Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wirginia Kukula-Koch | -- | 2415 | 2024-01-04 22:53:45 | | | |
| 2 | Fanny Huang | -1 word(s) | 2414 | 2024-01-09 09:21:13 | | | | |
| 3 | Fanny Huang | Meta information modification | 2414 | 2024-03-06 09:54:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Antoniadi, L.; Bartnik, M.; Angelis, A.; Wawruszak, A.; Halabalaki, M.; Kukula-Koch, W.; Skaltsounis, L.A. Pharmacological Properties of Gentiopicroside In Vivo. Encyclopedia. Available online: https://encyclopedia.pub/entry/53452 (accessed on 07 February 2026).
Antoniadi L, Bartnik M, Angelis A, Wawruszak A, Halabalaki M, Kukula-Koch W, et al. Pharmacological Properties of Gentiopicroside In Vivo. Encyclopedia. Available at: https://encyclopedia.pub/entry/53452. Accessed February 07, 2026.
Antoniadi, Lemonia, Magdalena Bartnik, Apostolis Angelis, Anna Wawruszak, Maria Halabalaki, Wirginia Kukula-Koch, Leandros A. Skaltsounis. "Pharmacological Properties of Gentiopicroside In Vivo" Encyclopedia, https://encyclopedia.pub/entry/53452 (accessed February 07, 2026).
Antoniadi, L., Bartnik, M., Angelis, A., Wawruszak, A., Halabalaki, M., Kukula-Koch, W., & Skaltsounis, L.A. (2024, January 04). Pharmacological Properties of Gentiopicroside In Vivo. In Encyclopedia. https://encyclopedia.pub/entry/53452
Antoniadi, Lemonia, et al. "Pharmacological Properties of Gentiopicroside In Vivo." Encyclopedia. Web. 04 January, 2024.
Copy Citation
Gentiopicroside (GPS) is a leading component of several plant species from the Gentianaceae botanical family. As a compound with plenty of biological activities and a component of herbal drugs, GPS has an important role in the regulation of physiological processes in humans. The results of recently published scientific studies underline a meaningful role of this molecule as an active factor in metabolic pathways and mechanisms, which may have an influence in the treatment of different diseases, including digestive tract disorders, malignant changes, neurological disorders, microbial infections, bone formation disorders, inflammatory conditions, and others.
gentiopicroside
Gentiana
in vivo
1. Introduction
It is generally believed that medicines (including natural ones) do not have to taste good, but they must be good for our health. The least pleasant taste for many people is the bitter taste of both food and medicines. Bitter-tasting plant compounds represent many different chemical structures, including alkaloids, polyphenolic compounds (coumarins, flavonoids), glucosinolates, and terpenoids. Belonging to the last group, secoiridoid glucoside gentiopicroside (gentiopicrin, GPS, CAS No 20831-76-9; Figure 1) occurs mainly in the Gentianales order, in the botanical family Gentianaceae [1]. Interestingly, GPS has also been found in plant species from the other botanical families, including Aster auriculatus Franch (Asteraceae) [2], Artocarpus heterophyllus Lam. (Moraceae) [3]), and Cephalaria kotschyi (Dipsaceae) [4]. In the Gentianaceae family, which contains about 400 species that are native to Europe, Asia, and North America, GPS is present in the genera Swertia, Centaurium, and Gentiana [5].
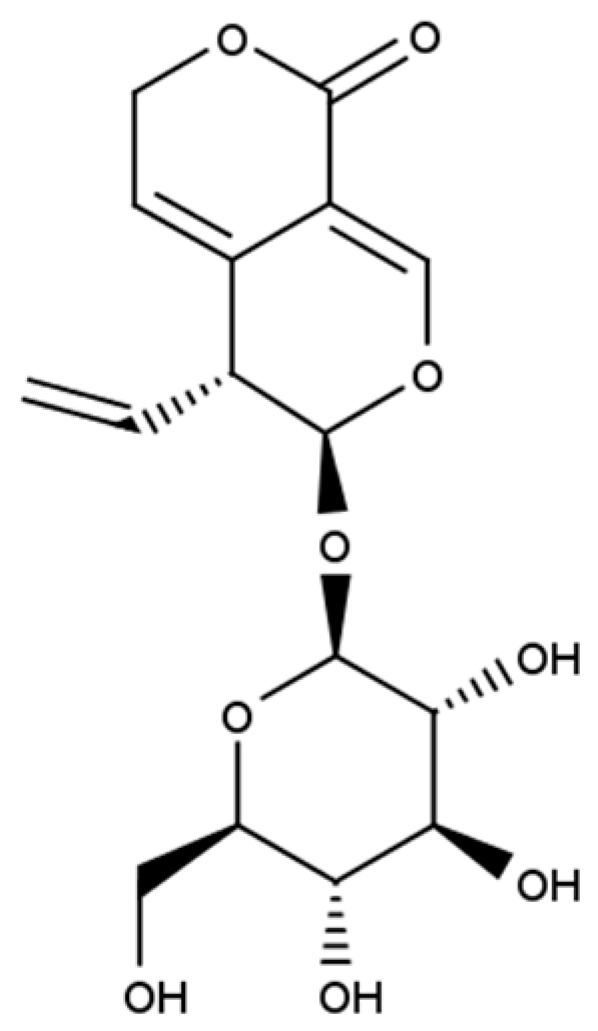
Figure 1. Chemical structure of gentiopicroside.
Gentian has been used in natural medicine for centuries, including traditional Chinese medicine (TCM) and Ayurvedic medicine. Plants of the Gentiana genus are also listed in many modern Pharmacopoeias, including the 10th edition of the European Pharmacopoeia, where a bitterness index of not less than 10,000 is required for the root of Gentiana lutea L. (compared to the bitterness index of quinine hydrochloride of 200,000) [6]. In this valuable medicinal plant, gentiopicroside is one of the best-known active substances and was first isolated from this plant source in 1862 [1]. Since then, the secoiridoid has been extensively studied for its biological activity in various in vitro and in vivo models, as well as in a few clinical trials. GPS was found to be non-toxic at the doses used and relatively stable, although it is not readily bioavailable.
2. Pharmacological Properties of Gentiopicroside In Vivo
According to scientific databases, recent years have brought the development of in vivo studies on gentiopicroside. Researchers performed in the Scopus database showed that the number of scientific articles that cover the topic of the in vivo testing of GPS reached eight annually, whereas, prior to 2014, one article was released annually. This confirms an increasing interest in in vivo studies on GPS that are conducted in various directions. The compound is researched for its antimicrobial, anti-inflammatory, central nervous system-targeting, osteogenic, anti-dermatophyte, anti-diabetic, or weight-reducing properties. The detailed characteristics of the aforementioned properties are presented below and in Figure 2.
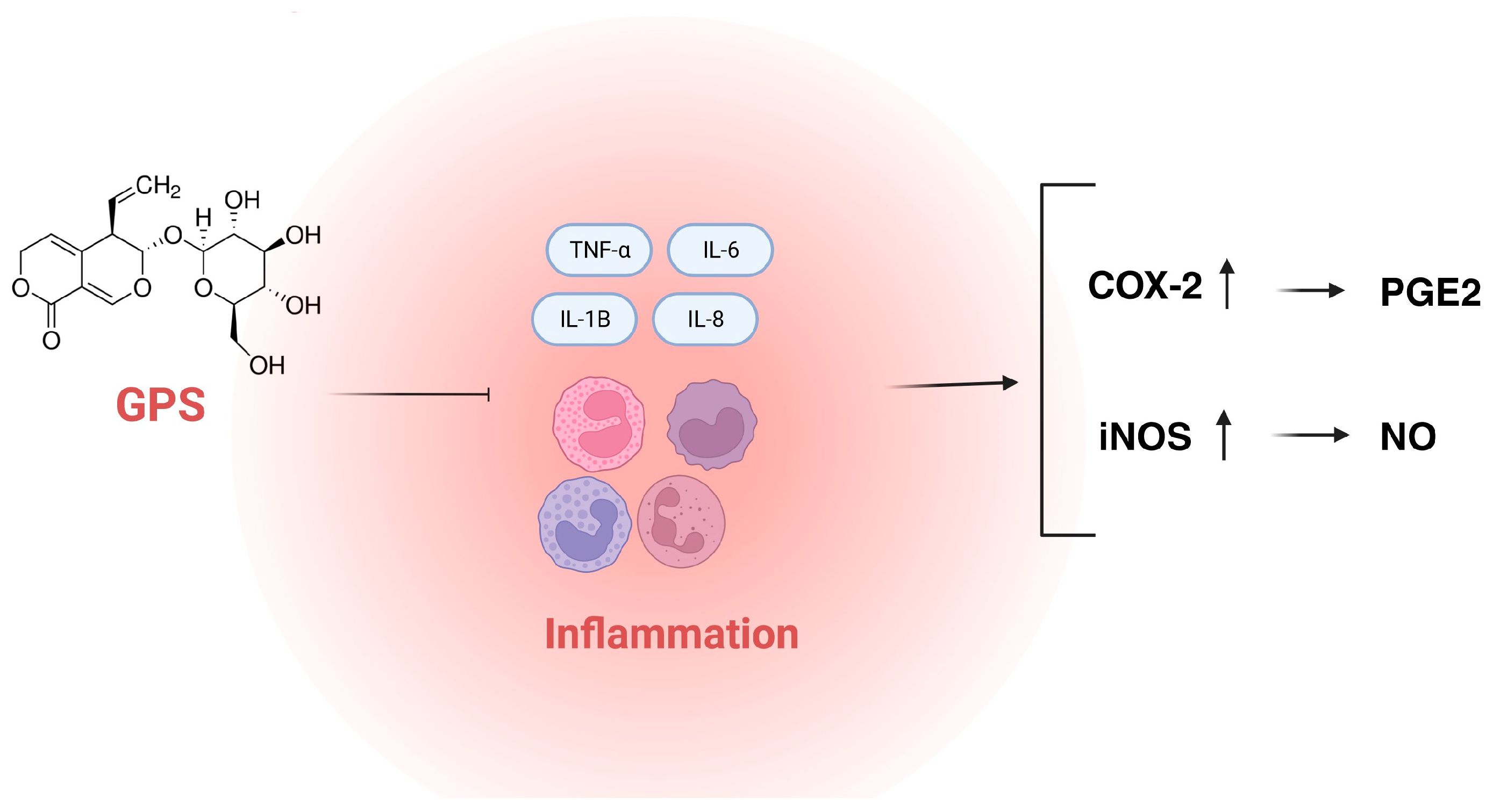
Figure 2. The main targets for anti-inflammatory activity of GPS (COX-2—cyclooxygenase 2; IL-1β—interleukin 1β; IL-6—interleukin 6; IL-8—interleukin 8; iNOS—inducible nitric oxide synthase; GPS—gentiopicroside; TNF-α—tumor necrosis factor α; PGE2—prostaglandin E2; NO—nitric oxide).
2.1. Anti-Inflammatory Properties of GPS In Vivo
In the study of Jia and co-investigators [7], the anti-inflammatory activity of GPS was investigated in male C57BL/6J mice that were administered GPS intragastrically at concentrations of 20 and 40 mg/kg of b.w. for a period of 30 days. As a consequence of GPS treatment, an inhibition in paw edema was observed for both tested doses; however, the therapeutic effects of the 40 mg/kg dose appeared earlier (8 days for 40 mg/kg vs. 22 days for 20 mg/kg). Both doses reduced the inflammatory infiltration, joint destruction, and bone damage thanks to the decreased serum cytokine levels (IL-1β, IL-6, and TNF-α) and suppressed CD147, p-p38, p-IκBα, MMP1, MMP2, and MMP3 in vivo.
The proven anti-inflammatory potential of GPS has encouraged scientists to study the properties of its semi-synthetic derivatives. In the study of Zhang and collaborators [8], some derivatives decreased the edema percentage by 34.17% after 7 days of administration of a dose of 0.28 mmol/kg of b.w.
The anti-inflammatory properties of GPS were also tested in a gouty arthritis model in male C57BL/6 mice [9]. The 24 h long treatment with 100 and 200 mg/kg of GPS p.o. showed a reduction in swelling, the occurrence of analgesic properties, and the inhibition of thermal hyperplasia. GPS inhibited the infiltration of neutrophils by alleviating the levels of interleukins IL-1β, IL-6, IL-18, TNF-α, caspase-1, NOD-like receptor protein 3, and ACS.
Another mechanism of anti-inflammatory action proposed by Wang et al. [10] included the GPS-based alleviation of the ROS-NF-κB-NLRP3 axis in synoviocytes, as well as the NF-κB pathway. As a consequence of a 14-day-long treatment with 200 mg/kg of GPS, a reduction in paw swelling and a decrease in the arthritis index value were observed. A histopathological analysis of joints showed reduced inflammation, bone destruction, synovial hyperplasia, and pannus formation, and no impact on body weight or spleen index, but a slight reduction in the thymus index value. GPS treatment inhibited IκBa degradation and p-IκBa and p-p65 expression levels in the synovial tissue, which suggests the joint-protective role of this natural product and confirms that its action is related to NFκB signaling.
The anti-inflammatory properties of GPS could find applications in the treatment of pulmonary fibrosis [11]. The compound administered to male SPF mice for 28 days decreased the concentration of hydroxyproline in the lung tissue and ameliorated fibrotic parameters (alveolar space narrowing, alveolar wall thickening, deposition of collagen) and inflammatory responses. The described properties occurred together with decreased levels of TNF-α, IL-1β, and TGF-β1.
GPS at a dose of 30 µM was found to exert anti-inflammatory properties in a zebrafish model of COX-2 enzyme inhibition [12]. GPS was found to inhibit the enzyme at 65 ± 5%, whereas the anti-inflammatory drug indometacin at a concentration of 0.9 µM was prone to inhibit the enzyme at 56 ± 1%.
2.2. GPS in the Treatment of Neuropathies In Vivo
Xu et al. [13] described the application of GPS in diabetic renal fibrosis—a symptom which is a consequence of neuropathy developed with the progression of diabetes mellitus and hyperglycemia that leads to an excessive deposition of extracellular matrix in the kidneys that disturbs their physiological functioning. The study was performed on 6-week-old male db/db mice that were intragastrically administered different doses of GPS (50, 100, and 200 mg/kg of b.w.) for 10 consecutive weeks. Valsartan (10 mg/kg of b.w.) was used as a positive control, and healthy male C57/BL6 mice as the control group. Animals treated with GPS were found to have an ameliorated metabolism of lipids and glucose that was confirmed by decreased levels of HbA1c, GSP, LDL-C, TG, and body weight at all tested doses and FBG (after 3 weeks of treatment). Also, the compound supported renal functions. The levels of creatinine, urea nitrogen, and albuminuria were significantly decreased in the treated animals. Staining tests showed an increased expansion in the lumen, glycogen accumulation in the renal cortex of the treated group, and a lowered degree of inflammation and fibrosis in the renal tubule. The latter effects were explained by an alleviated level of FN, α-SMA, and vimentin, and increased expression of E-cadherin after GPS treatment. As a result of these studies, the authors denoted that the secoiridoid suppresses the AT1R/CK2/NF-κB pathway.
According to Lu and collaborators [14], GPS decreased hyperalgesia in Sprague Dawley rats stimulated with hot, cold, and mechanical allodynia. The application of GPS in the treatment of diabetic peripheral neuropathy was suggested by the authors, as the compound was capable of restoring nerve blood flow, improving motor nerve conduction velocity and sensory nerve conduction velocity parameters, and regulating dyslipidemia. GPS influenced the expression of genes of the PPAR-γ/AMPK/ACC signaling pathway.
2.3. The Effect of GPS on the Metabolism of Glucose and Lipids In Vivo
GPS was also tested for its potential application in the treatment of alcoholic hepatosteatosis. The study of Li et al. [15] that was performed on male C57BL/6 mice that were treated with GPS and fed with ethanol in acute (3 days) and chronic (10 days) experiments showed several protective properties exhibited by the secoiridoid. A lower dose of 40 mg/kg of b.w. in the chronic administration prevented the increase in ALT and AST levels, as well as TG levels, in the serum and liver. The formed lipid droplets in the liver were smaller and less abundant. Also, increased LKB1 and AMPK phosphorylation levels were noted, SREBP1 protein expression was alleviated, the phosphorylation of ACC was restored, and PPARα regulatory genes were down-regulated. A protective role of GPS may be based on the inhibition of lipogenesis that occurs upon the influence of ethanol and through the elevation of lipid oxidation. GPS decreased the alcohol-induced expression of the receptors NLRP3 and P2X7, but only as a consequence of alcohol toxicity.
2.4. Central Nervous System-Targeting Activities of GPS In Vivo
GPS was proven to cross the blood–brain barrier and exhibit certain actions in the central nervous system. Several scientific papers describe its antidepressant activity. In the study of Liu et al. [16], who used a mouse model of reserpine-induced depression, GPS was administered intragastrically to male animals twice daily for 3 days and once on the fourth day before behavioral testing in three doses: 50, 100, and 200 mg/kg of b.w. GPS at the two highest doses decreased mechanical allodynia and immobility time in a forced swimming test and tail suspension test, and increased the traveled distance and the time in the center area in an open-field test. The observed behavioral changes in the studied animals were possibly triggered by the changes in the neuromodulator’s levels. GPS reversed the activity of reserpine that decreased the levels of norepinephrine, dopamine, and serotonin. However, when administered alone at a dose of 100 mg/kg, it did not cause any changes in neurotransmitter levels. Also, GPS reduced the intracellular levels of MDA production in the basolateral amygdala (BLA) of the treated mice; elevated the activity of catalase in their tissue, as well as Bcl-2 expression; and inhibited the activity of caspase-3 in a dose-dependent manner. The administration of reserpine led to an increased expression of the AMPA and NMDA subunits of BLA homogenates. In this study, GPS did not affect the expression of GluA1 and GluN2A subunits, but decreased the expression of GluN2B in reserpinized mice, which—according to the authors—may contribute to the attenuation of the depression dyad in tested mice.
The corticosterone-induced model of depression applied by Yao and co-investigators [17] on rats led to the recognition of the molecular mechanisms of GPS’s action, which was administered via gastric instillation at a dose of 100 mg/kg of b.w., 2 h prior to corticosterone, for 21 days. The authors proved a protective role of the compound against the steroid: the ability to inhibit apoptosis in the hippocampus that was caused by the steroid, to reduce the loss of Niss bodies, to increase the level of serotonin in the brain tissue, to elevate the expression of BDNF, and alleviate caspase 3 and Bax expression in the brain. An HPLC-MS-based chemometric analysis of the GPS-treated and untreated groups showed marked differences in the metabolite profiles between the groups. The former group was characterized with alleviated levels of sphinganine, stearoylethanolamide, and guanosine, and decreased levels of arachidonic acid, oxoadipic acid, L-phenylalanine, and thiamine. A pathway analysis provided evidence on the influence of GPS on sphingolipids, glycine/serine/threonine, and pyrimidine metabolism.
Deng et al. [18], in their study on BALB/C mice with lipopolysaccharide-induced depression that were administered with GPS (50 mg/kg of b.w. i.p.) once a day for 3 days, observed an alleviated activation of tryptophan metabolic pathways and a reduction in TNF-α and IL-1β levels in the brain (BLA) and plasma, and confirmed the expression of the GluN2B subunit of the NMDA receptor. Also, a down-regulation of indoleamine 2,3-oxygenase by GPS was noted. According to the authors, the antidepressant effect that was visualized in behavioral studies in forced swimming tests and tail suspension tests, as well as protection against the injected lipopolysaccharide, may be at least partially influenced by the ability of GPS to block different steps of the tryptophan-degrading pathway.
2.5. Other Activities
The secoiridoid administered orally at a dose of 50 mg/kg of b.w. for 12 weeks to mice fed with high-fat diet was proven to inhibit all key adipogenic transcription factors, like PPARγ, C/EBPα, and SREBP-1c, to down-regulate the expression of genes related to the transport of fatty acids, like the lipid uptake gene and the fatty acid (FABP4) and triglyceride (TG) transport-related gene, but also synthesis-related genes (DGAT2, FAS, SDC1) [19]. Also, the confirmed anti-inflammatory properties of GPS were expressed as regulatory towards the inflammatory genes, leading to a decreased cytokine release, which helped to reduce the weight of animals together with the visceral fat mass, decreasing the size of adipocytes.
According to Jiang et al., GPS promotes osteogenesis [20]. In a 3-month-long study on ovariectomized female C57BL/6 mice, the secoiridoid at a daily dose of 50 mg/kg of b.w. (oral gavage) promoted alkaline phosphatase activity and increased the level of osteogenic factors like Runx2, OCN, and BMP2, as well as the thickness and number of trabeculae, after the performed therapy, which was visualized in a micro-CT test. These observations provide scientific evidence on the possibility of GPS application in regulating bone metabolism.
As mentioned above, GPS shows antimicrobial potential. In the study published by Almukainzi and co-investigators [21], its antimicrobial properties were used in diabetic rats with infected wounds. GPS, together with thymoquinone, released from the constructed nanofibers led to a speedy recovery of the tissue and triggered the healing process. Another study of these authors [22] that was performed on streptozotocin-induced diabetic rats intended to histologically analyze the state of would incisions at different day intervals after the application of PLGA nanoparticles loaded with GPS. Interestingly, the tested formulation, orally administering 300 mg/kg of b.w. of GPS by increasing the bioavailability of GPS poorly dissolved in water, enhanced the antimicrobial effect in model animals (Sprague Dawley rats) in comparison with free GPS. The proposed treatment led to complete wound healing and wound closure within 12 days. Free GPS was found to induce wound-healing properties, too; however, the recovery time was longer and might have needed a more frequent administration. The regenerated tissue was normally organized, with developed mature collagen fibers, a minimal number of inflammatory cells, and physiological vasculatures.
Figure 3 summarizes the biological effects of gentiopicroside in in vivo studies conducted on animals (mice and rats).
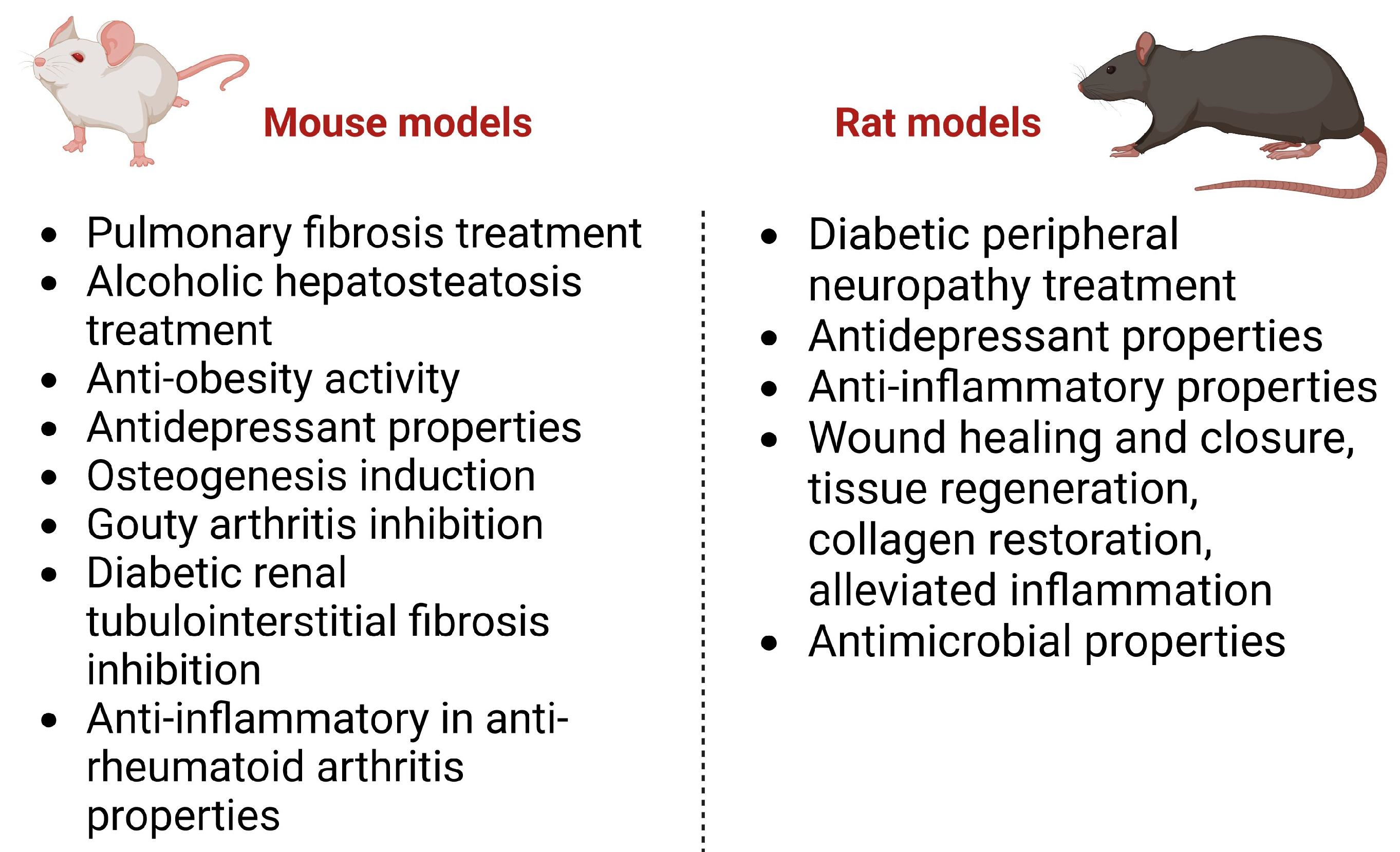
Figure 3. Biological effects of GPS, proven in in vivo studies.
References
- Jensen, S.R.; Schripsema, J. Chemotaxonomy and pharmacology of Gentianaceae. In Gentianaceae—Systematics and Natural History; Struwe, L., Albert, V., Eds.; Cambridge University Press: Cambridge, UK, 2002.
- Changzeng, W.; Dequan, Y. Diterpenoid, Sesquiterpenoid and Secoiridoid Glucosides from Aster auriculatus. Phytochemistry 1997, 45, 1483–1487.
- Daud, M.N.H.; Wibowo, A.; Abdullah, N.; Ahmad, R. Bioassay-Guided Fractionation of Artocarpus heterophyllus L. J33 Variety Fruit Waste Extract and Identification of Its Antioxidant Constituents by TOF-LCMS. Food Chem. 2018, 266, 200–214.
- Mustafayeva, K.; Di Giorgio, C.; Elias, R.; Kerimov, Y.; Ollivier, E.; De Méo, M. DNA-Damaging, Mutagenic, and Clastogenic Activities of Gentiopicroside Isolated from Cephalaria Kotschyi Roots. J. Nat. Prod. 2010, 73, 99–103.
- Jiang, M.; Cui, B.-W.; Wu, Y.-L.; Nan, J.-X.; Lian, L.-H. Genus Gentiana: A Review on Phytochemistry, Pharmacology and Molecular Mechanism. J. Ethnopharmacol. 2021, 264, 113391.
- EDQM. European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2019.
- Jia, N.; Ma, H.; Zhang, T.; Wang, L.; Cui, J.; Zha, Y.; Ding, Y.; Wang, J. Gentiopicroside Attenuates Collagen-Induced Arthritis in Mice via Modulating the CD147/P38/NF-κB Pathway. Int. Immunopharmacol. 2022, 108, 108854.
- Zhang, N.; Jiang, Y.; Mu, F.; Wu, H.; You, Q. Gentiopicrin Exerts Anti-Rheumatic Effect in Human Fibroblast-like Synoviocytes via Inhibition of P38MAPK/NF-κB Pathway. Cell. Mol. Biol. 2019, 65, 85–90.
- He, M.; Hu, C.; Chen, M.; Gao, Q.; Li, L.; Tian, W. Effects of Gentiopicroside on Activation of NLRP3 Inflammasome in Acute Gouty Arthritis Mice Induced by MSU. J. Nat. Med. 2022, 76, 178–187.
- Öztürk, N.; Korkmaz, S.; Öztürk, Y.; Başer, K.H.C. Effects of Gentiopicroside, Sweroside and Swertiamarine, Secoiridoids from Gentian (Gentiana lutea ssp. symphyandra), on Cultured Chicken Embryonic Fibroblasts. Planta Medica 2006, 72, 289–294.
- Chen, C.; Wang, Y.; Wang, Y.; Cheng, M.; Yin, J.; Zhang, X.; Hong, Z. Gentiopicroside Ameliorates Bleomycin-Induced Pulmonary Fibrosis in Mice via Inhibiting Inflammatory and Fibrotic Process. Biochem. Biophys. Res. Commun. 2018, 495, 2396–2403.
- Wang, Y.M.; Xu, M.; Wang, D.; Yang, C.R.; Zeng, Y.; Zhang, Y.J. Anti-inflammatory compounds of “Qin-Jiao”, the roots of Gentiana dahurica (Gentianaceae). J. Ethnopharmacol. 2013, 147, 341–348.
- Xu, Z.; Zhang, M.; Wang, Y.; Chen, R.; Xu, S.; Sun, X.; Yang, Y.; Lin, Z.; Wang, S.; Huang, H. Gentiopicroside Ameliorates Diabetic Renal Tubulointerstitial Fibrosis via Inhibiting the AT1R/CK2/NF-κB Pathway. Front. Pharmacol. 2022, 13, 848915.
- Lu, Y.; Yao, J.; Gong, C.; Wang, B.; Zhou, P.; Zhou, S.; Yao, X. Gentiopicroside Ameliorates Diabetic Peripheral Neuropathy by Modulating PPAR-Γ/AMPK/ACC Signaling Pathway. Cell. Physiol. Biochem. 2018, 50, 585–596.
- Li, X.; Zhang, Y.; Jin, Q.; Xia, K.-L.; Jiang, M.; Cui, B.-W.; Wu, Y.-L.; Song, S.-Z.; Lian, L.-H.; Nan, J.-X. Liver Kinase B1/AMP-Activated Protein Kinase-Mediated Regulation by Gentiopicroside Ameliorates P2X7 Receptor-Dependent Alcoholic Hepatosteatosis. Br. J. Pharmacol. 2018, 175, 1451–1470.
- Liu, S.; Zhao, R.; Li, X.; Guo, H.; Tian, Z.; Zhang, N.; Gao, G.; Zhao, M. Attenuation of Reserpine-Induced Pain/Depression Dyad by Gentiopicroside Through Downregulation of GluN2B Receptors in the Amygdala of Mice. NeuroMolecular Med. 2014, 16, 350–359.
- Yao, T.; Cui, Q.; Liu, Z.; Wang, C.; Zhang, Q.; Wang, G. Metabolomic Evidence for the Therapeutic Effect of Gentiopicroside in a Corticosterone-Induced Model of Depression. Biomed. Pharmacother. 2019, 120, 109549.
- Deng, Y.-t.; Zhao, M.-g.; Xu, T.-j.; Hou, J.; Li, X.-h. Gentiopicroside Abrogates Lipopolysaccharide-Induced Depressive-like Behavior in Mice through Tryptophan-Degrading Pathway. Metab. Brain Dis. 2018, 33, 1413–1420.
- Choi, R.-Y.; Nam, S.-J.; Lee, H.-I.; Lee, J.; Leutou, A.S.; Ri Ham, J.; Lee, M.-K. Gentiopicroside Isolated from Gentiana scabra Bge. Inhibits Adipogenesis in 3T3-L1 Cells and Reduces Body Weight in Diet-Induced Obese Mice. Bioorg. Med. Chem. Lett. 2019, 29, 1699–1704.
- Jiang, H.; Zhong, J.; Li, W.; Dong, J.; Xian, C.J.; Shen, Y.-K.; Yao, L.; Wu, Q.; Wang, L. Gentiopicroside Promotes the Osteogenesis of Bone Mesenchymal Stem Cells by Modulation of β-Catenin-BMP2 Signalling Pathway. J. Cell. Mol. Med. 2021, 25, 10825–10836.
- Almukainzi, M.; El-Masry, T.A.; Negm, W.A.; Elekhnawy, E.; Saleh, A.; Sayed, A.E.; Ahmed, H.M.; Abdelkader, D.H. Co-Delivery of Gentiopicroside and Thymoquinone Using Electrospun m-PEG/PVP Nanofibers: In Vitro and In Vivo Studies for Antibacterial Wound Dressing in Diabetic Rats. Int. J. Pharm. 2022, 625, 122106.
- Almukainzi, M.; El-Masry, A.T.; Negm, A.W.; Elekhnawy, E.; Saleh, A.; Sayed, E.A.; Khattab, A.M.; Abdelkader, H.D. Gentiopicroside PLGA Nanospheres: Fabrication, in vitro characterization, antimicrobial action, and in vivo effect for enhancing wound healing in diabetic rats. Int. J. Nanomed. 2022, 17, 1203–1225.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
891
Revisions:
3 times
(View History)
Update Date:
06 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




