
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Moganavelli Singh | -- | 3429 | 2024-01-08 08:04:50 | | | |
| 2 | Wendy Huang | Meta information modification | 3429 | 2024-01-08 08:19:38 | | |
Video Upload Options
The care and rehabilitation of acute and chronic wounds have a significant social and economic impact on patients and global health. This burden is primarily due to the adverse effects of infections, prolonged recovery, and the associated treatment costs. Chronic wounds can be treated with a variety of approaches, which include surgery, negative pressure wound therapy, wound dressings, and hyperbaric oxygen therapy. However, each of these strategies has an array of limitations. The existing dry wound dressings lack functionality in promoting wound healing and exacerbating pain by adhering to the wound. Hydrogels, which are commonly polymer-based and swell in water, have been proposed as potential remedies due to their ability to provide a moist environment that facilitates wound healing. Their unique composition enables them to absorb wound exudates, exhibit shape adaptability, and be modified to incorporate active compounds such as growth factors and antibacterial compounds.
1. Introduction
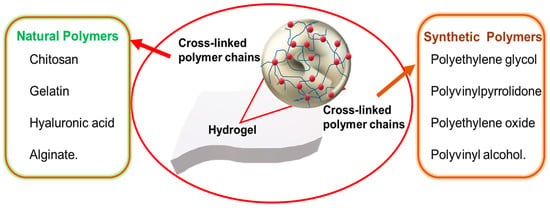
2. Natural Hydrogels
2.1. Chitosan
2.2. Gelatin
2.3. Hyaluronic Acid
2.4. Alginate
| Product | Company | Constituent | Use |
|---|---|---|---|
| DermaSyn® | DermaRite Industries (NJ, USA) |
Primary wound dressing with vitamin E | Partial and full-thickness chronic wounds |
| Neoheal® Hydrogel |
Kikgel | Polyethylene glycol, polyvinylpyrrolidone, Agar, and 90% water |
Low-exuding scabs, a abrasions, dry scabs, first, second-and third-degree burns, and ulcers |
| Restore Hydrogel |
Hollister Inc. (IL, USA) |
Gauze pad, Hyaluronic acid | Partial and full-thickness chronic wounds |
| ActivHeal® | Advanced Medical Solutions Ltd. (Oxon, UK) |
Primary wound dressing with 85% water |
Cavity wounds, pressure ulcers, diabetic foot ulcers, and leg ulcers |
| NU-GEL™ | Systagenix | Sodium alginate primary wound dressing |
Diabetic foot ulcers, leg ulcers, venous ulcers |
| Purilon® | Coloplast | Calcium alginate, sodium carboxymethyl cellulose |
Pressure ulcers, first and second degree burns, non-infected diabetic foot ulcers, leg ulcers |
| Simpurity™ Hydrogel |
Safe n’ Simple | Acrylate, polyvinyl alcohol, polyethylene oxide, polyurethane |
First and second-degree partial- thickness burns, low-exuding chronic wounds |
3. Synthetic Hydrogels
3.1. Polyethylene Glycol (PEG)
3.2. Polyvinyl Alcohol (PVA)
3.3. Polyvinylpyrrolidone (PVP)
References
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13, 5–15.
- Chen, Y.; Xiang, Y.; Zhang, H.; Zhu, T.; Chen, S.; Li, J.; Du, J.; Yan, X. A multifunctional chitosan composite aerogel based on high density amidation for chronic wound healing. Carbohydr. Polym. 2023, 321, 121248.
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620.
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609.
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127.
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377.
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719.
- Sheokand, B.; Vats, M.; Kumar, A.; Srivastava, C.M.; Bahadur, I.; Pathak, S.R. Natural polymers used in the dressing materials for wound healing: Past, present and future. J. Polym. Sci. 2023, 61, 1389–1414.
- Chandel, A.K.S.; Kannan, D.; Nutan, B.; Singh, S.; Jewrajka, S.K. Dually cross-linked injectable hydrogels of poly (ethylene glycol) and poly -b-poly (N-isopropyl acrylamide) as a wound healing promoter. J. Mater. Chem. B 2017, 5, 4955–4965.
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115.
- Chen, W.-H.; Chen, Q.-W.; Chen, Q.; Cui, C.; Duan, S.; Kang, Y.; Liu, Y.; Liu, Y.; Muhammad, W.; Shao, S. Biomedical polymers: Synthesis, properties, and applications. Sci. China Chem. 2022, 65, 1010–1075.
- Sahariah, P.; Kontogianni, G.-I.; Scoulica, E.; Sigurjonsson, O.E.; Chatzinikolaidou, M. Structure-activity relationship for antibacterial chitosan carrying cationic and hydrophobic moieties. Carbohyd. Polym. 2023, 312, 120796.
- Joseph, C.; Daniels, A.; Singh, S.; Singh, M. Histidine-tagged Folate-Targeted Gold Nanoparticles for enhanced transgene expression in Breast Cancer Cells in Vitro. Pharmaceutics 2022, 14, 53.
- Akinyelu, J.; Oladimeji, O.; Daniels, A.; Singh, M. Folate-Targeted Doxorubicin Delivery to Breast and Cervical Cancer cells using a Chitosan-Gold Nano-delivery System. J. Drug Deliv. Sci. Technol. 2022, 67, 102978.
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549.
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16.
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C 2019, 104, 109930.
- Hao, Y.; Zhao, W.; Zhang, H.; Zheng, W.; Zhou, Q. Carboxymethyl chitosan-based hydrogels containing fibroblast growth factors for triggering diabetic wound healing. Carbohydr. Polym. 2022, 287, 119336.
- Mousavi, S.; Khoshfetrat, A.B.; Khatami, N.; Ahmadian, M.; Rahbarghazi, R. Comparative study of collagen and gelatin in chitosan-based hydrogels for effective wound dressing: Physical properties and fibroblastic cell behavior. Biochem. Biophys. Res. Commun. 2019, 518, 625–631.
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the mechanical and thermal properties of gelatin hydrogels cross-linked by cellulose nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645.
- Xu, X.; Zhou, M. Antimicrobial gelatin nanofibers containing silver nanoparticles. Fibers Polym. 2008, 9, 685–690.
- Skopinska-Wisniewska, J.; Tuszynska, M.; Olewnik-Kruszkowska, E. Comparative Study of Gelatin Hydrogels Modified by Various Cross-Linking Agents. Materials 2021, 14, 396.
- Wang, Y.; Lv, Q.; Chen, Y.; Xu, L.; Feng, M.; Xiong, Z.; Li, J.; Ren, J.; Liu, J.; Liu, B. Bilayer hydrogel dressing with lysozyme-enhanced photothermal therapy for biofilm eradication and accelerated chronic wound repair. Acta Pharm. Sin. B 2023, 13, 284–297.
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701.
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40.
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464.
- Della Sala, F.; Longobardo, G.; Fabozzi, A.; di Gennaro, M.; Borzacchiello, A. Hyaluronic Acid-Based Wound Dressing with Antimicrobial Properties for Wound Healing Application. Appl. Sci. 2022, 12, 3091.
- Li, M.; Liang, Y.; Liang, Y.; Pan, G.; Guo, B. Injectable stretchable self-healing dual dynamic network hydrogel as adhesive anti-oxidant wound dressing for photothermal clearance of bacteria and promoting wound healing of MRSA infected motion wounds. Chem. Eng. J. 2022, 427, 132039.
- Sanchez, M.F.; Guzman, M.L.; Apas, A.L.; Alovero, F.d.L.; Olivera, M.E. Sustained dual release of ciprofloxacin and lidocaine from ionic exchange responding film based on alginate and hyaluronate for wound healing. Eur. J. Pharm. Sci. 2021, 161, 105789.
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428.
- Jang, J.; Seol, Y.-J.; Kim, H.J.; Kundu, J.; Kim, S.W.; Cho, D.-W. Effects of alginate hydrogel cross-linking density on mechanical and biological behaviors for tissue engineering. J. Mec. Behav. Biomed. Mater. 2014, 37, 69–77.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126.
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloid. Surf. B Biointerfaces 2023, 222, 113096.
- Rausch, M.K.; Parekh, S.H.; Dortdivanlioglu, B.; Rosales, A.M. Synthetic hydrogels as blood clot mimicking wound healing materials. Prog. Biomed. Eng. 2021, 3, 042006.
- Güiza-Argüello, V.R.; Solarte-David, V.A.; Pinzón-Mora, A.V.; Ávila-Quiroga, J.E.; Becerra-Bayona, S.M. Current Advances in the Development of Hydrogel-Based Wound Dressings for Diabetic Foot Ulcer Treatment. Polymers 2022, 14, 2764.
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels—A review. Am. J. Polym. Sci. 2014, 4, 25–31.
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21.
- Chen, S.L.; Fu, R.H.; Liao, S.F.; Liu, S.P.; Lin, S.Z.; Wang, Y.C. A PEG-Based Hydrogel for Effective Wound Care Management. Cell Transplant. 2018, 27, 275–284.
- Figueroa-Pizano, M.D.; Vélaz, I.; Martínez-Barbosa, M.E. A freeze-thawing method to prepare chitosan-poly (vinyl alcohol) hydrogels without cross-linking agents and diflunisal release studies. J. Vis. Exp. 2020, 14, e59636.
- Muchová, M.; Münster, L.; Capáková, Z.; Mikulcová, V.; Kuřitka, I.; Vícha, J. Design of dialdehyde cellulose cross-linked poly (vinyl alcohol) hydrogels for transdermal drug delivery and wound dressings. Mater. Sci. Eng. C 2020, 116, 111242.
- Li, X.; Jiang, Y.; Wang, F.; Fan, Z.; Wang, H.; Tao, C.; Wang, Z. Preparation of polyurethane/polyvinyl alcohol hydrogel and its performance enhancement via compositing with silver particles. RSC Adv. 2017, 7, 46480–46485.
- Liu, S.; Li, D.; Wang, Y.; Zhou, G.; Ge, K.; Jiang, L. Adhesive, antibacterial and double cross-linked carboxylated polyvinyl alcohol/chitosan hydrogel to enhance dynamic skin wound healing. Int. J. Biol. Macromol. 2023, 228, 744–753.
- Irmukhametova, G.S.; Shaikhutdinov, E.M.; Rakhmetullayeva, R.K.; Yermukhambetova, B.B.; Ishanova, A.K.; Temirkhanova, G.; Mun, G.A. Nanostructured Hydrogel Dressings on Base of Crosslinked Polyvinylpyrrolidone for Biomedical Application. Adv. Mater. Res. 2014, 875–877, 1467–1471.
- Shahrousvand, M.; Mirmasoudi, S.S.; Pourmohammadi-Bejarpasi, Z.; Feizkhah, A.; Mobayen, M.; Hedayati, M.; Sadeghi, M.; Esmailzadeh, M.; Mirkatoul, F.B.; Jamshidi, S. Polyacrylic acid/ polyvinylpyrrolidone hydrogel wound dressing containing zinc oxide nanoparticles promote wound healing in a rat model of excision injury. Heliyon 2023, 9, e19230.
- Xu, X.; Zeng, Y.; Chen, Z.; Yu, Y.; Wang, H.; Lu, X.; Zhao, J.; Wang, S. Chitosan-based multifunctional hydrogel for sequential wound inflammation elimination, infection inhibition, and wound healing. Int. J. Biol. Macromol. 2023, 235, 123847.
- Contardi, M.; Kossyvaki, D.; Picone, P.; Summa, M.; Guo, X.; Heredia-Guerrero, J.A.; Giacomazza, D.; Carzino, R.; Goldoni, L.; Scoponi, G.; et al. Electrospun polyvinylpyrrolidone (PVP) hydrogels containing hydroxycinnamic acid derivatives as potential wound dressings. Chem. Eng. J. 2021, 409, 128144.




