Eco-Friendly Denim Processing. In Handbook of Ecomaterials; Springer: Cham, Switzerland, 2019; Volume 3, pp. 1559–1579. ISBN 9783319682556.
- Raman, C.D.; Mkandawire, M. GIS Based Spatial and Temporal Investigation of Groundwater and Soil Quality along Noyyal River, Tiruppur, India. J. Geol. Soc. India 2021, 97, 1097–1104.
- Ram, S.; Dineshkumar, R.; Pancha, I.; Mishra, S. Prospects and Potential Role of Biological Treatment of Textile Effluent to Restore Water Reservoir. In Cost-efficient Wastewater Treatment Technologies; The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2023; Volume 118, pp. 199–212.
- Indian Textile and Apparel Industry Expected to Reach US $344.1 Billion by 2027. Available online: https://in.apparelresources.com/business-news/trade/indian-textile-apparel-industry-expected-reach-us-344-1-billion-2027/ (accessed on 26 August 2022).
- Periyasamy, A.P.; Ramamoorthy, S.K.; Rwawiire, S.; Zhao, Y. Sustainable Wastewater Treatment Methods for Textile Industry. In Sustainable Innovations in Apparel Production; Springer: Singapore, 2018; pp. 21–87.
- Mehra, S.; Singh, M.; Chadha, P. Adverse Impact of Textile Dyes on the Aquatic Environment as Well as on Human Beings. Toxicol. Int. 2021, 28, 165–176.
- Ali, A.E.; Chowdhury, Z.Z.; Devnath, R.; Ahmed, M.M.; Rahman, M.M.; Khalid, K.; Wahab, Y.A.; Badruddin, I.A.; Kamangar, S.; Hussien, M.; et al. Removal of Azo Dyes from Aqueous Effluent Using Bio-Based Activated Carbons: Toxicity Aspects and Environmental Impact. Separations 2023, 10, 506.
- Periyasamy, A.P.; Militky, J. Denim Processing and Health Hazards. In Sustainability in Denim; Elsevier: Amsterdam, The Netherlands, 2017; pp. 161–196.
- Pattnaik, P.; Dangayach, G.S.; Bhardwaj, A.K. A Review on the Sustainability of Textile Industries Wastewater with and without Treatment Methodologies. Rev. Environ. Health 2018, 33, 163–203.
- Bharagava, R.N.; Chowdhary, P. Emerging and Eco-Friendly Approaches for Waste Management. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Singapore, 2018; pp. 1–435.
- Motejadded Emrooz, H.B.; Maleki, M.; Rashidi, A.; Shokouhimehr, M. Adsorption Mechanism of a Cationic Dye on a Biomass-Derived Micro- and Mesoporous Carbon: Structural, Kinetic, and Equilibrium Insight. Biomass Convers. Biorefin 2021, 11, 943–954.
- Temesgen, F.; Gabbiye, N.; Sahu, O. Biosorption of Reactive Red Dye (RRD) on Activated Surface of Banana and Orange Peels: Economical Alternative for Textile Effluent. Surf. Interfaces 2018, 12, 151–159.
- Oyekanmi, A.A.; Ahmad, A.; Hossain, K.; Rafatullah, M. Adsorption of Rhodamine B Dye from Aqueous Solution onto Acid Treated Banana Peel: Response Surface Methodology, Kinetics and Isotherm Studies. PLoS ONE 2019, 14, e0216878.
- Harini, K.; Ramya, K.; Sukumar, M. Extraction of Nano Cellulose Fibers from the Banana Peel and Bract for Production of Acetyl and Lauroyl Cellulose. Carbohydr. Polym. 2018, 201, 329–339.
- Munagapati, V.S.; Yarramuthi, V.; Kim, Y.; Lee, K.M.; Kim, D.-S. Removal of Anionic Dyes (Reactive Black 5 and Congo Red) from Aqueous Solutions Using Banana Peel Powder as an Adsorbent. Ecotoxicol. Environ. Saf. 2018, 148, 601–607.
- Wekoye, J.N.; Wanyonyi, W.C.; Wangila, P.T.; Tonui, M.K. Kinetic and Equilibrium Studies of Congo Red Dye Adsorption on Cabbage Waste Powder. Environ. Chem. Ecotoxicol. 2020, 2, 24–31.
- Mashkoor, F.; Nasar, A. Facile Synthesis of Polypyrrole Decorated Chitosan-Based Magsorbent: Characterizations, Performance, and Applications in Removing Cationic and Anionic Dyes from Aqueous Medium. Int. J. Biol. Macromol. 2020, 161, 88–100.
- Zhao, F.; Yang, Z.; Wei, Z.; Spinney, R.; Sillanpää, M.; Tang, J.; Tam, M.; Xiao, R. Polyethylenimine-Modified Chitosan Materials for the Recovery of La(III) from Leachates of Bauxite Residue. Chem. Eng. J. 2020, 388, 124307.
- Subedi, N.; Lähde, A.; Abu-Danso, E.; Iqbal, J.; Bhatnagar, A. A Comparative Study of Magnetic Chitosan (Chi@Fe3O4) and Graphene Oxide Modified Magnetic Chitosan (Chi@Fe3O4GO) Nanocomposites for Efficient Removal of Cr(VI) from Water. Int. J. Biol. Macromol. 2019, 137, 948–959.
- Crini, G.; Torri, G.; Lichtfouse, E.; Kyzas, G.Z.; Wilson, L.D.; Morin-Crini, N. Dye Removal by Biosorption Using Cross-Linked Chitosan-Based Hydrogels. Environ. Chem. Lett. 2019, 17, 1645–1666.
- Shakoor, S.; Nasar, A. Removal of Methylene Blue Dye from Artificially Contaminated Water Using Citrus Limetta Peel Waste as a Very Low Cost Adsorbent. J. Taiwan. Inst. Chem. Eng. 2016, 66, 154–163.
- Mahato, N.; Sharma, K.; Sinha, M.; Baral, E.R.; Koteswararao, R.; Dhyani, A.; Hwan Cho, M.; Cho, S. Bio-Sorbents, Industrially Important Chemicals and Novel Materials from Citrus Processing Waste as a Sustainable and Renewable Bioresource: A Review; Cairo University: Giza, Egypt, 2020; Volume 23, ISBN 8201027988476.
- Tomar, V.; Prasad, S.; Kumar, D. Adsorptive Removal of Fluoride from Aqueous Media Using Citrus limonum (Lemon) Leaf. Microchem. J. 2014, 112, 97–103.
- Mohanraj, J.; Durgalakshmi, D.; Balakumar, S.; Aruna, P.; Ganesan, S.; Rajendran, S.; Naushad, M. Low Cost and Quick Time Absorption of Organic Dye Pollutants under Ambient Condition Using Partially Exfoliated Graphite. J. Water Process Eng. 2020, 34, 101078.
- Munagapati, V.S.; Kim, D.-S. Adsorption of Anionic Azo Dye Congo Red from Aqueous Solution by Cationic Modified Orange Peel Powder. J. Mol. Liq. 2016, 220, 540–548.
- Ahmed, M.; Mashkoor, F.; Nasar, A. Development, Characterization, and Utilization of Magnetized Orange Peel Waste as a Novel Adsorbent for the Confiscation of Crystal Violet Dye from Aqueous Solution. Groundw. Sustain. Dev. 2020, 10, 100322.
- Tahir, N.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Biopolymers Composites with Peanut Hull Waste Biomass and Application for Crystal Violet Adsorption. Int. J. Biol. Macromol. 2017, 94, 210–220.
- De Azevedo, A.C.N.; Vaz, M.G.; Gomes, R.F.; Pereira, A.G.B.; Fajardo, A.R.; Rodrigues, F.H.A. Starch/Rice Husk Ash Based Superabsorbent Composite: High Methylene Blue Removal Efficiency. Iran. Polym. J. 2017, 26, 93–105.
- Ashrafi, S.D.; Kamani, H.; Mahvi, A.H. The Optimization Study of Direct Red 81 and Methylene Blue Adsorption on NaOH-Modified Rice Husk. Desalination Water Treat. 2016, 57, 738–746.
- Shakoor, S.; Nasar, A. Adsorptive Decontamination of Synthetic Wastewater Containing Crystal Violet Dye by Employing Terminalia Arjuna Sawdust Waste. Groundw. Sustain. Dev. 2018, 7, 30–38.
- Tahir, H.; Sultan, M.; Akhtar, N.; Hameed, U.; Abid, T. Application of Natural and Modified Sugar Cane Bagasse for the Removal of Dye from Aqueous Solution. J. Saudi Chem. Soc. 2016, 20, S115–S121.
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and Equilibrium Study for the Adsorption of Textile Dyes on Coconut Shell Activated Carbon. Arab. J. Chem. 2017, 10, S3381–S3393.
- Gupta, S.A.; Vishesh, Y.; Sarvshrestha, N.; Bhardwaj, A.S.; Kumar, P.A.; Topare, N.S.; Raut-Jadhav, S.; Bokil, S.A.; Khan, A. Adsorption Isotherm Studies of Methylene Blue Using Activated Carbon of Waste Fruit Peel as an Adsorbent. Mater. Today Proc. 2022, 57, 1500–1508.
- Castillo, M.; de Guzman, M.J.K.; Aberilla, J.M. Environmental Sustainability Assessment of Banana Waste Utilization into Food Packaging and Liquid Fertilizer. Sustain. Prod. Consum. 2023, 37, 356–368.
- Farias, K.C.S.; Guimarães, R.C.A.; Oliveira, K.R.W.; Nazário, C.E.D.; Ferencz, J.A.P.; Wender, H. Banana Peel Powder Biosorbent for Removal of Hazardous Organic Pollutants from Wastewater. Toxics 2023, 11, 664.
- Khaleque, A.; Roy, D.K. Removing Reactive Dyes from Textile Effluent Using Banana Fibre. Int. J. Basic Appl. Sci. 2016, 16, 14–20.
- Das, E.; Rabha, S.; Talukdar, K.; Goswami, M.; Devi, A. Propensity of a Low-Cost Adsorbent Derived from Agricultural Wastes to Interact with Cationic Dyes in Aqueous Solutions. Environ. Monit. Assess. 2023, 195, 1044.
- Akbar, N.A.; Sabri, S.; Abu Bakar, A.A.; Azizan, N.S. Removal of Colour Using Banana Stem Adsorbent in Textile Wastewater. J. Phys. Conf. Ser. 2019, 1349, 12091.
- Karunakaran, G.; Periyasamy, A.P.; Tehrani, A. Extraction of Micro, Nanocrystalline Cellulose and Textile Fibers from Coffee Waste. J. Test. Eval. 2023, 51, 20220487.
- Malhotra, A.; Lokhande, R.S.; Sahu, R.; Jain, S.K.; Sharma, K.B.; Tripathi, B. Study on Adsorbent Characteristics of Coconut Coir as a Bio Sorbent for Removal of Methylene Blue Dye. Macromol. Symp. 2021, 399, 2100082.
- Le, P.T.; Bui, H.T.; Le, D.N.; Nguyen, T.H.; Pham, L.A.; Nguyen, H.N.; Nguyen, Q.S.; Nguyen, T.P.; Bich, N.T.; Duong, T.T.; et al. Preparation and Characterization of Biochar Derived from Agricultural By-Products for Dye Removal. Adsorpt. Sci. Technol. 2021, 2021, 9161904.
- Nasar, A. Utilization of Tea Wastes for the Removal of Toxic Dyes from Polluted Water—A Review. Biomass Convers. Biorefin 2023, 13, 1399–1415.
- Lin, D.; Wu, F.; Hu, Y.; Zhang, T.; Liu, C.; Hu, Q.; Hu, Y.; Xue, Z.; Han, H.; Ko, T.-H. Adsorption of Dye by Waste Black Tea Powder: Parameters, Kinetic, Equilibrium, and Thermodynamic Studies. J. Chem. 2020, 2020, 5431046.
- Jain, S.N.; Tamboli, S.R.; Sutar, D.S.; Jadhav, S.R.; Marathe, J.V.; Shaikh, A.A.; Prajapati, A.A. Batch and Continuous Studies for Adsorption of Anionic Dye onto Waste Tea Residue: Kinetic, Equilibrium, Breakthrough and Reusability Studies. J. Clean. Prod. 2020, 252, 119778.
- Kaisri, M.B. Application of Chitosan Derivatives as Promising Adsorbents for Treatment of Textile Wastewater; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081024911.
- Amari, A.; Alalwan, B.; Eldirderi, M.M.; Mnif, W.; Ben Rebah, F. Cactus Material-Based Adsorbents for the Removal of Heavy Metals and Dyes: A Review. Mater. Res. Express 2020, 7, 12002.
- Ingole, N.W. Colour Removal from Textile Effluent by Using Biomaterials—An Experimental Evaluation. J. Indian. Water Work. Assoc. 2020, LII.
- Khan, T.A.; Nazir, M.; Khan, E.A. Adsorptive Removal of Rhodamine B from Textile Wastewater Using Water Chestnut (Trapa natans L.) Peel: Adsorption Dynamics and Kinetic Studies. Toxicol. Environ. Chem. 2013, 95, 919–931.
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The Utilization of Leaf-Based Adsorbents for Dyes Removal: A Review. J. Mol. Liq. 2019, 276, 728–747.
- Deniz, F.; Saygideger, S.D. Equilibrium, Kinetic and Thermodynamic Studies of Acid Orange 52 Dye Biosorption by Paulownia Tomentosa Steud. Leaf Powder as a Low-Cost Natural Biosorbent. Bioresour. Technol. 2010, 101, 5137–5143.
- Guerrero-Coronilla, I.; Morales-Barrera, L.; Cristiani-Urbina, E. Kinetic, Isotherm and Thermodynamic Studies of Amaranth Dye Biosorption from Aqueous Solution onto Water Hyacinth Leaves. J. Environ. Manag. 2015, 152, 99–108.
- Deniz, F.; Karaman, S. Removal of Basic Red 46 Dye from Aqueous Solution by Pine Tree Leaves. Chem. Eng. J. 2011, 170, 67–74.
- Gupta, N.; Kushwaha, A.K.; Chattopadhyaya, M.C. Adsorption Studies of Cationic Dyes onto Ashoka (Saraca asoca) Leaf Powder. J. Taiwan. Inst. Chem. Eng. 2012, 43, 604–613.
- Mosoarca, G.; Vancea, C.; Popa, S.; Dan, M.; Boran, S. A Novel High-Efficiency Natural Biosorbent Material Obtained from Sour Cherry (Prunus cerasus) Leaf Biomass for Cationic Dyes Adsorption. Materials 2023, 16, 4252.
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective Removal of Anionic Textile Dyes Using Adsorbent Synthesized from Coffee Waste. Sci. Rep. 2020, 10, 2928.
- Babu, S.B.; Priyanka, S.V. Removal of Malachite Green Dye from Aqueous Solution Using Lemon Leaf Powder as an Adsorbent. J. Solid. Waste Technol. Manag. 2023, 49, 141–151.
- Rose, P.K.; Poonia, V.; Kumar, R.; Kataria, N.; Sharma, P.; Lamba, J.; Bhattacharya, P. Congo Red Dye Removal Using Modified Banana Leaves: Adsorption Equilibrium, Kinetics, and Reusability Analysis. Groundw. Sustain. Dev. 2023, 23, 101005.
- Paz, A.; Carballo, J.; Pérez, M.J.; Domínguez, J.M. Biological Treatment of Model Dyes and Textile Wastewaters. Chemosphere 2017, 181, 168–177.
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A Critical Review on Textile Wastewater Treatments: Possible Approaches. J. Environ. Manag. 2016, 182, 351–366.
- Ranganathan, K.; Karunagaran, K.; Sharma, D.C. Recycling of Wastewaters of Textile Dyeing Industries Using Advanced Treatment Technology and Cost Analysis—Case Studies. Resour. Conserv. Recycl. 2007, 50, 306–318.
- Balcik-Canbolat, C.; Sengezer, C.; Sakar, H.; Karagunduz, A.; Keskinler, B. Recovery of Real Dye Bath Wastewater Using Integrated Membrane Process: Considering Water Recovery, Membrane Fouling and Reuse Potential of Membranes. Environ. Technol. 2017, 38, 2668–2676.
- Van der Bruggen, B.; Canbolat, Ç.B.; Lin, J.; Luis, P. The Potential of Membrane Technology for Treatment of Textile Wastewater. In Sustainable Membrane Technology for Water and Wastewater Treatment; Figoli, A., Criscuoli, A., Eds.; Springer: Singapore, 2017; pp. 349–380. ISBN 978-981-10-5623-9.
- Cinperi, N.C.; Ozturk, E.; Yigit, N.O.; Kitis, M. Treatment of Woolen Textile Wastewater Using Membrane Bioreactor, Nanofiltration and Reverse Osmosis for Reuse in Production Processes. J. Clean. Prod. 2019, 223, 837–848.
- Tang, C.; Chen, V. Nanofiltration of Textile Wastewater for Water Reuse. Desalination 2002, 143, 11–20.
- Peng, Q.; Tan, X.; Xiong, X.; Wang, Y.; Novotná, J.; Shah, K.V.; Stempień, Z.; Periyasamy, A.P.; Kejzlar, P.; Venkataraman, M.; et al. Insights into the Large-size Graphene Improvement Effect of the Mechanical Properties on the Epoxy/Glass Fabric Composites. Polym. Compos. 2023, 44, 7430–7443.
- Hung, Y.-T.; Lo, H.H.; Wang, L.K.; Taricska, J.R.; Li, K.H. Granular Activated Carbon Adsorption. In Physicochemical Treatment Processes; Wang, L.K., Hung, Y.-T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2005; Volume 3, pp. 573–633. ISBN 978-1-59259-820-5.
- Belaid, K.D.; Kacha, S.; Kameche, M.; Derriche, Z. Adsorption Kinetics of Some Textile Dyes onto Granular Activated Carbon. J. Environ. Chem. Eng. 2013, 1, 496–503.
- Abou-Elela, S.I.; Ali, M.E.M.; Ibrahim, H.S. Combined Treatment of Retting Flax Wastewater Using Fenton Oxidation and Granular Activated Carbon. Arab. J. Chem. 2016, 9, 511–517.
- Arfin, T.; Varshney, N.; Singh, B. Ionic Liquid Modified Activated Carbon for the Treatment of Textile Wastewater. Green Mater. Wastewater Treat. Environ. Chem. A Sustain. World 2020, 38, 257–275.
- Bakht Shokouhi, S.; Dehghanzadeh, R.; Aslani, H.; Shahmahdi, N. Activated Carbon Catalyzed Ozonation (ACCO) of Reactive Blue 194 Azo Dye in Aqueous Saline Solution: Experimental Parameters, Kinetic and Analysis of Activated Carbon Properties. J. Water Process Eng. 2020, 35, 101188.
- Bilińska, L.; Gmurek, M.; Ledakowicz, S. Comparison between Industrial and Simulated Textile Wastewater Treatment by AOPs—Biodegradability, Toxicity and Cost Assessment. Chem. Eng. J. 2016, 306, 550–559.
- Kumar, P.S.; Yaashikaa, P.R. Sustainable Innovations in Apparel Production; Springer: Singapore, 2018; ISBN 978-981-10-8590-1.
- Yu, S.; Zhang, W.; Dong, X.; Wang, F.; Yang, W.; Liu, C.; Chen, D. A Review on Recent Advances of Biochar from Agricultural and Forestry Wastes: Preparation, Modification and Applications in Wastewater Treatment. J. Environ. Chem. Eng. 2024, 12, 111638.
- Pervez, M.N.; Fu, D.; Wang, X.; Bao, Q.; Yu, T.; Naddeo, V.; Tian, H.; Cao, C.; Zhao, Y. A Bifunctional-FeOOH@GCA Nanocomposite for Enhanced Adsorption of Arsenic and Photo Fenton-like Catalytic Conversion of As(III). Environ. Technol. Innov. 2021, 22, 101437.
- Patil, A.D.; Raut, P.D. Treatment of Textile Wastewater by Fenton’s Process as a Advanced Oxidation Process. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 29–32.
- Ramos, M.D.N.; Santana, C.S.; Velloso, C.C.V.; da Silva, A.H.M.; Magalhães, F.; Aguiar, A. A Review on the Treatment of Textile Industry Effluents through Fenton Processes. Process Saf. Environ. Prot. 2021, 155, 366–386.
- Ilhan, F.; Ulucan-Altuntas, K.; Dogan, C.; Kurt, U. Treatability of Raw Textile Wastewater Using Fenton Process and Its Comparison with Chemical Coagulation. Desalination Water Treat. 2019, 162, 142–148.
- Asghar, A.; Abdul Raman, A.A.; Wan Daud, W.M.A. Advanced Oxidation Processes for In-Situ Production of Hydrogen Peroxide/Hydroxyl Radical for Textile Wastewater Treatment: A Review. J. Clean. Prod. 2015, 87, 826–838.
- Yang, J.; Jia, K.; Lu, S.; Cao, Y.; Boczkaj, G.; Wang, C. Thermally Activated Natural Chalcopyrite for Fenton-like Degradation of Rhodamine B: Catalyst Characterization, Performance Evaluation, and Catalytic Mechanism. J. Environ. Chem. Eng. 2024, 12, 111469.
- Bethi, B.; Radhika, G.B.; Sonawane, S.H. Fundamentals of Advanced Oxidation Processes (AOPs) for Wastewater Treatment: Challenges and Opportunities. In Novel Approaches Towards Wastewater Treatment and Resource Recovery Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 209–220.
- Somensi, C.A.; Simionatto, E.L.; Bertoli, S.L.; Wisniewski, A.; Radetski, C.M. Use of Ozone in a Pilot-Scale Plant for Textile Wastewater Pre-Treatment: Physico-Chemical Efficiency, Degradation by-Products Identification and Environmental Toxicity of Treated Wastewater. J. Hazard. Mater. 2010, 175, 235–240.
- Sillanpää, M.E.T.; Agustiono Kurniawan, T.; Lo, W. Degradation of Chelating Agents in Aqueous Solution Using Advanced Oxidation Process (AOP). Chemosphere 2011, 83, 1443–1460.
- Sevimli, M.F.; Sarikaya, H.Z. Ozone Treatment of Textile Effluents and Dyes: Effect of Applied Ozone Dose, PH and Dye Concentration. J. Chem. Technol. Biotechnol. 2002, 77, 842–850.
- Selcuk, H. Decolorization and Detoxification of Textile Wastewater by Ozonation and Coagulation Processes. Dye. Pigment. 2005, 64, 217–222.
- Gähr, F.; Hermanutz, F.; Oppermann, W. Ozonation- An Important Technique to Comly with New German Laws for Textile Waste-Water Treatment. Water Sci. Technol. 1994, 30, 255–263.
- Arslan-Alaton, I.; Koba-Ucun, O. Treatment of Reactive Dye Hydrolysates with UV-C- and Ozone-Activated Percarbonate and Persulfate. Int. J. Environ. Res. 2023, 17, 58.
- Demirev, A.; Nenov, V. Ozonation of Two Acidic Azo Dyes with Different Substituents. Ozone Sci. Eng. 2005, 27, 475–485.
- Fanchiang, J.M.; Tseng, D.H. Degradation of Anthraquinone Dye, C.I. Reactive Blue 19 in Aqueous Solution by Ozonation. Chemosphere 2009, 77, 214–221.
- Iyer, S.R.S. Chapter 4—Physical Chemistry of Dyeing: Kinetics, Equilibrium, Dye-Fiber Affinity, and Mechanisms. In The Chemistry of Synthetic Dyes; Venkataraman, K., Ed.; Academic Press: Cambridge, MA, USA, 1974; pp. 115–275. ISBN 978-0-12-717007-7.
- Shuttleworth, L.; Weaver, M.A. The Chemistry and Application of Dyes; Waring, D.R., Hallas, G., Eds.; Springer: Boston, MA, USA, 1990; pp. 107–163. ISBN 978-1-4684-7715-3.
- Malik, S.N.; Ghosh, P.C.; Vaidya, A.N.; Mudliar, S.N. Hybrid Ozonation Process for Industrial Wastewater Treatment: Principles and Applications: A Review. J. Water Process Eng. 2020, 35, 101193.
- Bai, H.; Liang, L.; Cao, P.; Zhang, H.; Chen, S.; Yu, H.; Quan, X. MgAl2O4 Incorporated Catalytic Ceramic Membrane for Catalytic Ozonation of Organic Pollutants. Appl. Catal. B. 2024, 343, 123527.
- Guettaï, N.; Ait Amar, H. Photocatalytic Oxidation of Methyl Orange in Presence of Titanium Dioxide in Aqueous Suspension. Part I: Parametric Study. Desalination 2005, 185, 427–437.
- Guettaï, N.; Ait Amar, H. Photocatalytic Oxidation of Methyl Orange in Presence of Titanium Dioxide in Aqueous Suspension. Part II: Kinetics Study. Desalination 2005, 185, 439–448.
- Ebrahimi, I.; Parvinzadeh Gashti, M.; Sarafpour, M. Photocatalytic Discoloration of Denim Using Advanced Oxidation Process with H2O2/UV. J. Photochem. Photobiol. A Chem. 2018, 360, 278–288.
- Khosravi, A.; Karimi, M.; Ebrahimi, H.; Fallah, N. Sequencing Batch Reactor/Nanofiltration Hybrid Method for Water Recovery from Textile Wastewater Contained Phthalocyanine Dye and Anionic Surfactant. J. Environ. Chem. Eng. 2020, 8, 103701.
- Khouni, I.; Marrot, B.; Amar, R. Ben Treatment of Reconstituted Textile Wastewater Containing a Reactive Dye in an Aerobic Sequencing Batch Reactor Using a Novel Bacterial Consortium. Sep. Purif. Technol. 2012, 87, 110–119.
- Mendes, M.; Moreira, I.; Moreira, P.; Pintado, M.; Castro, P. Bioaugmentation of Aerobic Granular Sludge with Dye-Decolorizing Yeast for Textile Industrial Wastewater. Processes 2023, 11, 1654.
- Lourenço, N.D.; Novais, J.M.; Pinheiro, H.M. Effect of Some Operational Parameters on Textile Dye Biodegradation in a Sequential Batch Reactor. J. Biotechnol. 2001, 89, 163–174.
- Lacasse, K.; Baumann, W. Environmental Considerations for Textile Processes and Chemicals. In Textile Chemicals; Lacasse, K., Baumann, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 484–647. ISBN 978-3-642-18898-5.
- Siddique, K.; Rizwan, M.; Shahid, M.J.; Ali, S.; Ahmad, R.; Rizvi, H. Textile Wastewater Treatment Options: A Critical Review. In Enhancing Cleanup of Environmental Pollutants; Anjum, N.A., Gill, S.S., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 2, pp. 183–207. ISBN 9783319554235.
- Amuda, O.S.; Deng, A.; Alade, A.O.; Hung, Y.-T. Conversion of Sewage Sludge to Biosolids. In Biosolids Engineering and Management; Wang, L.K., Shammas, N.K., Hung, Y.-T., Eds.; Humana Press: Totowa, NJ, USA, 2008; Volume 7, pp. 65–119. ISBN 978-1-59745-174-1.
- Yadav, A.; Garg, V.K. Industrial Wastes and Sludges Management by Vermicomposting. Rev. Environ. Sci. Biotechnol. 2011, 10, 243–276.
- Karn, S.K.; Kumar, A. Hydrolytic Enzyme Protease in Sludge: Recovery and Its Application. Biotechnol. Bioprocess. Eng. 2015, 20, 652–661.
- He, L.; Du, P.; Chen, Y.; Lu, H.; Cheng, X.; Chang, B.; Wang, Z. Advances in Microbial Fuel Cells for Wastewater Treatment. Renew. Sustain. Energy Rev. 2017, 71, 388–403.
- Zhang, M.; Yang, C.; Jing, Y.; Li, J. Effect of Energy Grass on Methane Production and Heavy Metal Fractionation during Anaerobic Digestion of Sewage Sludge. Waste Manag. 2016, 58, 316–323.
- Yang, S.; Hai, F.I.; Price, W.E.; McDonald, J.; Khan, S.J.; Nghiem, L.D. Occurrence of Trace Organic Contaminants in Wastewater Sludge and Their Removals by Anaerobic Digestion. Bioresour. Technol. 2016, 210, 153–159.
- Wang, Q. A Roadmap for Achieving Energy-Positive Sewage Treatment Based on Sludge Treatment Using Free Ammonia. ACS Sustain. Chem. Eng. 2017, 5, 9630–9633.
- Sultana, S.; Khan, M.D.; Sabir, S.; Gani, K.M.; Oves, M.; Khan, M.Z. Bio-Electro Degradation of Azo-Dye in a Combined Anaerobic–Aerobic Process along with Energy Recovery. New J. Chem. 2015, 39, 9461–9470.
- Đurđević, D.; Blecich, P.; Jurić, Ž. Energy Recovery from Sewage Sludge: The Case Study of Croatia. Energies 2019, 12, 1927.
- Patinvoh, R.J.; Osadolor, O.A.; Sárvári Horváth, I.; Taherzadeh, M.J. Cost Effective Dry Anaerobic Digestion in Textile Bioreactors: Experimental and Economic Evaluation. Bioresour. Technol. 2017, 245, 549–559.
- Bahar, S.; Ciggin, A.S. A Simple Kinetic Modeling Approach for Aerobic Stabilization of Real Waste Activated Sludge. Chem. Eng. J. 2016, 303, 194–201.
- Sonai, G.G.; de Souza, S.M.A.G.U.; de Oliveira, D.; de Souza, A.A.U. The Application of Textile Sludge Adsorbents for the Removal of Reactive Red 2 Dye. J. Environ. Manag. 2016, 168, 149–156.
- Vigneswaran, C.; Ananthasubramanian, M.; Kandhavadivu, P.; Vigneswaran, C.; Ananthasubramanian, M.; Kandhavadivu, P. 5–Enzymes in Textile Effluents. In Bioprocessing of Textiles; Vigneswaran, C., Ananthasubramanian, M., Kandhavadivu, P.B.T.-B.T., Eds.; Woodhead Publishing: New Delhi, India, 2014; pp. 251–298. ISBN 9789380308425.
- Faubert, P.; Barnabé, S.; Bouchard, S.; Côté, R.; Villeneuve, C. Pulp and Paper Mill Sludge Management Practices: What Are the Challenges to Assess the Impacts on Greenhouse Gas Emissions? Resour. Conserv. Recycl. 2016, 108, 107–133.
- Volmajer Valh, J.; Majcen Le Marechal, A.; Vajnhandl, S.; Jerič, T.; Šimon, E. 4.20—Water in the Textile Industry. In Treatise on Water Science; Wilderer, P.B.T.-T.W.S., Ed.; Elsevier: Oxford, UK, 2011; Volume 4, pp. 685–706. ISBN 9780444531995.
- Kopperi, H.; Hemalatha, M.; Ravi Kiran, B.; Santhosh, J.; Venkata Mohan, S. Sustainable Consideration for Traditional Textile Handloom Cluster/Village in Pollution Abatement—A Case Study. Environ. Pollut. 2023, 324, 121320.
- Jahan, N.; Tahmid, M.; Shoronika, A.Z.; Fariha, A.; Roy, H.; Pervez, M.N.; Cai, Y.; Naddeo, V.; Islam, M.S. A Comprehensive Review on the Sustainable Treatment of Textile Wastewater: Zero Liquid Discharge and Resource Recovery Perspectives. Sustainability 2022, 14, 15398.
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.-S.; Jeon, B.-H.; Park, Y.-K. A Review on Recent Advances in the Treatment of Dye-Polluted Wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19.
- Andooz, A.; Eqbalpour, M.; Kowsari, E.; Ramakrishna, S.; Ansari Cheshmeh, Z. A Comprehensive Review on Pyrolysis from the Circular Economy Point of View and Its Environmental and Social Effects. J. Clean. Prod. 2023, 388, 136021.
- Periyasamy, A.P.; Militky, J. Denim and Consumers’ Phase of Life Cycle. In Sustainability in Denim; Muthu, S.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 257–282.
- Periyasamy, A.P.; Wiener, J.; Militky, J. Life-Cycle Assessment of Denim. In Sustainability in Denim; Elsevier: Amsterdam, The Netherlands, 2017; pp. 83–110.
- Periyasamy, A.P.; Duraisamy, G. Carbon Footprint on Denim Manufacturing. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–18. ISBN 978-3-319-48281-1.
- Periyasamy, A.P. Life Cycle Assessment of Denim: A Review. Colourage 2019, 66, 54–62.
More
Information
Subjects:
Polymer Science
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.4K
Revisions:
2 times
(View History)
Update Date:
09 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




