Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Konlayutt Punyawudho | -- | 1645 | 2024-01-05 15:27:13 | | | |
| 2 | Camila Xu | Meta information modification | 1645 | 2024-01-09 02:26:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Payattikul, L.; Chen, C.; Chen, Y.; Raja Pugalenthi, M.; Punyawudho, K. Non-Precious Carbon-Based Nanomaterials as ORR Electrocatalysts. Encyclopedia. Available online: https://encyclopedia.pub/entry/53488 (accessed on 07 February 2026).
Payattikul L, Chen C, Chen Y, Raja Pugalenthi M, Punyawudho K. Non-Precious Carbon-Based Nanomaterials as ORR Electrocatalysts. Encyclopedia. Available at: https://encyclopedia.pub/entry/53488. Accessed February 07, 2026.
Payattikul, Laksamee, Chen-Yu Chen, Yong-Song Chen, Mariyappan Raja Pugalenthi, Konlayutt Punyawudho. "Non-Precious Carbon-Based Nanomaterials as ORR Electrocatalysts" Encyclopedia, https://encyclopedia.pub/entry/53488 (accessed February 07, 2026).
Payattikul, L., Chen, C., Chen, Y., Raja Pugalenthi, M., & Punyawudho, K. (2024, January 05). Non-Precious Carbon-Based Nanomaterials as ORR Electrocatalysts. In Encyclopedia. https://encyclopedia.pub/entry/53488
Payattikul, Laksamee, et al. "Non-Precious Carbon-Based Nanomaterials as ORR Electrocatalysts." Encyclopedia. Web. 05 January, 2024.
Copy Citation
Electrochemical applied potentials have a significant impact on oxygen reduction reactions (ORRs) reactions and their kinetics. The thermodynamic potential of 1.229 V vs. standard hydrogen electrode (RHE) for ORR is indicated as a specific potential in an electrochemical reaction.
carbon electrocatalyst
heteroatoms
single-atom metals
metal–organic framework
energy storage
1. Introduction
It has become urgent to find new, efficient, and clean energy sources due to the impact of environmental ruin and energy depletion caused by the use of fossil fuels [1]. Proton exchange membrane fuel cells (PEMFCs), which make electricity through an electrochemical process involving hydrogen (H2) and oxygen (air), have many benefits over conventional Li-ion batteries, including practically equivalent performance, a high energy/power density, rapid refueling, and a lack of CO2 emissions [2]. Researchers are becoming increasingly interested in the oxygen reduction reaction (ORR), which is a crucial electrode reaction that occurs in fuel cells and rechargeable metal–air batteries to convert chemical energy into electrical power [3]. However, the efficiencies of fuel cells and metal–air batteries are severely constrained by the slow ORR reaction mechanism [3]. In the past, research has shown that there are two potential routes for the ORR process: a direct four-electron (4e−) pathway and a slow two-electron (2e−) process within standard potentials [4]. Direct four-electron activity seems to exhibit superior activity over the use of unfavorable two-electron reactions, which indicates that designing efficient electrocatalysts is required to facilitate good ORR [4][5]. In view of the search for excellent electrocatalytic performance, platinum (Pt)-based nanomaterials show outstanding potential as ORR electrocatalysts [6]. However, significant efforts have been made to discover non-precious metal (Pt-free) electrocatalysts to replace Pt-based electrocatalysts due to the disadvantages of the cost, scarcity, and deprived stability of Pt [7]. Carbon-based electrocatalysts, including heteroatom-doped carbon electrocatalysts, [8] single-metal-atom-based carbon electrocatalysts (M-N-Cs), [9], and metal–organic frameworks (MOFs), have also been explored significantly in the past decades [10] due to their inexpensive cost and outstanding electrocatalytic efficiency. Since it alters the electron density of carbon atoms within the region of heteroatom dopants, the heteroatom doping of carbon has been accepted as a successful method to adjust the characteristics of carbon nanomaterials and augment ORR performance [11]. It may also alter the chemisorption of reactants and control the chemical properties of electrocatalysts. Via the strategy of doping heteroatoms, numerous groups have recently attempted to boost the ORR activity of carbon-based nanomaterials such as carbon nanotubes and graphene [12]. The structural, electronic, and electrochemical characteristics of carbon nanomaterials have been successfully modified via heteroatom doping [13]. Graphene-doped sulfur (S), boron (B), and nitrogen (N)-doped carbon nanotubes, as well as N-doped graphene, have all been the subject of extensive research in the past [14][15][16][17]. Additionally, N- and S-doped graphene has proven to be an extraordinary non-metal ORR electrocatalyst. Furthermore, heteroatom doping has produced exciting characteristics that have led to increased attention on the imperfections of carbon nanomaterials.
According to the second law of thermodynamics, natural carbon nanomaterials, such as carbon nanotubes, graphite, and graphene, have always exhibited numerous disordered structures or imperfections on their surfaces or the edges of carbon [18]. Such defects, which are caused by the absence of specific atoms or an adjustment within the crystalline structure, definitely disrupt the electron–hole pairs and increase the ORR activity. According to the results of physical characterization and density functional theory (DFT), heteroatom doping provides edge carbon atoms with a higher electron density than pure carbon atoms, which have a similar effect.
Because of their improved ORR performance, single-metal-atom-based carbon nanomaterials (M-N-Cs) have been found to be extremely good electrocatalysts for replacing Pt-based nanomaterials [19]. Due to the importance of reducing metal particle sizes and controlling their dispersion on carbon supports, a wide range of methods have been used to prepare relatively stable and superior ORR-active metal-based electrocatalysts. Such techniques enhance the interplay between supports through the formation of chemical bonds between the metal and its related interface and the carbon support interface, as well as the charge carrier between both the carbon framework and metal species. The precise active centers in the metal-enclosed electrocatalyst could alter ORR activity and stability and, finally, increase ORR [20]. Metal atoms are typically attached to nitrogen sites, which noticeably changes the electronic structure of carbon atoms. Because of their exceptional electrocatalytic activity, better selectivity, high stability, and maximum atom exploitation efficiency, single-atom electrocatalysts (SAECs) have a great potential for substituting Pt-based electrocatalysts in ORR, according to DFT and experimentation calculations [19]. Three crucial elements (N, C, and M) are simultaneously present in the metal–organic frameworks (MOFs), which are chemically and structurally distinctive. Researchers now exploring them as effective precursors present the single or bi-metallic active sites, which are atomically distributed within nitrogen structures and stabilized by carbon supports [21][22][23]. The M-N-C electrocatalysts have the notable performance improvement due to these unique features. Various scientific studies of SAECs show their excellent ORR performance and comparability to the commercial Pt electrocatalyst, which have the outstanding catalytic reactions in the field of electrocatalysts. Additionally, there are significant efforts being made to create a wide range of electrochemical applications, such as fuel cells, metal–air batteries, electrochemical water splitting, solar cells, and supercapacitors [8][10][11][24].
2. Fundamentals of Electrocatalysis Kinetics
Electrochemical applied potentials have a significant impact on ORR reactions and their kinetics. The thermodynamic potential of 1.229 V vs. standard hydrogen electrode (RHE) for ORR is indicated as a specific potential in an electrochemical reaction. The least standard potential for the chemical reaction to take place while the reactant gas is delivered is regarded as the onset potential at the anode (small) and cathode electrodes (high). Over-potential (η) is described as the additional potential present in addition to the standard potential during reactions.
In Equation (1), the term is the Tafel slope. The smaller Tafel slopes contribute to the higher current density of electrocatalysis with the same η. The current density (j0) is calculated through the Tafel plot (η = 0).
3. General ORR Mechanism
The ORR reaction is classified into two types: the four-electron (4e−) and two-electron (2e−) pathways. During the four-electron pathway, O2 is converted to H2O (4H+ + 4e− + O2→2H2O in acidic media) or OH− (2H2O + 4e− +O2→4OH− in alkaline media). In another case, O2 is converted to hydrogen peroxide (H2O2) following the two-electron (2e−) pathway that is not required for fuel cells application. Depending on the oxygen dissociation capability of the electrocatalyst surface, ORR took place via various mechanisms (dissociative or associative) in a four-electron pathway [24].
Dissociative mechanism:


Associative mechanism:
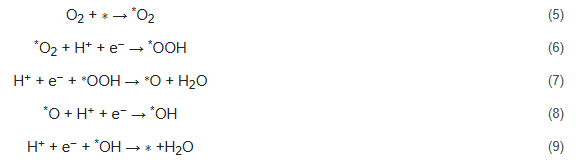
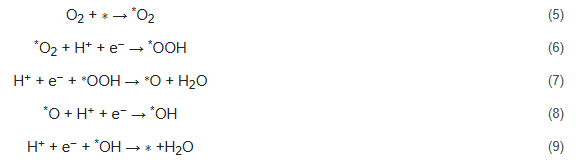
The above reaction applies to acidic medium and ∗ represents active sites. In alkaline condition, the H+ is substituted with water (H2O). For direct four-electron pathways,
O2 + 4e− + 2H2O → 4OH−
The mechanism referred to as the series 2-electron pathway, or alternatively known as the indirect four electron (4e−) pathway, involves the formation of H2O2 as an intermediary. Hydrogen peroxide is first produced by a two-electron (2e−) route, which is followed by further reduction to H2O with the addition of an additional two electrons.
Distinguished researchers Griffith, Pauling, and Bridge discovered the three distinct type of O2 adsorption [25][26][27]. The Griffith model shows the existence of a strong bond between the atoms of an unfilled dz2 orbital as well as the σ orbital causing the interaction of an oxygen molecule (O2) with a single atom on the surface of the metal substrate [28]. The necessary electron density in the σ orbital to the dz2 acceptor orbital is created by end-on adsorption through the single-type bond of the oxygen molecule, according to the Pauling model. For the Pt-based electrocatalyst, the Bridge model describes how two strong bonds interact with two different active sites.
4. Performance Analysis of ORR Electrocatalyst
The common approaches to investigate the potential of ORR electrocatalysts is the evaluation of power density through membrane electrode assembly (MEA). However, so much work is required to construct the improved electrodes for the intensive research of ORR electrocatalysts through MEA. As a consequence, the performance of the electrochemical cell has been examined using the two-half-cell method. Both rotating ring disk electrodes (RRDEs) and rotating disk electrodes (RDEs) enable the disk electrode to rotate at a great speed of 1600 rpm, which can be used to transfer mass [29][30][31][32][33]. The screen-printed carbon electrode (GCE) surface was shielded by a fine layer of electrocatalytic materials, confirming laminar flow during rotation and lowering the resistance of mass transfer via Nafion [34][35][36]. The atomically thin and homogeneous electrocatalytic can be coated on the GCE of RDEs to investigate the ORR reaction through polarization curves for accurate measurements.
4.1. RDE and RRDE Method
The RDE and RRDE prototype is a useful tool for evaluating ORR electrocatalyst efficiency and also avoiding the insufficient oxygen transport [37]. Three distinct regions with a variety of reaction kinetics can be identified in the ORR polarization curve (diffusion, kinetic, and mixed kinetic diffusion controlled) of the RDE measurement. These associated ORR parameters were calculated via these equations (Equations (11) and (12)) with onset potential (Eonset), limited current (JL), kinetic current density (JK), half-wave potential (E1/2), hydrogen peroxide yield (H2O2−%), electron transfer number (n), double-layer capacitance (Cdl), electrochemical surface area (ECSA), resistance (R), and so on.

In Equation (12), ‘F’ is the faraday constant; ‘ω’ is the angular velocity (rad/s); ‘D0’ and ‘C0’ indicate the oxygen diffusion coefficient and oxygen bulk concentration, respectively. ‘ν’ is the known kinetic viscosity.
4.2. Membrane Electrode Assembly (MEA) Test
The fuel cell test that contains the two-electrode and electrolyte system, which reveals how the electrocatalyst is used in actual devices [38]. Electrocatalytic layers, which have been composed of electrocatalysts, carbon black, and binder, are often needed for MEA analysis. The electrocatalyst-coated membrane (ECCM) or electrocatalyst-coated substrate (ECCS) techniques are presently used to produce MEA. In general, the US Energy standard equations were used to correct the fuel cells’ current density [39][40][41]. The MEA methodology can display the real condition of fuel cells with electrocatalysts. However, its complicated fuel cell components, ambiguous electrocatalyst active sites, as well as slow mass transport in electrocatalyst layers describe the complexity associated to assessing the specific activity of electrocatalysts [42].
References
- Dou, S.; Tao, L.; Huo, J.; Wang, S.; Dai, L. Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis. Energy Environ. Sci. 2016, 9, 1320–1326.
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491.
- Wei, Q.; Xiong, F.; Tan, S.; Huang, L.; Lan, E.H.; Dunn, B.; Mai, L. Porous One-Dimensional Nanomaterials: Design, Fabrication and Applications in Electrochemical Energy Storage. Adv. Mater. 2017, 29, 1602300.
- Shi, H.; Shen, Y.; He, F.; Li, Y.; Liu, A.; Liu, S.; Zhang, Y. Recent advances of doped carbon as non-precious catalysts for oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 15704–15716.
- Zhang, Z.; Gao, X.; Dou, M.; Ji, J.; Wang, F. Biomass Derived N-Doped Porous Carbon Supported Single Fe Atoms as Superior Electrocatalysts for Oxygen Reduction. Small 2017, 13, 1604290.
- Wu, R.; Chen, S.; Zhang, Y.; Wang, Y.; Nie, Y.; Ding, W.; Qi, X.; Wei, Z. Controlled synthesis of hollow micro/meso-pore nitrogen-doped carbon with tunable wall thickness and specific surface area as efficient electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 2433–2437.
- Wan, K.; Tan, A.-D.; Yu, Z.-P.; Liang, Z.-X.; Piao, J.-H.; Tsiakaras, P. 2D nitrogen-doped hierarchically porous carbon: Key role of low dimensional structure in favoring electrocatalysis and mass transfer for oxygen reduction reaction. Appl. Catal. B Environ. 2017, 209, 447–454.
- Yu, H.; Fisher, A.; Cheng, D.; Cao, D. Cu,N-codoped Hierarchical Porous Carbons as Electrocatalysts for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 21431–21439.
- Chen, Y.; Ji, S.; Wang, Y.; Dong, J.; Chen, W.; Li, Z.; Shen, R.; Zheng, L.; Zhuang, Z.; Wang, D.; et al. Isolated Single Iron Atoms Anchored on N-Doped Porous Carbon as an Efficient Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2017, 56, 6937–6941.
- Xia, W.; Zou, R.; An, L.; Xia, D.; Guo, S. A metal–organic framework route to in situ encapsulation of Co@Co3O4@C core@bishell nanoparticles into a highly ordered porous carbon matrix for oxygen reduction. Energy Environ. Sci. 2015, 8, 568–576.
- Shi, P.-C.; Yi, J.-D.; Liu, T.-T.; Li, L.; Zhang, L.-J.; Sun, C.-F.; Wang, Y.-B.; Huang, Y.-B.; Cao, R. Hierarchically porous nitrogen-doped carbon nanotubes derived from core–shell ZnO@zeolitic imidazolate framework nanorods for highly efficient oxygen reduction reactions. J. Mater. Chem. A 2017, 5, 12322–12329.
- Dou, S.; Shen, A.; Tao, L.; Wang, S. Molecular doping of graphene as metal-free electrocatalyst for oxygen reduction reaction. Chem. Commun. 2014, 50, 10672–10675.
- Song, H.; Li, H.; Wang, H.; Key, J.; Ji, S.; Mao, X.; Wang, R. Chicken bone-derived N-doped porous carbon materials as an oxygen reduction electrocatalyst. Electrochim. Acta 2014, 147, 520–526.
- Ma, Z.; Dou, S.; Shen, A.; Tao, L.; Dai, L.; Wang, S. Sulfur-Doped Graphene Derived from Cycled Lithium-Sulfur Batteries as a Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2015, 54, 1888–1892.
- Liu, J.; Shen, A.; Wei, X.; Zhou, K.; Chen, W.; Chen, F.; Xu, J.; Wang, S.; Dai, L. Ultrathin Wrinkled N-Doped Carbon Nanotubes for Noble-Metal Loading and Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2015, 7, 20507–20512.
- Wang, S.; Zhang, L.; Xia, Z.; Roy, A.; Chang, D.W.; Baek, J.; Dai, L. BCN Graphene as Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2012, 51, 4209–4212.
- Wang, X.; Wang, J.; Wang, D.; Dou, S.; Ma, Z.; Wu, J.; Tao, L.; Shen, A.; Ouyang, C.; Liu, Q.; et al. One-pot synthesis of nitrogen and sulfur co-doped graphene as efficient metal-free electrocatalysts for the oxygen reduction reaction. Chem. Commun. 2014, 50, 4839–4842.
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural Defects in Graphene. ACS Nano 2011, 5, 26–41.
- Yin, P.; Yao, T.; Wu, Y.; Zheng, L.; Lin, Y.; Liu, W.; Ju, H.; Zhu, J.; Hong, X.; Deng, Z.; et al. Single Cobalt Atoms with Precise N-Coordination as Superior Oxygen Reduction Reaction Catalysts. Angew. Chem. Int. Ed. 2016, 55, 10800–10805.
- Bayatsarmadi, B.; Zheng, Y.; Vasileff, A.; Qiao, S. Recent Advances in Atomic Metal Doping of Carbon-based Nanomaterials for Energy Conversion. Small 2017, 13, 1700191.
- Zhang, H.; Osgood, H.; Xie, X.; Shao, Y.; Wu, G. Engineering nanostructures of PGM-free oxygen-reduction catalysts using metal-organic frameworks. Nano Energy 2017, 31, 331–350.
- Barkholtz, H.M.; Liu, D.-J. Advancements in rationally designed PGM-free fuel cell catalysts derived from metal–organic frameworks. Mater. Horiz. 2017, 4, 20–37.
- Zhang, H.; Li, J.; Tan, Q.; Lu, L.; Wang, Z.; Wu, G. Metal–Organic Frameworks and Their Derived Materials as Electrocatalysts and Photocatalysts for CO2 Reduction: Progress, Challenges, and Perspectives. Chem.—A Eur. J. 2018, 24, 18137–18157.
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998.
- Griffith, J.S.; Roughton, F.J.W. On the magnetic properties of some haemoglobin complexes, Proceedings of the Royal Society of London. Ser. A Math. Phys. Sci. 1997, 235, 23–36.
- Weiss, J.J. Nature of the Iron–Oxygen Bond in Oxyhæmoglobin. Nature 1964, 203, 183.
- Yeager, E. Recent Advances in the Science of Electrocatalysis. J. Electrochem. Soc. 1981, 128, 160C.
- Adžić, R.R.; Wang, J.X. Configuration and Site of O2 Adsorption on the Pt(111) Electrode Surface. J. Phys. Chem. B 1998, 102, 8988–8993.
- Du, C.; Sun, Y.; Shen, T.; Yin, G.; Zhang, J. Applications of RDE and RRDE Methods in Oxygen Reduction Reaction. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Elsevier: Amsterdam, The Netherlands, 2014; pp. 231–277.
- Biddinger, E.J.; von Deak, D.; Singh, D.; Marsh, H.; Tan, B.; Knapke, D.S.; Ozkan, U.S. Examination of Catalyst Loading Effects on the Selectivity of CNx and Pt/VC ORR Catalysts Using RRDE. J. Electrochem. Soc. 2011, 158, B402.
- Nagai, T.; Jahn, C.; Jia, H. Improved Accelerated Stress Tests for ORR Catalysts Using a Rotating Disk Electrode. J. Electrochem. Soc. 2019, 166, F3111–F3115.
- Kocha, S.S.; Shinozaki, K.; Zack, J.W.; Myers, D.J.; Kariuki, N.N.; Nowicki, T.; Stamenkovic, V.; Kang, Y.; Li, D.; Papageorgopoulos, D. Best Practices and Testing Protocols for Benchmarking ORR Activities of Fuel Cell Electrocatalysts Using Rotating Disk Electrode. Electrocatalysis 2017, 8, 366–374.
- Mills, A.; O’Rourke, C. Wireless rotating disk electrode (wRDE) for assessing heterogeneous water oxidation catalysts (WOCs). Chem. Commun. 2016, 52, 7727–7730.
- Martens, S.; Asen, L.; Ercolano, G.; Dionigi, F.; Zalitis, C.; Hawkins, A.; Bonastre, A.M.; Seidl, L.; Knoll, A.C.; Sharman, J.; et al. A comparison of rotating disc electrode, floating electrode technique and membrane electrode assembly measurements for catalyst testing. J. Power Sources 2018, 392, 274–284.
- Bernt, M.; Hartig-Weiß, A.; Tovini, M.F.; El-Sayed, H.A.; Schramm, C.; Schröter, J.; Gebauer, C.; Gasteiger, H.A. Current Challenges in Catalyst Development for PEM Water Electrolyzers. Chem. Ing. Technol. 2020, 92, 31–39.
- Zhong, G.; Xu, S.; Liu, L.; Zheng, C.Z.; Dou, J.; Wang, F.; Fu, X.; Liao, W.; Wang, H. Effect of Experimental Operations on the Limiting Current Density of Oxygen Reduction Reaction Evaluated by Rotating-Disk Electrode. ChemElectroChem 2020, 7, 1107–1114.
- Ratera, I.; Veciana, J. Playing with organic radicals as building blocks for functional molecular materials, Chem. Soc. Rev. 2012, 41, 303–349.
- Kang, S.-F.; Chang, H.-M. Coagulation of textile secondary effluents with fenton’s reagent. Water Sci. Technol. 1997, 36, 215–222.
- Lefèvre, M.; Proietti, E.; Jaouen, F.; Dodelet, J.-P. Iron-Based Catalysts with Improved Oxygen Reduction Activity in Polymer Electrolyte Fuel Cells. Science 2009, 324, 71–74.
- Neyerlin, K.C.; Gu, W.; Jorne, J.; Gasteiger, H.A. Determination of Catalyst Unique Parameters for the Oxygen Reduction Reaction in a PEMFC. J. Electrochem. Soc. 2006, 153, A1955.
- Jaouen, F.; Goellner, V.; Lefèvre, M.; Herranz, J.; Proietti, E.; Dodelet, J. Oxygen reduction activities compared in rotating-disk electrode and proton exchange membrane fuel cells for highly active FeNC catalysts. Electrochim. Acta 2013, 87, 619–628.
- Gasteiger, H.A.; Kocha, S.S.; Sompalli, B.; Wagner, F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 2005, 56, 9–35.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
513
Revisions:
2 times
(View History)
Update Date:
09 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




