Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | NIKUNJ TYAGI | -- | 5753 | 2024-01-05 07:25:50 | | | |
| 2 | Rita Xu | -3 word(s) | 5750 | 2024-01-05 07:44:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jamwal, S.; Jena, M.K.; Tyagi, N.; Kancharla, S.; Kolli, P.; Mandadapu, G.; Kumar, S.; Mohanty, A.K. Molecular Dynamics of Early Pregnancy in Farm Animals. Encyclopedia. Available online: https://encyclopedia.pub/entry/53466 (accessed on 10 March 2026).
Jamwal S, Jena MK, Tyagi N, Kancharla S, Kolli P, Mandadapu G, et al. Molecular Dynamics of Early Pregnancy in Farm Animals. Encyclopedia. Available at: https://encyclopedia.pub/entry/53466. Accessed March 10, 2026.
Jamwal, Shradha, Manoj Kumar Jena, Nikunj Tyagi, Sudhakar Kancharla, Prachetha Kolli, Gowtham Mandadapu, Sudarshan Kumar, Ashok Kumar Mohanty. "Molecular Dynamics of Early Pregnancy in Farm Animals" Encyclopedia, https://encyclopedia.pub/entry/53466 (accessed March 10, 2026).
Jamwal, S., Jena, M.K., Tyagi, N., Kancharla, S., Kolli, P., Mandadapu, G., Kumar, S., & Mohanty, A.K. (2024, January 05). Molecular Dynamics of Early Pregnancy in Farm Animals. In Encyclopedia. https://encyclopedia.pub/entry/53466
Jamwal, Shradha, et al. "Molecular Dynamics of Early Pregnancy in Farm Animals." Encyclopedia. Web. 05 January, 2024.
Copy Citation
Infertility is a major problem in farm animals, which has a negative economic effect on farm industries. Infertility can be defined as the inability of animals to achieve a successful pregnancy. Early pregnancy is crucial to establish a successful pregnancy, and it is reported that 70–80% and 20–30% of total embryonic loss occur in cattle and pigs, respectively, during the first month of pregnancy.
early pregnancy
proteomics
mass spectrometry
embryo implantation
1. Introduction
Implantation is a highly coordinated and intricately timed mechanism that requires an essential cross-signaling between blastocyst and maternal endometrium to establish a successful pregnancy in mammals, including domestic animals (cattle, sheep, pigs, and goats), humans, and primates [1]. The process of implantation varies among species, and it has been divided into three categories based on the series of events taking place during interaction between blastocyst and uterine cells. The three categories are centric, eccentric, and interstitial types of implantation [2]. The centric type of implantation occurs in domestic animals, where blastocysts grow in size and fuse with the apical membrane of luminal epithelia without invading it. On the other hand, in eccentric and interstitial types of implantations, blastocysts invade the luminal epithelia partially and fully, respectively [3]. The eccentric type of implantation occurs in rodents, including rats, mice, and hamsters, whereas humans and guinea pigs involve an interstitial type of implantation [2]. The general mechanism of implantation (Figure 1) is conserved across mammals and follows the shedding of the zona pellucida, orientation, apposition, attachment, and adhesion of the blastocyst to the endometrium; however, after fertilization, the timing of the blastocyst’s development and subsequent series of implantation stages vary among species, most importantly the window of implantation (WOI), where the uterus allows the blastocyst to attach and fuse [3][4]. In cattle, blastocysts are formed after 7–8 days post-fertilization and do not implant until 30 days post-fertilization. In pigs, blastocysts are formed after 6 days post-fertilization, and implantation takes place 20–22 days post-fertilization [5]. In sheep, blastocysts are formed after 6 days post-fertilization, and implantation takes place after 22 days post-fertilization [3].
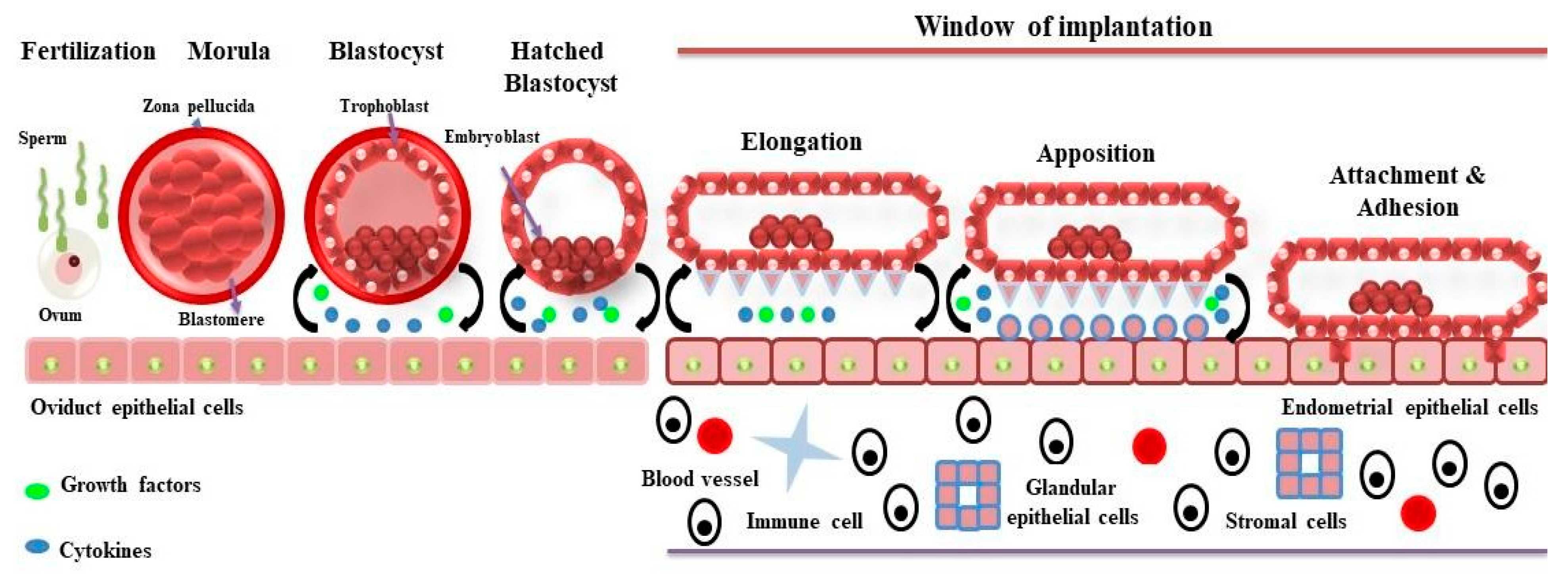
Figure 1. The process of implantation into the maternal endometrium in domestic ruminants occurs in various steps that involve fertilization, blastocyst hatching, elongation, apposition, attachment, and adhesion. During these phases, the conceptus and/or endometrium release several growth factors and cytokines, including interferon tau, into the uterine microenvironment. These molecules act in an autocrine and paracrine manner so that proper communication can occur between the conceptus and the uterine endometrium for the establishment of a successful pregnancy.
Infertility is a major problem in farm animals, which has a negative economic effect on farm industries. It can be defined as the inability of animals to achieve a successful pregnancy. Various factors are implicated in infertility, such as nutrition, genetics, environmental factors, and maternal factors like incompetent embryo, loss of embryo, lack of signals during cross-talk between embryo and uterine endometrium, and non-receptive state of uterine endometrium [6]. The peri-implantation period is considered critical for embryo development and endometrium receptivity in ruminants, as most of the pregnancy losses occur in this period [7]. In cattle, early embryonic mortality is explained as the death of fertilized ova and embryos up to day 28 of gestation [8]. After a successful insemination, 70–80% of total embryonic loss occurs in cattle during the first three weeks of post-insemination [9], whereas in pigs, the prenatal death rate accounts for 30–40% on average, with the largest loss of 20–30% during the first month of gestation [10]. It is necessary to improve the reproductive efficiency of female animals to reduce the economic loss in farm industries. Thus, an in-depth understanding of molecular mechanisms and signaling pathways involved in the processes of post-hatching development, endometrial receptivity, and implantation is essential to develop therapeutic strategies for successful pregnancy in livestock.
“Omics”-based high-throughput techniques have emerged as promising tools to understand the underlying mechanisms of a biological system, which provide information on the global contents of proteins, lipids, RNAs, and metabolites in cells or tissues [11]. Based on the study of cellular contents, “omics”-based studies have been categorized as follows: Proteomics (global profiling of proteins), transcriptomics (global profiling of RNA transcripts), metabolomics (global profiling of metabolites), and lipidomics (global profiling of lipids) [12] Proteins are the primary functional biomolecules of cells [13], and the expression and state of proteins give rise to different physiological processes at the cellular level [14]. The term ‘proteome’ is defined as the total protein content present in a cell or an organism throughout their entire lifespan [15][16]. A single gene encodes a single protein or variants of proteins; however, a single protein exhibits different functions depending on expression, cellular localization, and post-translational modification (PTM) [16].
The term ‘proteoform’ is being used to define the complexity of ‘proteome’ due to variation in single proteins [17]. Thus, a ‘proteome’ can be defined as a set of total proteoforms expressed in biological material (specific tissue, fluids, cell type, or organelle) at a particular time point [16]. The proteomic approaches provide systematic analysis of proteoforms, which constitute a parts of the proteome, as well as quantitative analysis of protein abundance [18][19]. The mass-spectrometry (MS)-based approaches, namely top-down and bottom-up proteomics, are used for proteomic analysis [18][20]. The top-down approach is demonstrated by 2-D DIGE (2-dimensional Difference Gel Electrophoresis), and MS/MS techniques involve separation of intact proteins and their proteoforms on the basis of their pI (isoelectric point) and MW (molecular weight), followed by excision of protein gel, proteolytic digestion, and MS analysis [18]. The bottom-up proteomics is a more widely used technique that involves proteolytic digestion of proteins into small peptides, separation by liquid chromatography, ionization, and MS analysis [20]. Proteomics-based technologies have been adopted to understand the early phase of pregnancy in farm animals. A generalized overview of the workflow for top-down and bottom-up proteomics for proteomic analysis of early pregnancy is shown in Figure 2.
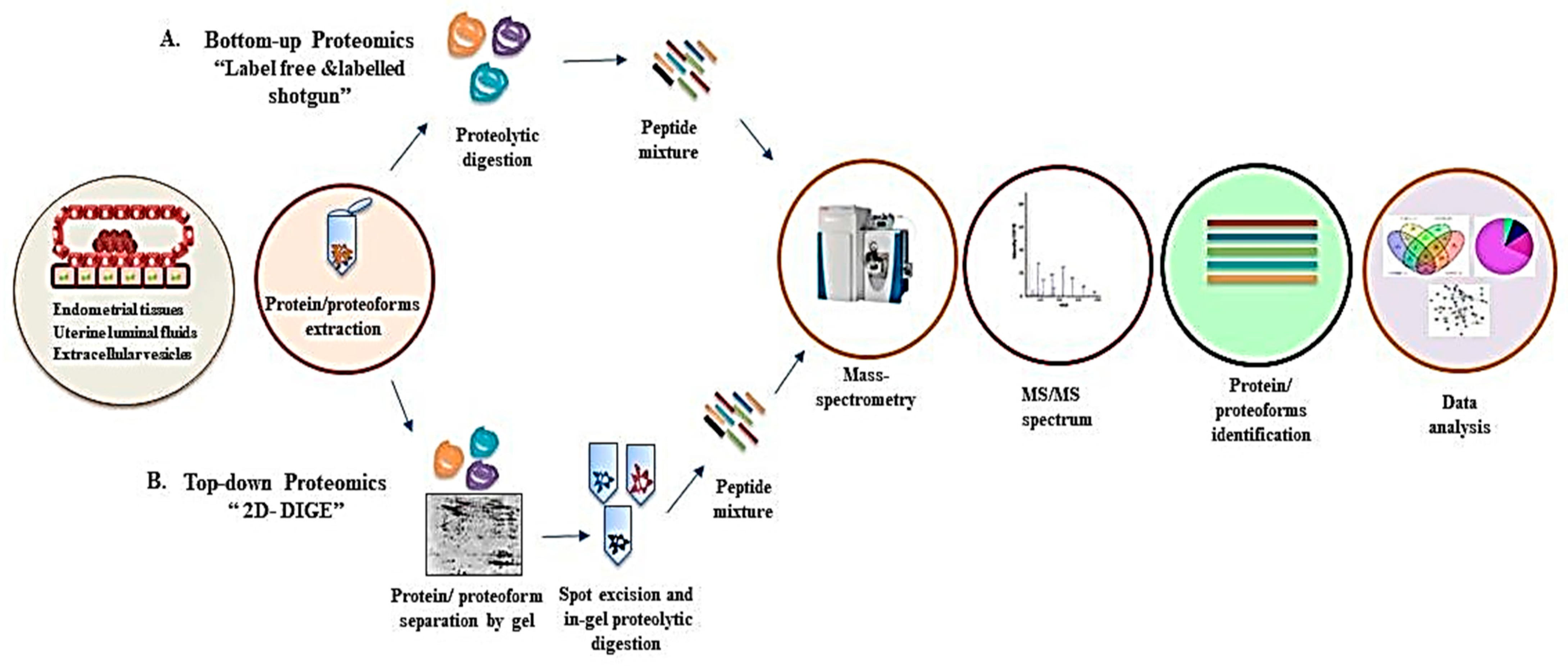
Figure 2. A generalized overview of mass spectrometry-based proteomics analysis of different sources from the early phase of pregnancy shows various steps involved in proteome analysis by two approaches, which are as follows: (A) Bottom-up proteomics: protein extraction, enzymatic digestion of protein/proteoforms followed by mass spectrometry analysis, identification of proteins, and data analysis using different bioinformatics tools. (B) Top-down proteomics: protein/proteoform extraction, gel separation, spot excision, proteolytic digestion followed by identification by mass spectrometry, and data analysis.
2. Proteomics of Bovine Uterus Milieu to Decipher the Molecular Landscape of Successful Pregnancy
Receptive endometrium is vital for appropriate fetal-maternal communication to establish a successful pregnancy. Bovine endometrium, based on its structure and function, is divided into two layers, namely the basal layer and the functional layer with pseudostratified columnar/simple columnar epithelial cells [21]. The endometrium undergoes morphological and functional changes throughout the estrous cycle to receive a viable competent blastocyst [22] and remains under the influence of ovarian hormones (estradiol-17β and progesterone). Progesterone plays a key role in the maintenance of pregnancy [23]. The endometrium is transformed from a non-receptive to a receptive state when it comes in contact with floating blastocysts and is known to be affected by various signaling molecules released from blastocysts and endometrial cells [24]. To this date, it is unclear how embryo implantation is regulated in ruminants [25]. Therefore, it is necessary to identify molecules and signaling pathways associated with endometrial receptivity and embryo implantation. This may lead to the discovery of molecular markers as predictors of implantation and pregnancy, which would further pave the way for finding therapeutic solutions to treat reproductive disorders.
2.1. Proteome Profiling of Endometrial Tissue
A study on cow endometrial tissue from pregnant and non-pregnant animals at the onset of the pre-attachment period, specifically on the 18th day of pregnancy, using quantitative proteomics-based approaches (2-D DIGE) and matrix-assisted laser desorption ionization-time-of-flight/time-of-flight mass spectrometry (MALDI-TOF/TOF MS) revealed interesting findings [26]. Four proteins, namely Rho GDP dissociation inhibitor beta (Rho GDI), 20 alpha-hydroxysteroid dehydrogenase (20 alpha HSD), isocitrate dehydrogenase (ICD), and acyl-CoA binding protein (ACBP), were identified with a two-fold increased abundance in pregnant animals in comparison to non-pregnant animals [26]. The abundance of these proteins suggests that they play an important role during the implantation process in ruminants. Isocitrate dehydrogenase (ICD) is an enzyme of the Krebs cycle, and Rho GDI is a member of the Rho guanosine diphosphate dissociation inhibitors family, which inhibits the GDP/GTP exchange [27]. Acyl-CoA binding protein (ACBP), also known as diazepam binding inhibitor, is an acyl-CoA binding protein that plays an essential role in lipid metabolism. The absence of the ACBP gene induces embryonic lethality during the early pre-implantation stage [28]. The enzyme 20-Alpha-HSD is involved in prostaglandin catabolism in bovine endometrium [26]. It is suggested in the mice study that maternal and fetal 20-Alpha-HSD are essential for the maintenance of pregnancy. The deletion of enzyme 20-Alpha-HSD increased the progesterone level, which is lethal for fetus survival [29].
The impact of embryonic mortality varies among species; for instance, early embryonic mortality is more prevalent in cattle, which occurs before Day 19 of pregnancy, whereas it has limited effects on buffaloes [30]. Late embryonic mortality (from Day 25 to Day 32) is a major cause of poor reproductive efficiency in buffaloes [30][31]. Till 2013, the proteome of the buffalo embryo-uterine interaction or uterine environment was unknown. For the first time, Balestrieri et al. employed 2D-DIGE and MALDI-ToF mass spectrometry techniques to elucidate the proteomic profiles of the chorioamnions and uterine caruncles in the water buffalo [32]. They compared the difference in protein expression of the chorioamnion and the corresponding caruncles of retarded embryos to those of normal embryos (on day 27 of gestation) [32]. A total of 95 differentially expressed protein spots (p < 0.05) were identified in tissue from normal and retarded embryos by 2D-DIGE analysis, and 93 differentially expressed protein spots (p < 0.05) in normal and retarded car uncles [32]. Further analysis by MALDI-ToF mass spectrometry revealed a total of 18 proteins (normal and retarded embryos) and 17 proteins (normal caruncles and retarded caruncles) with a significant total ion score confidence interval. Gene ontology (GO) of differentially expressed proteins (DEPs) in terms of molecular functions in the chorioamnions revealed their association with different molecular functions such as DNA and RNA binding, cell redox homeostasis, protein folding, cytoskeletal organization, proteolysis regulation, chromosome segregation, protein transport, and calcium binding. The molecular function analysis of identified proteins in caruncles showed their involvement in protein folding, hemoglobin binding, proteolysis regulation, and the nucleoside metabolic process [32].
2.2. Proteome Profiling of Uterine Lumen Fluid (ULF)
Endometrial tissue is widely used in the study of reproductive biology; however, obtaining an endometrial biopsy could have a detrimental effect on embryo implantation. There fore, less invasive methods such as uterine fluids can be a great alternative to exploring the process of implantation and other reproduction mechanisms [33]. In addition, there is a possibility that the results obtained from the biopsy will not match the subsequent cycles of pregnancy [34]. Uterine lumen fluid (ULF) is highly dynamic and contains various components such as hormones, enzymes, cytokines, growth factors, protein carriers, amino acids, and other substances collectively known as histotroph. These substances are synthesized and secreted by uterine cells, namely epithelial cells (luminal and glandular cells) and stromal cells [35]. These secretions are crucial for the establishment and maintenance of pregnancy in mammals. In a recent report, Simintiras et al. used high-throughput untargeted semi-quantitative metabolomic profiling on ULF and showed that uterine fluid is metabolically semi-autonomous and has a role in the active metabolic pathways due to the presence of active enzymes [36]. Their study found 317 and 7 more metabolites on days 12 and 16, respectively, in ULF from cyclic heifers through metabolomic profiling, and these metabolites were associated with fertility and pregnancy [36]. Various methodologies from different studies have been summarized and reviewed in detail by Itze-Mayrhofer and Brem, revealing the processes for sample preparation and the workflow of proteomics analysis of uterine fluids (UF) in different farm animals [37].
2.2.1. Proteomics of Uterine Lumen Fluid (ULF) in In Vivo Studies
The first study using ULF was reported by Ledgard et al., who compared the ULF proteome of pregnant cows with that of nonpregnant cows during the peri-attachment period (specifically on days 16 and 18) [38]. By using the 2D-DIGE approach, 9 DEPs with high abundance were identified in pregnant cows, whereas 4 proteins had low abundance. The abundant proteins were mainly involved in biosynthetic pathways, cytoskeleton organization, the Kreb cycle, and antioxidant activity. The less abundant proteins were found to be associated with endometrial remodeling. The proteins obtained in endometrial biopsy proteome analysis by Berendt et al. showed the least similarity with this study, and only one common protein, isocitrate dehydrogenase (ICD), was observed [26]. A study on the effect of the uterine environment at different stages of the oestrous cycle on blastocyst development post-hatch using the 2D-DIGE approach identified 10 DEPs on ULF between Day 5 and Day 9 after oestrus [39].
With advancements in proteomics-based approaches, Faulkner et al. employed isobaric tags for relative and absolute quantitation (iTRAQ)-based quantitative proteomics to characterize bovine endometrium [40]. In their study, they compared the UF with blood plasma from beef heifers on day 7 of the oestrous cycle. In total, 112 proteins were identified in UF and plasma, out of which 53 (in UF, 35 proteins upregulated and 18 proteins downregulated) DEPs (minimum fold change of ±1.5 or greater and FDR < 0.10) were selected for analysis. The identified DEPs were mostly metabolic enzymes, anti-oxidants, and immune response modulators, which were associated with 12 different biological processes, demonstrating the multifunctional role of the uterine proteome [40]. Using the same quantitative proteomics-based approach, Faulkner et al. investigated the effects of progesterone and stage of the oestrous cycle on the uterine proteome in beef heifers on day 5 of the oestrous cycle [41]. For proteome profiling, the UF were collected from animals at days 7 and 15 of the oestrous cycle and divided into low and high progesterone groups. A total of 169 proteins were identified on each day of the cycle, out of which 40 were DEPs identified on Day 15 compared to Day 7. There were 20 up-regulated proteins (5.9-fold higher, p < 0.05, FDR < 0.10) and 20 down-regulated proteins (2.3-fold lower, p < 0.05, FDR < 0.10) present in the uterine proteome on Day 15, which were associated with different biological processes including apoptosis, cell communication, cell cycle, cellular processes, developmental process, immune system, cellular transport, homeostatic process, response to stimuli, and metabolic process [41]. It was observed that progesterone has an effect on protein expression at different days of the cycle (days 3–7 or 15) [41]. Similarly, the study by Mullen et al., using a gel-free approach to globally characterize the bovine uterine proteome at different stages of the oestrous cycle, revealed 300 proteins (on Day 7) and 510 proteins (on Day 13), identified in high-fertility cattle by utilizing a label-free liquid chromatography−tandem mass spectrometry (LC−MS/MS) approach [42]. The bioinformatics analysis showed that proteins from both groups were majorly involved in metabolism, multicellular development, transport, and signal transduction [42]. This study revealed two novel proteins in UF on Day 13, namely S100-A8 and S100-A9, which belong to the S100 family of molecules, are inflammatory proteins with affinity to Ca2+ ions [43], and play an important role at the maternal-fetal interface. The altered expression of these proteins is known to be lethal for pregnancy establishment [44]. These above-mentioned studies [40][41][42] described the proteome profile of uterine fluid in pregnant cows at a single or two points on days 5, 7, 13, 16, 15, and 18. A study on the global protein content in UF from days 10, 13, 16, and 19 by using iTRAQ-based quantitative analysis revealed proteins such as RPB4 (DNA-dependent RNA polymerase), TIMP2 (tissue inhibitor of metalloprotease-2), IFNT (interferon Tau), ALDOA (aldolase A), cytochrome C oxidase, GSN (gelsolin), HSP90A1 (heat shock protein 90-alpha), SERPINA31 (alpha-1 antitrypsin), PNP (purine nucleoside phosphorylase), and HSPA8 (heat shock protein family A (Hsp70) member 8) proteins present during the pre-implantation period of pregnancy in cows [45].
In another attempt to explore the protein profile of UF in relation to embryo growth and development, Beltmen et al. compared the UF of beef heifers with that of normal and degenerate embryos on Day 7 after insemination using LC-MS technology [46]. Out of 40 identified proteins, six up-regulated proteins were found in viable embryos, and one up-regulated protein was found in degenerate embryos [46]. Unique proteins such as platelet-activating factor acetyl hydrolase 1b catalytic subunit 3 (PAFAH1B3) were found in the animal group with viable embryos. The protein PAFAH1B3 is a subunit of platelet-activating factor acetyl hydrolase (PAF-AH), which plays crucial roles in various physiological processes such as apoptosis, wound healing, fertilization, implantation, and embryonic development [47][48][49]. S100-A4, the only highly abundant protein found in groups with degenerate embryos, is also known as metastasin 1 (Mts1) or fibroblast-specific protein 1 (Fsp1) and is involved in a variety of biological functions, such as cell motility, adhesion, and invasion [50]. This protein is also known to be involved in the progression and metastasis of cancer [51]. It is not clear how the S100-A4 protein regulates embryonic development and endometrial function during the onset of implantation, which needs further study. In another study, Fortes et al. employed RNA-sequencing on uterine tissue (for transcriptome profiling) and MS for proteome profiling of UF to relate the protein and gene expression patterns of the uterus and its secretion during puberty and unravel the molecular dynamics during implantation and pregnancy [52]. This study made a comparison of the proteome and transcriptome data of pre-pubertal and post-pubertal cycling Bos indicus heifers and found 4 DEPs encoded by differentially expressed genes in post-pubertal heifers: OVGP1 (oviduct-specific glycoprotein), GRP (gastrin-releasing peptide), CAP1 (cyclase-associated actin cytoskeleton regulatory protein 1), and HBA (hemoglobin alpha 2). It is suggested that these proteins may help the uterus prepare for a successful pregnancy. OVGP1 (oviduct-specific glycoprotein 1) is an estrogen-dependent protein that is found in the oviductal fluid of different mammalian species, including bovine. OVGP1 (oviduct-specific glycoprotein 1) plays an important role in the processes of fertilization, sperm capacitation, and embryonic development [53][54]. During fertilization, OVGP1 binds to the zona pellucida of oocytes and modifies the matrix structure to allow sperm penetration [55]. In a study with a human endometrial epithelial cell line, it was found that OVGP1 regulates the expression of receptivity-related genes, including cytokines, matrix metalloproteases, and tissue inhibitors of matrix metalloproteases [56]. CAPs (cyclase-associated actin cytoskeleton regulatory protein) are an actin-binding protein that, together with ADF/cofilin, regulates actin dynamics [57]. Cofilin, also an actin-binding protein (a component of actin cytoskeleton proteins), plays an important role in endometrium remodeling during blastocyst implantation [58]. The protein GRP is released by uterine glandular cells into the uterine lumen and regulates pregnancy through autocrine and paracrine modes of signaling [59]. Moraes et al. compared the UF from high fertility to sub-fertile or infertile heifers on Day 17 [60]. In total, 221 DEPs were found in pregnant high-fertile and sub-fertile heifers, out of which 142 were up-regulated and 79 were down-regulated [60]. These DEPs were associated with vitamin B6 metabolism, energy metabolism, the p38 MAPK pathway, cytoskeletal regulation, hemostasis, the plasminogen activating cascade, and blood coagulation pathways [60]. The differential expression of proteins among these two groups indicated that these proteins alter the uterine environment and influence the uterine receptivity for successful implantation and the survival of the fetus.
To enhance the milk yield in dairy cows, single trait selection has been extensively used; however, it has led to a decline in fertility in high-yielding dairy cows [61][62]. In a recent study, Gegenfurtner et al. investigated the effect of genetic merit for fertility on the proteome of the bovine uterine luminal fluid from Day 19 of pregnancy [62]. Using nanoLC-MS/MS coupled with a label-free quantification approach, they identified 597 DEPs between the three groups: heifers with a low fertility index (Holstein) and two groups of heifers with a high fertility index (Holstein and Montbéliarde) [62]. Sorbitol dehydrogenase (SORD) was highly abundant (with a 14.3- and 11.1-fold expression) in high fertility groups (Holstein and Montbéliarde) in comparison to low fertility groups (Holstein) [62]. The protein SORD (sorbitol dehydrogenase) is required for fructose synthesis from glucose. Fructose is the most abundant hexose sugar in the conceptus and endometrium of porcines and may play an important role in the growth and development of the fetus [63]. SORD (sorbitol dehydrogenase) is expressed by porcine uterine epithelial cells during the peri-implantation stage, indicating its role in implantation [62].
The postpartum and peripartum periods are critical stages for reproductive health and are decisive factors for fertility [64]. Aranciaga et al. explored the ULF from early to mid-postpartum (first to third estrus) to analyze the effects of these molecular changes on embryonic development [65]. LC–MS/MS (Liquid chromatography with tandem mass spectrometry) analysis identified a total of 1563 proteins, of which 472 were novel in bovine ULF at day 7 of pregnancy at the first and third estrus postpartum. Gene ontology (GO) revealed these proteins to be involved in metabolic processes, regulatory pathways, responses to stress, and immunomodulation [65]. The proteins that were identified in ULF and have an impact on embryo quality are eukaryotic translation initiation factor 5A1, plasminogen, membrane-associated guanylate kinase inverted 3, phospholipase A2 activating protein (PLAA), triosephosphate isomerase (TIM), delta-amino levulinic acid dehydratase (ALADH), costars family protein (ABRACL) dihydropteridine reductase, cystatin B, acyl-CoA-binding protein (DBI), protein S100A2, Unc-80 homolog and NALCN channel complex subunit, ATP synthase subunit beta, macrophage migration inhibitory factor (MIF), pyruvate kinase (PKM2), alpha-1-antiproteinase (SERPINA1), prostaglandin reductase 1 (PTGR1), and myostatin (MSTN) [65].
2.2.2. Proteomics of Uterine Lumen Fluid (ULF) in In Vitro Studies
In vitro models utilizing the co-culture of endometrial cells and embryos or trophoblast cells provide a great opportunity to study the implantation process at different time intervals, which is difficult to investigate in in vivo studies. Moreover, the in vitro models are easy to manipulate and readily available [66]. Several powerful techniques have been developed over the past several decades, and one of them is microfluidic technology, with a broad range of applications in cell biology research [67]. A study by De Bem et al. used the endometrium-on-a-chip microfluidics approach, which can mimic the bovine endometrium in vitro, to study the transcriptome and proteomic secretome of endometrial cells (epithelial and stromal cells) under the influence of maternal metabolic factors [68]. It has been observed that metabolic factors such as glucose and insulin alter the transcriptome and protein secretome content of the uterine luminal fluid. In a transcriptomics study, 21 genes in epithelial and 191 genes in stromal cells were altered under high glucose concentrations, while there was limited change in gene expression under the influence of insulin. In a quantitative proteomics study, 1 and 23 proteins were altered in epithelial and stromal cells, respectively, under the presence of glucose, while 196 proteins were found in the secretome under the influence of insulin [68]. The proteins identified in this study were involved in different biological processes; and the pathways which were regulated by different concentrations of insulin were as follows: synthesis of amino acids, carbon metabolism, synthesis of antibodies, metabolic pathways, complement and coagulation cascades, protein processing in endoplasmic reticulum, and amoebiasis, protein digestion and absorption, extracellular matrix (ECM)–receptor interaction, and proteoglycans in cancer (Proteoglycans are present on the cell surface and can interact with both ligands and receptors, thus, execute multiple functions in cancer), while two major pathways like platelet and lysosome pathways were up-regulated by glucose treatment [68]. Altogether, this study demonstrated the use of a novel in vitro model to study uterine functions and fetal-uterine interaction.
2.2.3. Proteomics of Exosomal microRNAs in Uterine Lumen Fluid (ULF)
MicroRNAs (miRNAs) are small non-coding RNAs that act as post-transcriptional regulators of gene expression and are present in multiple subcellular compartments, including the rough endoplasmic reticulum, processing (P)-bodies, trans-Golgi network, lysosomes, mitochondria, and nucleus [69]. The miRNAs that are released by the endometrium are known to be involved in the implantation and pregnancy processes, and their abnormal expression is associated with a lack of fetal-maternal cross-talk and pregnancy failure. The miRNAs may serve as a better alternative to invasive biomarkers for analysis of implantation or reproductive health in animals, as invasive methods pose a threat to embryo implantation [70][71][72]. Recently, Kusama et al. used iTRAQ-based analysis to investigate the global protein content and exosomal miRNAs in the UF of cows at Day 7 of pregnancy [72]. A total of 260 proteins were up-regulated among the total identified 336 proteins. These proteins were mainly involved in innate immunity and, more specifically, in neutrophil-mediated immune responses [72]. The isolated exosomes from UF expressed 37 miRNAs, of which 3 miRNAs were low-abundant and six miRNAs were high-abundant in artificially inseminated cows.
Principal component analysis showed that miRNAs and proteins found in UF have a strong relationship and identified a unique protein called suppressor of the G2 allele of the SKP1 homolog (SUGT1), associated with embryonic development [69]. SUGT1 (suppressor of the G2 allele of the SKP1 homolog) is a highly conserved protein involved in multiple biological processes such as innate immune response, centrosome organization, and cytokinesis [73]. Further studies are needed to understand the role of this protein in regulating the uterine environment during pregnancy. In addition to this study, Koh et al. compared the exosomes extracted from the blood plasma of high- and low-fertility heifers using mass spectrometry and identified 4 unique proteins in high-fertility heifers and 31 unique proteins in low-fertility heifers [74].
The data presented in the proteomics-based studies on endometrial tissues and UF gives an insight into the complex mechanisms of pregnancy, including implantation, embryo development, uterine molecular signatures at the postpartum period, and puberty. However, further investigation is needed to understand the complex process of pregnancy, which can address issues related to reproductive failure in animals.
2.3. Proteome Profiling of Oviductal Fluids (OF)
The oviduct is the part of the reproductive tract in mammalian species where several crucial events take place, such as sperm storage, transportation of sperm and oocytes, fertilization, and embryonic development, which are indispensable for a successful pregnancy. Fertilization occurs when oocytes are released by the process of ovulation into the oviduct, which allows the stored sperm to fertilize with the oocytes. The oviduct remains under the influence of different factors and undergoes physiological and morphological changes [75][76]. The oviductal fluid (OF) is secreted by oviductal luminal epithelial cells and is composed of complex mixtures of ions, proteins, metabolites, lipids, and glycans [77][78]. It is essential to understand how the OF affects the mechanisms of fertilization, oocyte-sperm interaction, and early embryonic development. In recent years, several attempts have been made to understand the global contents of OF using “omics”-based studies.
The proteome analysis of the bovine OF revealed many DEPs according to the site of ovulation, the stage of the oestrous cycle, and progesterone concentration using the label-free method [79]. The OF (oviductal fluid) was analyzed at four stages of the oestrous cycle in cyclic cows: pre-ovulatory, post-ovulatory, mid-, and late-luteal phases. A total of 482 proteins were present on oviductal secretions during the oestrous cycle, and anexin A1 was the most abundant protein at the pre-ovulatory stage, whereas heat shock proteins (HSP) were upregulated at the post-ovulatory bovine ipsilateral OF [79].
Annexins, a family of calcium-dependent phospholipid-binding proteins, are a highly conserved group of proteins present in all eukaryotes, including humans, animals, and plants. There are more than 100 types of annexins discovered so far across all species. The common composition of annexins involves two main domains: the head (NH2-terminal) and the core (COOH-terminal). The core region is conserved, while the head is divergent among the members of the annexin family, and the C-terminal interacts with calcium, further mediating canonical membrane binding properties [80][81]. Annexins are involved in different biological processes, including exocytosis, endocytosis, stabilization of the plasma membrane at a specific site, RNA-binding, and nucleotide-binding functions. However, the functions of all annexins are not fully understood [82]. Several annexins are found to be associated with female reproduction and are expressed at different sites of the uterus. Annexins A2, A1, and A7 are expressed by endometrial cells and regulate endometrial receptivity and embryo implantation in humans and mice [83][84][85].
In another study, Pillai et al. used secretions from in vitro models of bovine oviductal epithelial cells (OEC) and ex vivo OF; a total of 2087 proteins were identified, of which 266 were secretory in nature [86]. In terms of functions, proteins were active in immune homeostasis, gamete maturation, fertilization, and early embryonic development. The OF (oviductal fluid) also contains exosomes, which may act as key modulators of embryonic development and survival. Alminana et al. proposed that the exosomes play a vital role in early embryonic development and gamete-oviduct interactions and characterized the molecular signatures of bovine oviductal extracellular vesicles (oEVs) derived from in vivo and in vitro samples at different stages of the bovine estrous cycle using mass-spectrometry-based approaches [87][88]. In the first study, a total of 319 proteins were found in oviductal extracellular vesicles (oEVs) at the post-ovulatory stage. Then, they compared the protein content of in vivo and in vitro oEVs; 186 DEPs were identified in oEVs. This study revealed important proteins that regulate the gamete/embryo-oviduct interactions, which are oviductal glycoprotein 1, HSP90, A8, and 70, gelsolin (GSN), and ezrin (EZR) [87]. In the second study, they analyzed the oEVs at different stages of the bovine estrous cycle. A total of 336 proteins were identified in this study, out of which 170 were differentially expressed across the estrous cycle (p-value < 0.05, ratio < 0.5, or >2). GO analysis showed overrepresented terms related to vesicles, focal adhesion, cytoskeleton, cell surface metalloexopeptidase activity, and the innate immune system [88].
In general, OF is obtained through invasive surgery, a labor-intensive and time-consuming process that is stressful for the animals. Papp et al. developed a novel method to retrieve the OF using a less invasive method of transvaginal endoscopy for proteome profiling [89]. In total, 3001 proteins were identified (FDR ≤ 1%) in heifers at two different stages of the estrous cycle (Day 1 and Day 3), and it was observed that common proteins like oviductal glycoprotein 1 and HSP identified in this study have also been reported by previous groups [89]. Comparative analysis of the OF (oviductal fluid) proteome between pregnant and cyclic heifers at different oviductal regions, including the ampulla and isthmus, on Day 3 post-estrus revealed many interesting findings [90]. Some important proteins, such as serum albumin, serotransferrin, stress-induced phosphoprotein 1, phosphoglycerate kinase 1, and serpina1, were in high abundance in the presence of embryos, indicating a potential role in embryonic development [90]. Another study with the bovine OF proteome, taking into account three factors such as the anatomical oviduct region (isthmus vs. ampulla), the peri-ovulatory stage (pre-ovulatory vs. post-ovulatory), and the proximity of the ovulating ovary (ipsi- lateral vs. contralateral side) using the nano-LC-MS/MS approach, identified many DEPs [77]. This study identified a total of 3760 proteins in the bovine OF, which are the most comprehensive proteomes published until now. They performed a quantitative analysis using a label-free proteomic approach and found differentially abundant proteins involved in a broad range of biological functions like protein binding, response to stress, cellular adhesion, calcium homeostasis, and immune system modulation [77]. Many abundant proteins were identified in bovine OF, including several HSPs, myosin, CD109, complement C3, and oviductin, as described in other studies as well. These studies covered various factors that influence the bovine OF, including the stage of the cycle, the presence of gametes, the anatomical region of the oviduct, the sides of ovulation, and the in vitro secretome of the OF. However, there are several other factors, such as fertility index, metabolic stress, and the effects of lactation, that can modulate the proteome of bovine OF, thus affecting embryonic development and early implantation [91]. In an attempt to analyze the effects of fertility index and metabolic stress on dairy cows, Gegenfurtner et al. compared OF from two animal models: a metabolic model (postpartum lactating and non-lactating cows) and a genetic model (low- and high-fertility index) using nano-liquid chromatography tandem mass spectrometry analysis and label-free quantification [91]. A total of 1976 proteins were present in OF, of which, in the metabolic model, a total of 37 significantly abundant proteins were found (p-value < 0.05, log2-fold change > 0.6), while in the genetic model, 110 proteins were significantly abundant (p-value < 0.05, log2-fold change > 0.6) in pair-wise comparisons. The analysis of GO terms for abundant proteins revealed associations with actin binding, translation, and immune system processes [91].
2.4. Proteome Profiling of Cows with Endometritis
Endometritis is a common disease with a major impact on the reproductive health of animals, which burdens the farm industry. The pathogenic bacteria often cause inflammation in the endometrial lining during parturition and coitus [92]. The endometrium plays an important role in reproduction, nourishment of the early embryo, and sperm transit. The inflammation in the endometrium hinders sperm mobility and embryo implantation, leading to pregnancy failure [93]. Postpartum bacterial infections affect 40% of dairy cows worldwide [94]. The endometrium consists of epithelial and stromal cells that express pathogen recognition receptors that show an innate immune response against microbes such as Escherichia coli, Fusobacterium necrophorum, Prevotella species, and Trueperella pyogenes (isolated from cows with endometritis) [92][94].
Over the past two decades, extensive research has been performed to identify the biomarkers related to endometritis. Advancements in the field of proteomics have renewed interest in the treatment of reproductive disorders in animals [95]. High-throughput proteomics-based approaches are great tools to explore the plethora of protein signatures associated with endometritis. Various sources, such as endometrial tissues, blood plasma, exosomes, and uterine secretions, have been used to investigate the protein profile associated with endometritis using proteomics approaches like 2-D gel electrophoresis and iTRAQ-based mass spectrometry.
Cairoli et al. investigated the protein expression pattern in cows during pregnancy and the peripartum period, with and without postpartum uterine infection, using a gel-based approach [96]. They identified a high difference in acute-phase proteins, including orosomucoid and haptoglobin, during the last phase of pregnancy and early postpartum. A very high level of orosomucoid was also observed in cows with postpartum endometritis after 2 weeks of calving. Orosomucoid is also known as α-1-acid glycoprotein (AGP), which is an anti-inflammatory protein, and its role is unclear [97]. The acute phase proteins are components of the natural defensive system and play a crucial role in the prevention of infections and the regeneration of tissues. In bovines, haptoglobin and serum amyloid A are found to be associated with reproduction physiology [98]. The acute phase proteins are sensitive and non-specific indicators of inflammation or infection, and recently they have been used as therapeutic targets in veterinary medicine in large animals for restoration of homeostasis [99].
Based on 2D-gel electrophoresis followed by protein identification by mass spectrometry, Choe et al. and Legard et al. compared cows with a normal uterus to cows with an endometritic uterus [93][100]. The former group identified 12 DEPs in endometrium with endometritis, and they suggested that desmin, α-actin, HSP27, HSP70, and MHC-I may play an important role in endometrium for successful implantation [93]. The latter group identified 12 proteins with high abundance in cows infected with Trueperella pyogenes in comparison to normal cows [100]. They also compared protein expression in relation to the percentage of polymorphonuclear neutrophils (PMNs) (0–35% PMNs) in lactating cows at 15 and 42 days postpartum [100]. The PMNs are the first line of defense and are released in the uterus after a pathogen attack. The detection of PMNs in uterine samples is frequently used as a diagnostic tool for the identification of uterine health. Few indicator proteins were found in the study that had a positive correlation with the percentage of PMNs (>10% PMN), namely cathelicidin, peptidoglycan recognition protein 1, serine protease inhibitor B1, and calprotectin at 15 days postpartum. The expression of these proteins was high in cows with an increased presence of T. pyogenes and an elevated PMN percentage [100].
References
- Bowen, J.A.; Burghardt, R.C. Cellular mechanisms of implantation in domestic farm animals. Semin. Cell Dev. Biol. 2000, 11, 93–104.
- Lee, K.Y.; DeMayo, F.J. Animal models of implantation. Reproduction 2004, 128, 679–695.
- Spencer, T.E.; Johnson, G.A.; Bazer, F.W.; Burghardt, R.C. Implantation mechanisms: Insights from the sheep. Reproduction 2004, 128, 657–668.
- MacIntyre, D.M.; Lim, H.C.; Ryan, K.; Kimmins, S.; Small, J.A.; MacLaren, L.A. Implantation-associated changes in bovine uterine ex-pression of integrins and extracellular matrix. Biol. Reprod. 2002, 66, 1430–1436.
- Paulson, E.E.; Comizzoli, P. Endometrial receptivity and embryo implantation in carnivores-commonalities and differences with other mammalian species. Biol. Reprod. 2021, 104, 771–783.
- Bajaj, N.K.; Sharma, N. Endocrine Causes of Early Embryonic Death: An Overview. Curr. Res. Dairy Sci. 2011, 3, 1–24.
- Binelli, M.; Silva, F.A.C.C.; Rocha, C.C.; Martins, T.; Sponchiado, M.; Van Hoeck, V.; Cordeiro, A.; Campbell, M.; Leroy, J.L.M.R.; Peñagaricano, F.; et al. Endometrial receptivity in cattle: The mutual reprogramming paradigm. Anim. Reprod. 2022, 19, e20220097.
- Reese, S.T.; Franco, G.A.; Poole, R.K.; Hood, R.; Fernadez Montero, L.; Oliveira Filho, R.V.; Cooke, R.F.; Pohler, K.G. Pregnancy loss in beef cattle: A meta-analysis. Anim. Reprod. Sci. 2020, 212, 106251.
- Diskin, M.G.; Morris, D.G. Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 2008, 43 (Suppl. S2), 260–267.
- Spencer, T.E. Early pregnancy: Concepts, challenges, and potential solutions. Anim. Front. 2013, 3, 48–55.
- La, Y.; Tang, J.; Guo, X.; Zhang, L.; Gan, S.; Zhang, X.; Zhang, J.; Hu, W.; Chu, M. Proteomic analysis of sheep uterus reveals its role in prolificacy. J. Proteom. 2020, 210, 103526.
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83.
- Smith, L.M.; Agar, J.N.; Chamot-Rooke, J.; Danis, P.O.; Ge, Y.; Loo, J.A.; Paša-Tolić, L.; Tsybin, Y.O.; Kelleher, N.L. Consortium for Top-Down Proteomics. The Human Proteoform Project: Defining the human proteome. Sci. Adv. 2021, 7, eabk0734.
- Cox, J.; Mann, M. Is proteomics the new genomics? Cell 2007, 130, 395–398.
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2017, 55, 182–196.
- Carbonara, K.; Andonovski, M.; Coorssen, J.R. Proteomes Are of Proteoforms: Embracing the Complexity. Proteomes 2021, 9, 38.
- Smith, L.M.; Kelleher, N.L.; Consortium for Top Down Proteomics. Proteoform: A single term describing protein complexity. Nat. Methods 2013, 10, 186–187.
- Ercan, H.; Resch, U.; Hsu, F.; Mitulovic, G.; Bileck, A.; Gerner, C.; Yang, J.W.; Geiger, M.; Miller, I.; Zellner, M. A Practical and Analytical Comparative Study of Gel-Based Top-Down and Gel-Free Bottom-Up Proteomics Including Unbiased Proteoform Detection. Cells 2023, 12, 747.
- Haider, S.; Pal, R. Integrated analysis of transcriptomic and proteomic data. Curr. Genom. 2013, 14, 91–110.
- Bludau, I.; Frank, M.; Dörig, C.; Cai, Y.; Heusel, M.; Rosenberger, G.; Picotti, P.; Collins, B.C.; Röst, H.; Aebersold, R. Systematic detection of functional proteoform groups from bottom-up proteomic datasets. Nat. Commun. 2021, 12, 3810.
- Thasmi, C.N.; Siregar, T.N.; Wahyuni, S.; Aliza, D.; Panjaitan, B.; Nazaruddin, N.; Sabila, F.N.; Fallatanza, M. Anatomical and histological changes of uterine horn of Aceh cattle with repeat breeding. J. Adv. Vet. Anim. Res. 2018, 5, 445–453.
- Arai, M.; Yoshioka, S.; Tasaki, Y.; Okuda, K. Remodeling of bovine endometrium throughout the estrous cycle. Anim. Reprod. Sci. 2013, 142, 1–9.
- Lonergan, P.; Forde, N. Maternal-embryo interaction leading up to the initiation of implantation of pregnancy in cattle. Animal 2014, 8 (Suppl. S1), 64–69.
- Salilew-Wondim, D.; Hölker, M.; Rings, F.; Ghanem, N.; Ulas-Cinar, M.; Peippo, J.; Tholen, E.; Looft, C.; Schellander, K.; Tesfaye, D. Bovine pretransfer endometrium and embryo transcriptome fingerprints as predictors of pregnancy success after embryo transfer. Physiol. Genom. 2010, 42, 201–218.
- Li, X.; Yao, X.; Xie, H.; Zhang, G.; Deng, M.; Deng, K.; Gao, X.; Bao, Y.; Li, K.; Wang, F. PPP2R2A affects embryonic implantation by regulating the proliferation and apoptosis of Hu sheep endometrial stromal cells. Theriogenology 2021, 176, 149–162.
- Berendt, F.J.; Fröhlich, T.; Schmidt, S.E.; Reichenbach, H.D.; Wolf, E.; Arnold, G.J. Holistic differential analysis of embryo-induced alter-ations in the proteome of bovine endometrium in the preattachment period. Proteomics 2005, 5, 2551–2560.
- Liu, S.; Cui, H.; Li, Q.; Zhang, L.; Na, Q.; Liu, C. RhoGDI2 is expressed in human trophoblasts and involved in their migration by inhibiting the activation of RAC1. Biol. Reprod. 2014, 90, 88.
- Landrock, D.; Atshaves, B.P.; McIntosh, A.L.; Landrock, K.K.; Schroeder, F.; Kier, A.B. Acyl-CoA binding protein gene ablation induces pre-implantation embryonic lethality in mice. Lipids 2010, 45, 567–580.
- Choi, J.H.; Ishida, M.; Matsuwaki, T.; Yamanouchi, K.; Nishihara, M. Involvement of 20α-hydroxysteroid dehydrogenase in the maintenance of pregnancy in mice. J. Reprod. Dev. 2008, 54, 408–412.
- Campanile, G.; Neglia, G.; Gasparrini, B.; Galiero, G.; Prandi, A.; Di Palo, R.; D’Occhio, M.J.; Zicarelli, L. Embryonic mortality in buffaloes synchronized and mated by AI during the seasonal decline in reproductive function. Theriogenology 2005, 63, 2334–2340.
- Russo, M.; Vecchio, D.; Neglia, G.; Pacelli, C.; Prandi, A.; Gasparrini, B.; Zicarelli, L.; D’Occhio, M.J.; Campanile, G. Corpus luteum function and pregnancy outcome in buffaloes during the transition period from breeding to non-breeding season. Reprod. Domest. Anim. 2010, 45, 988–991.
- Balestrieri, M.L.; Gasparrini, B.; Neglia, G.; Vecchio, D.; Strazzullo, M.; Giovane, A.; Servillo, L.; Zicarelli, L.; D’Occhio, M.J.; Campanile, G. Proteomic Profiles of the Embryonic Chorioamnion and Uterine Caruncles in Buffaloes (Bubalus bubalis) with Normal and Retarded Embryonic Development. Bio. Reprod. 2013, 88, 1–14.
- Boomsma, C.M.; Kavelaars, A.; Eijkemans, M.J.; Amarouchi, K.; Teklenburg, G.; Gutknecht, D.; Fauser, B.J.; Heijnen, C.J.; Macklon, N.S. Cytokine profiling in endometrial secretions: A non-invasive window on endometrial receptivity. Reprod. Biomed. Online 2009, 18, 85–94.
- Guo, X.; Li, T.C.; Chen, X. The endometrial proteomic profile around the time of embryo implantation. Biol. Reprod. 2021, 104, 11–26.
- Bazer, F.W.; Wu, G.; Johnson, G.A.; Kim, J.; Song, G. Uterine histotroph and conceptus development: Select nutrients and secreted phosphoprotein 1 affect mechanistic target of rapamycin cell signaling in ewes. Biol. Reprod. 2011, 85, 1094–1107.
- Simintiras, C.A.; Drum, J.N.; Liu, H.; Sofia Ortega, M.; Spencer, T.E. Uterine lumen fluid is metabolically semi-autonomous. Commun. Biol. 2022, 5, 191.
- Itze-Mayrhofer, C.; Brem, G. Quantitative proteomic strategies to study reproduction in farm animals: Female reproductive flu-ids. J. Proteom. 2020, 225, 103884.
- Ledgard, A.M.; Lee, R.S.; Peterson, A.J. Bovine endometrial legumain and TIMP-2 regulation in response to presence of a conceptus. Mol. Reprod. Dev. 2009, 76, 65–74.
- Ledgard, A.M.; Berg, M.C.; McMillan, W.H.; Smolenski, G.; Peterson, A.J. Effect of asynchronous transfer on bovine embryonic devel-opment and relationship with early cycle uterine proteome profiles. Reprod. Fertil. Dev. 2012, 24, 962–972.
- Faulkner, S.; Elia, G.; Mullen, M.P.; O’Boyle, P.; Dunn, M.J.; Morris, D. A comparison of the bovine uterine and plasma proteome using iTRAQ proteomics. Proteomics 2012, 12, 2014–2023.
- Faulkner, S.; Elia, G.; O’Boyle, P.; Dunn, M.; Morris, D. Composition of the bovine uterine proteome is associated with stage of cycle and concentration of systemic progesterone. Proteomics 2013, 13, 3333–3353.
- Mullen, M.P.; Elia, G.; Hilliard, M.; Parr, M.H.; Diskin, M.G.; Evans, A.C.; Crowe, M.A. Proteomic characterization of histotroph during the preimplantation phase of the estrous cycle in cattle. J. Proteome Res. 2012, 11, 3004–3018.
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298.
- Nair, R.R.; Khanna, A.; Singh, K. Role of inflammatory proteins S100A8 and S100A9 in pathophysiology of recurrent early preg-nancy loss. Placenta 2013, 34, 824–827.
- Forde, N.; McGettigan, P.A.; Mehta, J.P.; O’Hara, L.; Mamo, S.; Bazer, F.W.; Spencer, T.E.; Lonergan, P. Proteomic analysis of uterine fluid during the pre-implantation period of pregnancy in cattle. Reproduction 2014, 147, 575–587.
- Beltman, M.E.; Mullen, M.P.; Elia, G.; Hilliard, M.; Diskin, M.G.; Evans, A.C.; Crowe, M.A. Global proteomic characterization of uterine histotroph recovered from beef heifers yielding good quality and degenerate day 7 embryos. Domest. Anim. Endocrinol. 2014, 46, 49–57.
- Minhas, B.S.; Ripps, B.A.; Zhu, Y.P.; Kim, H.N.; Burwinkel, T.H.; Gleicher, N. Platelet activating factor and conception. Am. J. Reprod. Immunol. 1996, 35, 267–271.
- Tang, S.; Ni, J.; Chen, B.; Sun, F.; Huang, J.; Ni, S.; Tang, Z. PAFAH1B3 predicts poor prognosis and promotes progression in lung adenocarcinoma. BMC Cancer 2022, 22, 525.
- Xu, W.; Lu, X.; Liu, J.; Chen, Q.; Huang, X.; Huang, K.; Liu, H.; Zhu, W.; Zhang, X. Identification of PAFAH1B3 as Candidate Prognosis Marker and Potential Therapeutic Target for Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 700.
- Wu, Y.; Zhang, J.; Qin, Y. S100A4 promotes the development of lipopolysaccharide-induced mouse endometritis. Biol. Reprod. 2018, 99, 960–967.
- Fei, F.; Qu, J.; Li, C.; Wang, X.; Li, Y.; Zhang, S. Role of metastasis-induced protein S100A4 in human non-tumor pathophysiologies. Cell Biosci. 2017, 7, 64.
- Fortes, M.R.S.; Zacchi, L.F.; Nguyen, L.T.; Raidan, F.; Weller, M.M.D.C.A.; Choo, J.J.Y.; Reverter, A.; Rego, J.P.A.; Boe-Hansen, G.B.; Porto-Neto, L.R.; et al. Pre- and post-puberty expression of genes and proteins in the uterus of Bos indicus heifers: The luteal phase effect post-puberty. Anim. Genet. 2018, 49, 539–549.
- Rottmayer, R.; Ulbrich, S.E.; Kölle, S.; Prelle, K.; Neumueller, C.; Sinowatz, F.; Meyer, H.H.; Wolf, E.; Hiendleder, S. A bovine oviduct epithelial cell suspension culture system suitable for studying embryo-maternal interactions: Morphological and functional characterization. Reproduction 2006, 132, 637–648.
- Avilés, M.; Gutiérrez-Adán, A.; Coy, P. Oviductal secretions: Will they be key factors for the future ARTs? Mol. Hum. Reprod. 2010, 16, 896–906.
- Algarra, B.; Han, L.; Soriano-Úbeda, C.; Avilés, M.; Coy, P.; Jovine, L.; Jiménez-Movilla, M. The C-terminal region of OVGP1 remodels the zona pellucida and modifies fertility parameters. Sci. Rep. 2016, 6, 32556.
- Laheri, S.; Ashary, N.; Bhatt, P.; Modi, D. Oviductal glycoprotein 1 (OVGP1) is expressed by endometrial epithelium that regulates receptivity and trophoblast adhesion. J. Assist. Reprod. Genet. 2018, 35, 1419–1429.
- Bertling, E.; Hotulainen, P.; Mattila, P.K.; Matilainen, T.; Salminen, M.; Lappalainen, P. Cyclase-associated protein 1 (CAP1) promotes cofilin-induced actin dynamics in mammalian nonmuscle cells. Mol. Biol. Cell 2004, 15, 2324–2334.
- Jalali, B.; Likszo, P.; Skarzynski, D.J. Proteomic and network analysis of pregnancy-induced changes in the porcine endometrium on Day 12 of gestation. Mol. Reprod. Dev. 2016, 83, 827–841.
- Budipitoj, T.; Matsuzaki, S.; Cruzana, M.B.; Baltazar, E.T.; Hondo, E.; Sunaryo, S.; Kitamura, N.; Yamada, J. Immunolocalization of gastrin-releasing peptide in the bovine uterus and placenta. J. Vet. Med. Sci. 2001, 63, 11–15.
- Moraes, J.G.N.; Behura, S.K.; Bishop, J.V.; Hansen, T.R.; Geary, T.W.; Spencer, T.E. Analysis of the uterine lumen in fertility-classified heifers: II. Proteins and metabolites †. Biol. Reprod. 2020, 102, 571–587.
- Berry, D.P.; Wall, E.; Pryce, J.E. Genetics and genomics of reproductive performance in dairy and beef cattle. Animal 2014, 8, 105–121.
- Gegenfurtner, K.; Fröhlich, T.; Flenkenthaler, F.; Kösters, M.; Fritz, S.; Desnoës, O.; Le Bourhis, D.; Salvetti, P.; Sandra, O.; Charpigny, G.; et al. Genetic merit for fertility alters the bovine uterine luminal fluid proteome †. Biol. Reprod. 2020, 102, 730–739.
- Steinhauser, C.B.; Landers, M.; Myatt, L.; Burghardt, R.C.; Vallet, J.L.; Bazer, F.W.; Johnson, G.A. Fructose Synthesis and Transport at the Uterine-Placental Interface of Pigs: Cell-Specific Localization of SLC2A5, SLC2A8, and Components of the Polyol Pathway. Biol. Reprod. 2016, 95, 108.
- Gumen, A.; Keskin, A.; Yilmazbas-Mecitoglu, G.; Karakaya, E.; Wiltbank, M. Dry period management and optimization of post-partum reproductive management in dairy cattle. Reprod. Domest. Anim. 2011, 46, 11–17.
- Aranciaga, N.; Morton, J.D.; Maes, E.; Gathercole, J.L.; Berg, D.K. Proteomic determinants of uterine receptivity for pregnancy in early and mid-postpartum dairy cows. Biol. Reprod. 2021, 105, 1458–1473.
- Jhamat, N.; Niazi, A.; Guo, Y.; Chanrot, M.; Ivanova, E.; Kelsey, G.; Bongcam-Rudloff, E.; Andersson, G.; Humblot, P. LPS-treatment of bovine endometrial epithelial cells causes differential DNA methylation of genes associated with inflammation and endometrial function. BMC Genom. 2020, 21, 385.
- Smith, G.D.; Takayama, S. Application of microfluidic technologies to human assisted reproduction. Mol. Hum. Reprod. 2017, 23, 257–268.
- De Bem, T.H.C.; Tinning, H.; Vasconcelos, E.J.R.; Wang, D.; Forde, N. Endometrium On-a-Chip Reveals Insulin-and Glucose-induced Alterations in the Transcriptome and Proteomic Secretome. Endocrinology 2021, 162, bqab054.
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402.
- Goharitaban, S.; Abedelahi, A.; Hamdi, K.; Khazaei, M.; Esmaeilivand, M.; Niknafs, B. Role of endometrial microRNAs in repeated implantation failure (mini-review). Front. Cell Dev. Biol. 2022, 10, 936173.
- Liang, J.; Wang, S.; Wang, Z. Role of microRNAs in embryo implantation. Reprod. Biol. Endocrinol. 2017, 15, 90.
- Kusama, K.; Rashid, M.B.; Kowsar, R.; Marey, M.A.; Talukder, A.K.; Nagaoka, K.; Shimada, M.; Khatib, H.; Imakawa, K.; Miyamoto, A. Day 7 Embryos Change the Proteomics and Exosomal Micro-RNAs Content of Bovine Uterine Fluid: Involvement of Innate Immune Functions. Front. Genet. 2021, 12, 676791.
- Niikura, Y.; Kitagawa, K. Functions of SGT1, a Co-chaperone. In Heat Shock Protein 90 in Human Diseases and Disorders; Asea, A., Kaur, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 317–370.
- Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; Almughlliq, F.B.; Meier, S.; Burke, C.R.; Roche, J.R.; Reed, C.B.; Arachchige, B.J.; Reed, S.; et al. Proteome profiling of exosomes derived from plasma of heifers with divergent genetic merit for fertility. J. Dairy Sci. 2018, 101, 6462–6473.
- Coy, P.; García-Vázquez, F.A.; Visconti, P.E.; Avilés, M. Roles of the oviduct in mammalian fertilization. Reproduction 2012, 144, 649–660.
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrin. 2017, 232, R1–R26.
- Mahé, C.; Lavigne, R.; Com, E.; Pineau, C.; Locatelli, Y.; Zlotkowska, A.M.; Almiñana, C.; Tsikis, G.; Mermillod, P.; Schoen, J.; et al. Spatiotemporal profiling of the bovine oviduct fluid proteome around the time of ovulation. Sci. Rep. 2022, 12, 4135.
- Saint-Dizier, M.; Schoen, J.; Chen, S.; Banliat, C.; Mermillod, P. Composing the Early Embryonic Microenvironment: Physiology and Regulation of Oviductal Secretions. Int. J. Mol. Sci. 2019, 21, 223.
- Lamy, J.; Labas, V.; Harichaux, G.; Tsikis, G.; Mermillod, P.; Saint-Dizier, M. Regulation of the bovine oviductal fluid proteome. Reproduction 2016, 152, 629–644.
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371.
- Mirsaeidi, M.; Gidfar, S.; Vu, A.; Schraufnagel, D. Annexins family: Insights into their functions and potential role in pathogenesis of sarcoidosis. J. Transl. Med. 2016, 14, 89.
- Rescher, U.; Gerke, V. Annexins–unique membrane binding proteins with diverse functions. J. Cell Sci. 2004, 117 Pt 13, 2631–2639.
- Alauddin, M.; Salker, M.S.; Umbach, A.T.; Rajaxavier, J.; Okumura, T.; Singh, Y.; Wagner, A.; Brucker, S.Y.; Wallwiener, D.; Brosens, J.J.; et al. Annexin A7 Regulates Endometrial Receptivity. Front. Cell Dev. Biol. 2020, 8, 770.
- Wang, B.; Shao, Y. Annexin A2 acts as an adherent molecule under the regulation of steroids during embryo implantation. Mol. Hum. Reprod. 2020, 26, 825–836.
- Wang, B.; Ye, T.M.; Lee, K.F.; Chiu, P.C.; Pang, R.T.; Ng, E.H.; Yeung, W.S. Annexin A2 Acts as an Adhesion Molecule on the Endometrial Epithelium during Implantation in Mice. PLoS ONE 2015, 10, e0139506.
- Pillai, V.V.; Weber, D.M.; Phinney, B.S.; Selvaraj, V. Profiling of proteins secreted in the bovine oviduct reveals diverse functions of this luminal microenvironment. PLoS ONE 2017, 12, e0188105.
- Almiñana, C.; Corbin, E.; Tsikis, G.; Alcântara-Neto, A.S.; Labas, V.; Reynaud, K.; Galio, L.; Uzbekov, R.; Garanina, A.S.; Druart, X.; et al. Oviduct extracellular vesicles protein content and their role during oviduct-embryo cross-talk. Reproduction 2017, 154, 153–168.
- Almiñana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implica-tions for the gametes-oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622.
- Papp, S.M.; Fröhlich, T.; Radefeld, K.; Havlicek, V.; Kösters, M.; Yu, H.; Mayrhofer, C.; Brem, G.; Arnold, G.J.; Besenfelder, U. A novel approach to study the bovine oviductal fluid proteome using transvaginal endoscopy. Theriogenology 2019, 132, 53–61.
- Rodríguez-Alonso, B.; Maillo, V.; Acuña, O.S.; López-Úbeda, R.; Torrecillas, A.; Simintiras, C.A.; Sturmey, R.; Avilés, M.; Lonergan, P.; Rizos, D. Spatial and Pregnancy-Related Changes in the Protein, Amino Acid, and Car-bohydrate Composition of Bovine Oviduct Fluid. Int. J. Mol. Sci. 2020, 21, 1681.
- Gegenfurtner, K.; Fröhlich, T.; Kösters, M.; Mermillod, P.; Locatelli, Y.; Fritz, S.; Salvetti, P.; Forde, N.; Lonergan, P.; Wolf, E.; et al. Influence of metabolic status and genetic merit for fertility on proteomic composition of bovine oviduct fluid. Biol. Reprod. 2019, 101, 893–905.
- Pascottini, O.B.; Van Schyndel, S.J.; Spricigo, J.F.W.; Rousseau, J.; Weese, J.S.; LeBlanc, S.J. Dynamics of uterine microbiota in postpartum dairy cows with clinical or subclinical endometritis. Sci. Rep. 2020, 10, 12353.
- Choe, C.; Park, J.W.; Kim, E.S.; Lee, S.G.; Park, S.Y.; Lee, J.S.; Cho, M.J.; Kang, K.R.; Han, J.; Kang, D. Proteomic analysis of differentially expressed proteins in bovine endometrium with endome-tritis. Korean J. Physiol. Pharmacol. 2010, 14, 205–212.
- Turner, M.L.; Cronin, J.G.; Healey, G.D.; Sheldon, I.M. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology 2014, 155, 1453–1465.
- Almeida, A.M.; Bassols, A.; Bendixen, E.; Bhide, M.; Ceciliani, F.; Cristobal, S.; Eckersall, P.D.; Hollung, K.; Lisacek, F.; Mazzucchelli, G.; et al. Animal board invited review: Advances in proteomics for animal and food sciences. Animal 2015, 9, 1–17.
- Cairoli, F.; Battocchio, M.; Veronesi, M.C.; Brambilla, D.; Conserva, F.; Eberini, I.; Wait, R.; Gianazza, E. Serum protein pattern during cow pregnancy: Acute-phase proteins increase in the peripartum period. Electrophoresis 2006, 27, 1617–1625.
- Brown, W.E.; Garcia, M.; Mamedova, L.K.; Christman, K.R.; Zenobi, M.G.; Staples, C.R.; Leno, B.M.; Overton, T.R.; Whitlock, B.K.; Daniel, J.A.; et al. Acute-phase protein α-1-acid glycoprotein is negatively associated with feed intake in postpartum dairy cows. J. Dairy Sci. 2021, 104, 806–817.
- Tóthová, C.; Nagy, O.; Kovác, G. Changes in the concentrations of selected acute phase proteins and variables of energetic profile in dairy cows after parturition. J. Appl. Anim. Res. 2014, 42, 278–283.
- Fazio, E.; Bionda, A.; Liotta, L.; Amato, A.; Chiofalo, V.; Crepaldi, P.; Satué, K.; Lopreiato, V. Changes of acute-phase proteins, glucose, and lipid metabolism during pregnancy in lactating dairy cows. Arch. Anim. Breed. 2022, 65, 329–339.
- Ledgard, A.M.; Smolenski, G.A.; Henderson, H.; Lee, R.S. Influence of pathogenic bacteria species present in the postpartum bovine uterus on proteome profiles. Reprod. Fertil. Dev. 2015, 27, 395–406.
More
Information
Subjects:
Reproductive Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
579
Revisions:
2 times
(View History)
Update Date:
05 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





