Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manish Vinayak | -- | 2949 | 2024-01-04 15:08:14 | | | |
| 2 | Fanny Huang | Meta information modification | 2949 | 2024-01-10 06:35:47 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Serrao, G.; Vinayak, M.; Nicolas, J.; Subramaniam, V.; Lai, A.C.; Laskey, D.; Kini, A.; Seethamraju, H.; Scheinin, S. Coronary Artery Disease in the Lung Transplant Patient. Encyclopedia. Available online: https://encyclopedia.pub/entry/53434 (accessed on 07 March 2026).
Serrao G, Vinayak M, Nicolas J, Subramaniam V, Lai AC, Laskey D, et al. Coronary Artery Disease in the Lung Transplant Patient. Encyclopedia. Available at: https://encyclopedia.pub/entry/53434. Accessed March 07, 2026.
Serrao, Gregory, Manish Vinayak, Johny Nicolas, Varsha Subramaniam, Ashton C. Lai, Daniel Laskey, Annapoorna Kini, Harish Seethamraju, Scott Scheinin. "Coronary Artery Disease in the Lung Transplant Patient" Encyclopedia, https://encyclopedia.pub/entry/53434 (accessed March 07, 2026).
Serrao, G., Vinayak, M., Nicolas, J., Subramaniam, V., Lai, A.C., Laskey, D., Kini, A., Seethamraju, H., & Scheinin, S. (2024, January 04). Coronary Artery Disease in the Lung Transplant Patient. In Encyclopedia. https://encyclopedia.pub/entry/53434
Serrao, Gregory, et al. "Coronary Artery Disease in the Lung Transplant Patient." Encyclopedia. Web. 04 January, 2024.
Copy Citation
Lung transplantation can greatly improve quality of life and extend survival in those with end-stage lung disease. In order to derive the maximal benefit from such a procedure, patients must be carefully selected and be otherwise healthy enough to survive a high-risk surgery and sometimes prolonged immunosuppressive therapy following surgery. Patients therefore must be critically assessed prior to being listed for transplantation with close attention paid towards assessment of cardiovascular health and operative risk. One of the biggest dictators of this is coronary artery disease.
coronary artery disease
lung transplant evaluation
1. Introduction
Lung transplantation can truly be a life-saving procedure for the end-stage lung disease (ESLD) population. However, as with any organ allocation, comorbid conditions are important considerations prior to undertaking such a resource-intensive endeavor. Although there are many factors that go into the decision for lung transplantation, one of the more important considerations is concomitant coronary artery disease (CAD). Patient not only need to tolerate a complex transplantation procedure but they will also need to live long enough from a cardiovascular standpoint in order derived benefit from the transplantation.
Thus, coronary artery disease remains a relative contraindication to lung transplantation; however, with improvements in contemporary percutaneous and surgical revascularization strategies, the morbidity and mortality of underlying CAD in ESLD patients hopefully can now be successfully managed prior to, or even during, transplantation [1][2]. However, patients remain high-risk for recurrent coronary artery disease even after transplantation, as there is an accelerated atherosclerotic process seen in transplant recipients that is likely attributable to the immunosuppressive medications used [3]. Thus, surveillance, diagnosis, and treatment strategies need to be implemented even after lung transplantation.
Furthermore, there are significant interactions between CAD, end-stage lung disease, and lung transplantation that complicate a simplistic approach to clinical decision making. Therefore, given the high prevalence and significant morbidity and mortality of CAD in ESLD patients, careful attention to risk mitigation via pharmacological, procedural, and surgical approaches is important prior to, during and even after lung transplantation [4]. Given the complexity of management over such a long period of time, a multidisciplinary team is of the utmost importance. The involvement of a cardiologist is crucial in the lung transplantation process: diagnosis of CAD with the appropriate anatomical and/or functional stress test is fraught with nuisances: the proper management of CAD with the appropriate stent, antiplatelet therapy, anti-anginal therapy, and surgical revascularization approach are not trivial clinical considerations in the ESLD or transplanted population.
2. Prevalence of Coronary Artery Disease among Lung Transplant Candidates
The pathophysiological mechanisms that establish a connection between coronary artery disease (CAD) and advanced lung diseases remain inadequately understood. However, the relationship between these two medical conditions is principally attributed to overlapping risk factors, as illustrated in Figure 1. Despite an overall decrease in CAD in the general population, it is believed that its prevalence has increased among lung transplant candidates as more patients with advanced lung disease are now eligible for transplant compared to decades ago [5]. The prevalence of CAD among candidates for lung transplantation demonstrates considerable variation across different studies, ranging from 5% to 24% [1][6][7][8][9]. This substantial discrepancy can largely be attributed to the lack of a unified methodology for categorizing the severity of the disease. In most studies, CAD is defined based on coronary angiography, specifically as a luminal stenosis of either at least 50% or at least 70%. When the criteria are limited to patients exhibiting a 70% luminal narrowing in a major coronary artery, or a 50% narrowing in the left main coronary artery, the prevalence of CAD is approximately 10% [9]. The prevalence also appears to be influenced by the specific type of pulmonary disease; for instance, patients with fibrotic lung disease have twice the risk of developing CAD compared to those with non-fibrotic lung disease [10]. Moreover, the prevalence of CAD is significantly higher in patients with lung fibrosis than in those with emphysema (28.6% vs. 9.8%), even though the latter group demonstrates a higher prevalence of smoking. Risk stratification based on the presence of various comorbidities has revealed that individuals with at least three risk factors are more likely to exhibit obstructive CAD upon angiographic examination [7][9]. Specifically, diabetes mellitus and chest pain are associated with the highest positive predictive values, while hyperlipidemia and smoking are linked to the lowest [11]. One study, utilizing the Prospective Cardiovascular Münster (PROCAM) score to predict the risk of atherosclerotic disease in lung transplant candidates, found no correlation between various comorbidities and CAD. Most notably, this study revealed that nearly one-third of asymptomatic candidates over the age of 50 years were afflicted with undetected coronary artery disease [4]. Due to the inconsistencies in published reports and the absence of robust risk stratification tools, there is no uniform approach to the routine screening of occult CAD among patients undergoing pre-transplant evaluations. Nonetheless, most transplant centers currently screen lung transplant candidates who are above the age of 45 years.
Figure 1. Common risk factors for coronary artery disease and lung disease.
3. Impact of Coronary Artery Disease on Lung Transplant Candidates
According to the most recent 2021 guidelines from the International Society for Heart and Lung Transplantation, having acute coronary syndrome within one month prior to transplant surgery, or possessing significant CAD that cannot be treated with revascularization, constitutes an important but not definitive disqualifier for undergoing a transplant [12]. The presence of CAD in lung transplant candidates introduces a complex layer of medical considerations, potentially increasing morbidity and mortality in the short and long term. As in any other non-cardiac surgery, CAD acts as a powerful negative prognosticator, imposing heightened risks for perioperative complications such as myocardial infarction, cardiac arrhythmias, and hemodynamic instability [13][14]. In the long term, immunosuppression accelerates the progression of atherosclerotic disease [15]. Despite that, data on the effects of preoperative CAD on mortality in lung transplant recipients are controversial. Several retrospective analyses conducted in the last half-decade indicate that lung transplant candidates with mild to moderate CAD, or those who have received revascularization procedures for CAD, may not necessarily experience increased perioperative complications or poorer survival outcomes compared to individuals without CAD [1][5][16][17]. In 210 lung transplant recipients, there were no mortality differences in the first 5 years after transplant between patients with mild-to-moderate CAD and patients without CAD [18]. A similar finding was reported in another study, including a cohort of 539 lung transplant candidates of whom one-third had moderate CAD [19]. Conversely, Jones et al. reported higher mortality rates in patients with CAD than in those without CAD following the transplant; however, the investigators used a broader definition of CAD, including those with a prior history of MI and revascularization [20]. To note, patients in these studies were meticulously selected and underwent more often a single-lung transplant. With respect to other post-transplant outcomes and complications, such as primary graft failure, prolonged hospital stay, myocardial infarction, and stroke, several studies have shown no differences between those with and without CAD, though the former group had higher rates of revascularization [2][21][22]. In one study including 539 lung transplant candidates, 33% had mild-to-moderate CAD and were more likely to undergo coronary revascularization following transplantation, as compared to those without CAD at baseline (PCI: 4.0% vs. 0.3%; CABG: 2.3% vs. 0.3%) [19]. Other cardiovascular events, not limited to coronary events but also occurring in other vascular beds, have been described. Few analyses report an increased incidence of non-fatal cardiovascular events, such as ischemic stroke and dysrhythmias, in lung transplant patients with CAD [8][16][23][24]. In a cohort of 280 lung transplant candidates, 48 patients with advanced CAD had an event rate of 12%, which consisted of 2% peripheral artery disease events, 4% coronary events, and 6% cerebrovascular events [16].
4. Medical Therapy for the Prevention or Treatment of CAD in Lung Transplant Candidates
Medical therapy aimed at treating and preventing CAD in lung transplant patients is of paramount importance, not only to improve perioperative cardiovascular risks but also to optimize long-term post-transplant survival (Figure 2). Lifestyle modifications, including smoking cessation, healthy diet, and exercise, can have a direct impact on cardiovascular risk in these patients. The cornerstone of medical management includes pharmacotherapeutic interventions such as antiplatelet agents (e.g., aspirin), lipid-lowering therapies, antihypertensive medications, and glucose-lowering agents in diabetic patients. These therapies aim to mitigate the underlying risk factors for CAD and have been shown to be effective in reducing the rates of myocardial infarction, stroke, and cardiovascular mortality. It is imperative that individualized medical regimens be tailored for each candidate, taking into account their specific cardiovascular and pulmonary pathology, and that continuous monitoring be instituted to assess treatment efficacy and adjust dosages as required. In patients with established CAD, low-dose aspirin is recommended for primary prevention [25]. The decision to initiate low-dose aspirin should be an individualized one, made in conjunction with a healthcare provider based on risk-benefit assessment. When aspirin is contraindicated, an adenosine diphosphate receptor (P2Y12) inhibitor may be used [26]. Statin treatment is the principal therapy focused on lowering cholesterol levels for reducing the risk of cardiovascular events in individuals with stable CAD, and it should be given at a dose that attains a targeted low-density lipoprotein cholesterol level [27][28]. When cholesterol levels are still above target, other agents such as ezetimibe and proprotein convertase subtilisin/kexin type 9 inhibitors may be added [29][30][31]. A recommended goal for systolic and diastolic blood pressure in individuals with CAD is below 130/80 mm Hg. The guidelines advise the preferential use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for reducing the likelihood of cardiovascular incidents, with the prescription of β-blockers for patients with angina [32][33]. A tight control of sugar levels is an essential part in mitigating the cardiovascular risk; antidiabetic agents such sodium-glucose cotransporter 2 inhibitors (SGLT-2i) or glucagon-like peptide 1 receptor agonists have been shown to have a direct impact on the cardiovascular risk and should be preferred [34]. Lastly, antianginal medications, such as β-blockers and calcium channel blockers, are essential in symptomatic treatment. The effectiveness of various antianginal medications is generally modest and relatively comparable across different drug classes [35][36]. Consequently, the choice of medication is often determined by the presence of comorbid conditions and the likelihood of adverse effects. For individuals with left ventricular dysfunction or a history of myocardial infarction, β-blockers are particularly recommended. Newer agents, such as SGLT-2i and angiotensin receptor–neprilysin inhibitors, have also been shown to decrease mortality and hospitalization for heart failure and are currently recommended by guidelines for patients with left ventricular dysfunction [37].
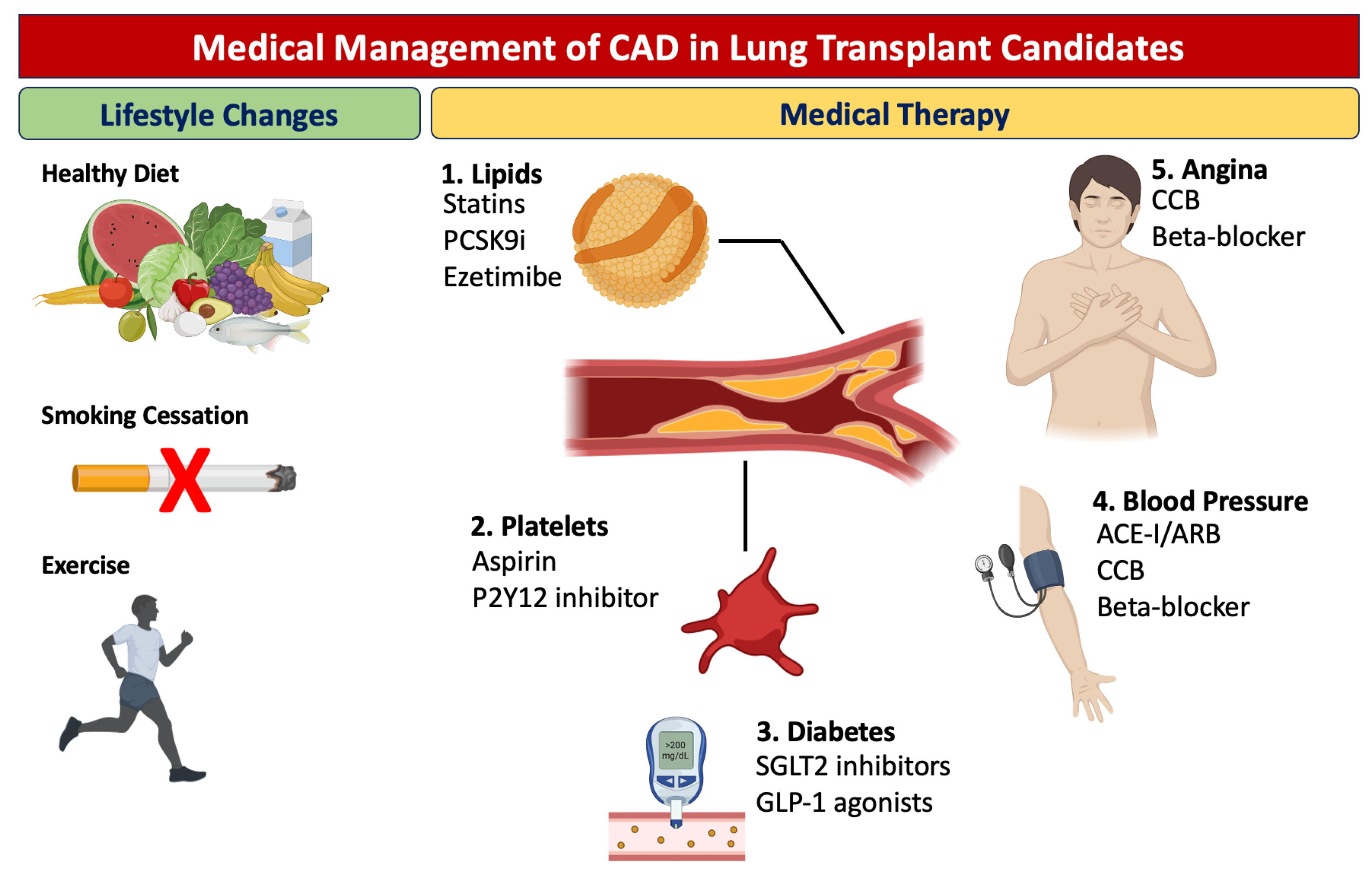
Figure 2. Medical management of coronary artery disease in lung transplant candidates.
5. CAD Screening in Lung Transplantation Candidates
Due to the scarcity of the donor lungs and concerns about the potential progression of underlying atherosclerosis with immunosuppressive therapies, guidelines have historically considered CAD as an absolute contraindication for the lung transplantation [38]. However, in light of advancements in procedural techniques, the 2014 update of the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation guidelines considers CAD as an absolute contraindication only if there is uncorrected atherosclerosis accompanied by end-organ dysfunction and/or CAD that is not amenable to revascularization [39]. Subsequently, the 2021 update of these guidelines considers acute coronary syndrome or myocardial infarction within 30 days as the only absolute contraindication [12].
The evaluation prior to transplantation commences with an accurate history, a clinical examination, and an assessment of traditional cardiac risk factors, including age > 65 years, smoking, dyslipidemia, hypertension, diabetes, and a history of prior established cardiovascular disease. The presence of ≥2 cardiac risk factors correlates with a high likelihood of having underlying CAD [19]. Furthermore, a number of screening modalities (non-invasive and invasive) are available for the assessment of CAD risk, each with their own limitations (Figure 3).
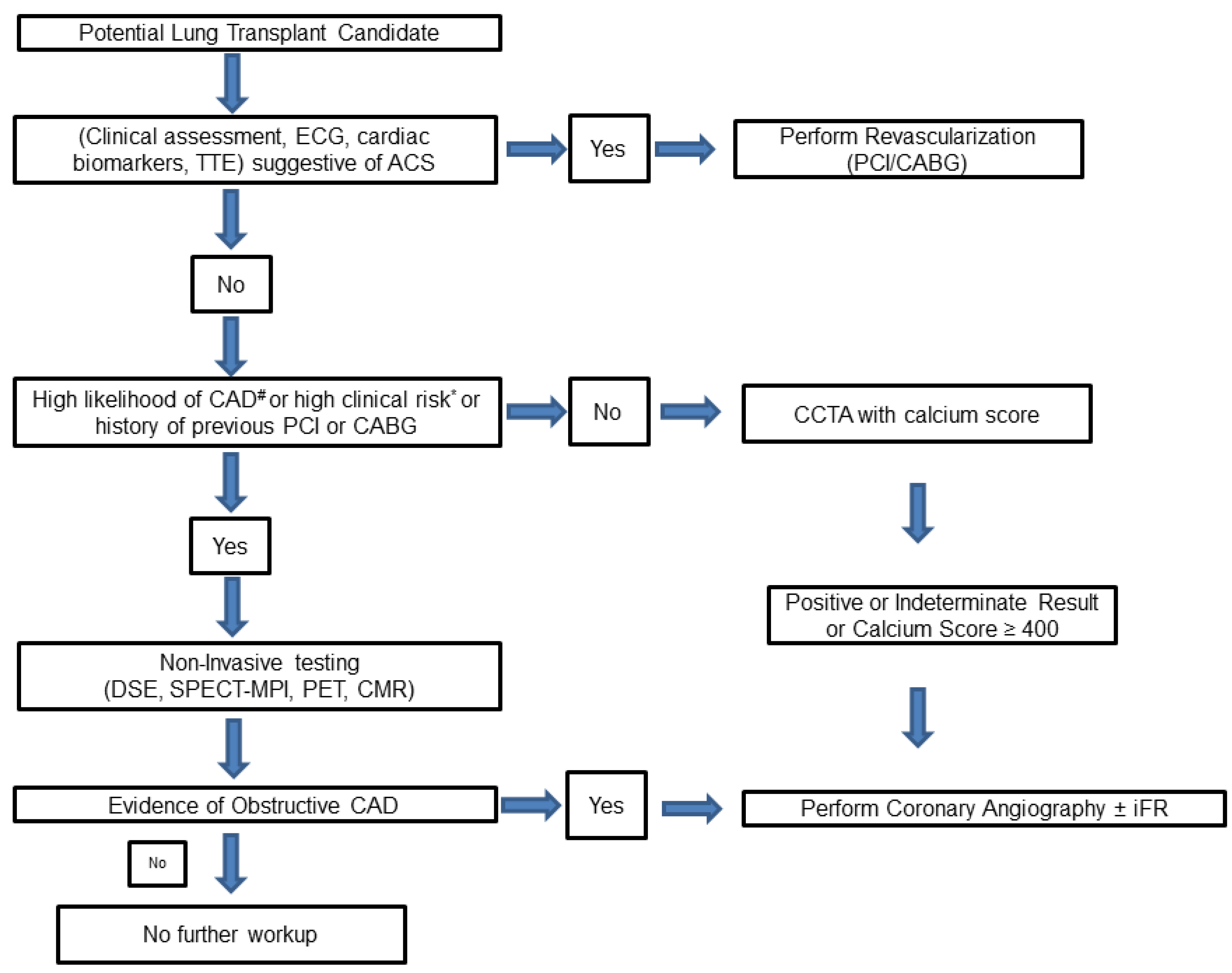
Figure 3. Proposed algorithm for protocolized assessment of coronary artery disease in lung transplant candidates. #: Two or more risk factors for cardiovascular disease (CVD): dyslipidemia, diabetes, hypertension, smoking, family history of CVD, prior established CVD. *: One or more clinical risk factor according to the Revised Cardiac Risk Index (RCRI): ischemic heart disease, cerebrovascular disease, history of congestive heart failure, serum creatinine level > 2 mg/dl, diabetes requiring insulin therapy. CABG: Coronary Artery Bypass Grafting, CCTA: Coronary Computed Tomography Angiography, CMR: Cardiac Magnetic Resonance, DSE: Dobutamine Stress Echocardiogram, ECG: Electrocardiogram, iFR: instantaneous wave-free pressure ratio, PCI: Percutaneous Coronary Intervention, PET: Positron Emission Tomography, SPECT-MPI: Single Positron Emission Tomography-Myocardial Perfusion Imaging, TTE: Trans-Thoracic Echocardiogram.
5.1. Exercise Testing
In terms of evaluating functional status, exercise testing has limited value due to poor functional capacity caused by underlying pulmonary disease, leading to an inability to achieve the target heart rate.
5.2. Dobutamine Stress Echocardiogram (DSE)
Pharmacological stress testing with DSE is an accepted method for the preoperative assessment of cardiac risk in patients undergoing non-cardiac surgery. The utility of DSE in patients with severe lung disease has been evaluated in patients undergoing lung volume reduction surgery, where DSE exhibited limited positive predictive value (25%) but excellent negative predictive value (NPV) (100%) for perioperative and long-term cardiac events [40].
5.3. Myocardial Perfusion Imaging (MPI)
Myocardial perfusion imaging with single positron emission tomography (SPECT) is another modality for diagnosing CAD in the general population. The sensitivity of MPI is as low as 43% with a reasonable specificity of 82% reported in a study on 349 patients undergoing lung transplant evaluation [41]. Furthermore, the role of pharmacological SPECT-MPI testing with vasodilators such as adenosine and dipyridamole is limited, due to possible receptor-mediated bronchoconstriction in patients with severe lung disease. Similar to DSE, dobutamine thallium MPI scan has limited specificity in lung transplant candidates with reported false-positive rates of up to 50% [42]. However, SPECT-MPI with regadenoson (a selective A2A receptor agonist) has been shown to be well tolerated in patients with end-stage lung disease with a negative predictive value (NPV) of 85% and a positive predictive value (PPV) of 75% [43]. Nonetheless, positron emission tomography (PET) for myocardial perfusion using rubidium-82 has a high sensitivity and specificity of 90% and 88%, respectively, in detecting CAD in the general population and is recommended as one of the imaging modalities for patients undergoing solid organ transplantation, including lung transplantation [44].
5.4. Stress Cardiac Magnetic Resonance (CMR)
Stress CMR imaging along with late gadolinium enhancement has been shown to predict myocardial ischemia, along with assessment of myocardial viability and cardiac function. A meta-analysis involving 2841 patients with CAD revealed that stress CMR exhibits a pooled sensitivity of 89% and a specificity of 76% in predicting CAD with ≥50% stenosis on coronary angiography [45].
5.5. Coronary Computed Tomography Angiography (CCTA)
Coronary computed tomography angiography (CCTA) is validated as a potential alternative to coronary angiography for diagnosing and grading the severity of CAD. A meta-analysis of studies evaluating the ability of CCTA and coronary artery calcium score (CACS) to predict perioperative cardiovascular events in non-cardiac surgery has demonstrated a threefold and eightfold increased risk of perioperative major adverse cardiovascular events (MACE) for individuals with single-vessel and multivessel obstructive CAD on CCTA, respectively, as compared to those with no CAD. Furthermore, a rising CACS showed a correlation with a progressively elevated trend in perioperative MACE (CACS ≥ 100, odds ratio (OR) of 5.2; ≥1000, OR of 10.4; both with a p-value < 0.01) [46]. While one study assessed the usefulness of CACS assessment on high-resolution computed tomography (HRCT) for patients with idiopathic pulmonary fibrosis, there is a paucity of data regarding the predictive ability of CCTA and CACS with respect to perioperative outcomes in patients undergoing lung transplantation [47].
5.6. Coronary Angiography
Invasive coronary angiography is the gold standard for CAD detection but is associated with procedural risks, including radiation and contrast exposure. Newer assessment strategies such as physiology assessment with fractional flow reserve (FFR), plaque characterization with intra-vascular ultrasound (IVUS), and optical coherence tomography (OCT) improve diagnostic accuracy. Moreover, newer techniques such as the instantaneous wave-free pressure ratio (iFR) and the Resting flow ratio (RFR), which do not require adenosine, offer an additional advantage in assessing the significance of intermediate stenosis in lung transplant candidates, as adenosine has the potential to induce bronchospasm [48].
The choice between the invasive versus non-invasive screening modalities remains an elusive one. The 2022 ESC guidelines for cardiovascular assessment in non-cardiac surgery clearly categorize invasive coronary angiography as a Class III recommendation for patients undergoing low- or intermediate-risk non-cardiac surgery [49]. However, in the case of lung transplant, which is categorized as high surgical risk (30-day risk of cardiovascular death, myocardial infarction (MI), and stroke > 5%), there is a lack of clear guideline recommendations regarding the use of invasive coronary angiography as part of preoperative evaluation in this specific population. Due to the lack of guideline recommendations, as well as the questionable sensitivity and specificity of non-invasive testing, many lung transplant centers continue to favor coronary angiography as the screening investigation of choice, particularly for individuals over the age of 40. Figure 1 represents a possible approach to CAD screening in lung transplantation candidates.
6. Indications for Revascularization
The quandary of whether to undergo coronary angiography extends to the question of whether revascularization should be considered for asymptomatic patients. The occurrence of CAD in patients undergoing non-cardiac surgeries places them at higher risk of ischemic events during the perioperative period. However, there are conflicting data regarding the benefit offered by prophylactic PCI before non-cardiac surgery in reducing perioperative ischemic events [50]. Routine revascularization prior to non-cardiac surgery in order to reduce perioperative cardiovascular events is currently not recommended under the 2014 ACC/AHA guidelines and the 2022 ESC guidelines (unless undergoing carotid endarterectomy) [49][50][51]. Therefore, the recommendations for cardiac revascularization before non-cardiac surgeries remain the same as they would be if surgery was not a factor [51].
Various studies have reported similar rates of survival with no differences in perioperative morbidity between patients with obstructive CAD requiring revascularization, either by PCI or previous or concomitant CABG, compared to those with no underlying CAD [5][52]. Similarly, for patients with moderate CAD (angiographic epicardial stenosis > 20% but <70%), Zanotti et al. and Choong et al. have reported that there is no significant increase in perioperative morbidity or mortality in patients with moderate CAD who do not undergo preoperative revascularization before lung transplantation compared to patients with no underlying CAD. However, among moderate-CAD patients, 6% to 18% developed symptomatic artery disease requiring post-transplant revascularization by either CABG or PCI, highlighting the importance of initiating statins and aspirin to prevent cardiovascular events in patients with underlying CAD [18][19].
References
- Koprivanac, M.; Budev, M.M.; Yun, J.J.; Kelava, M.; Pettersson, G.B.; McCurry, K.R.; Johnston, D.R.; Mangi, A.A.; Houghtaling, P.L.; Blackstone, E.H.; et al. How important is coronary artery disease when considering lung transplant candidates? J. Heart Lung Transplant. 2016, 35, 1453–1461.
- Makey, I.A.; Sui, J.W.; Huynh, C.; Das, N.A.; Thomas, M.; Johnson, S. Lung transplant patients with coronary artery disease rarely die of cardiac causes. Clin. Transplant. 2018, 32, e13354.
- Miller, L.W. Cardiovascular Toxicities of Immunosuppressive Agents. Am. J. Transplant. 2002, 2, 807–818.
- Wild, J.; Arrigo, M.; Isenring, B.D.; Buergi, U.; Kurowski, T.; Schuurmans, M.M.; Huber, L.C.; Benden, C. Coronary artery disease in lung transplant candidates: Role of routine invasive assessment. Respiration 2015, 89, 107–111.
- Khandhar, S.J.; Althouse, A.D.; Mulukutla, S.; Kormos, R.; Toma, C.; Marroquin, O.; Volz, E.; Tefera, L.; Bermudez, C. Postoperative outcomes and management strategies for coronary artery disease in patients in need of a lung transplantation. Clin. Transplant. 2017, 31, e13026.
- Ben-Dor, I.; Shitrit, D.; Kramer, M.R.; Iakobishvili, Z.; Sahar, G.; Hasdai, D. Is routine coronary angiography and revascularization indicated among patients undergoing evaluation for lung transplantation? Chest 2005, 128, 2557–2562.
- Kaza, A.K.; Dietz, J.F.; Kern, J.A.; Jones, D.R.; Robbins, M.K.; Fiser, S.M.; Long, S.M.; Bergin, J.D.; Kron, I.L.; Tribble, C.G. Coronary risk stratification in patients with end-stage lung disease. J. Heart Lung Transplant. 2002, 21, 334–339.
- Seoane, L.; Arcement, L.M.; Valentine, V.G.; McFadden, P.M. Long-term survival in lung transplant recipients after successful preoperative coronary revascularization. J. Thorac. Cardiovasc. Surg. 2005, 130, 538–541.
- Jones, R.M.; Enfield, K.B.; Mehrad, B.; Keeley, E.C. Prevalence of obstructive coronary artery disease in patients undergoing lung transplantation: Case series and review of the literature. Catheter. Cardiovasc. Interv. 2014, 84, 1–6.
- Kizer, J.R.; Zisman, D.A.; Blumenthal, N.P.; Kotloff, R.M.; Kimmel, S.E.; Strieter, R.M.; Arcasoy, S.M.; Ferrari, V.A.; Hansen-Flaschen, J. Association between pulmonary fibrosis and coronary artery disease. Arch. Intern. Med. 2004, 164, 551–556.
- Snell, G.I.; Richardson, M.; Griffiths, A.P.; Williams, T.J.; Esmore, D.S. Coronary artery disease in potential lung transplant recipients > 50 years old: The role of coronary intervention. Chest 1999, 116, 874–879.
- Leard, L.E.; Holm, A.M.; Valapour, M.; Glanville, A.R.; Attawar, S.; Aversa, M.; Campos, S.V.; Christon, L.M.; Cypel, M.; Dellgren, G.; et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2021, 40, 1349–1379.
- Chassot, P.G.; Delabays, A.; Spahn, D. Preoperative evaluation of patients with, or at risk of, coronary artery disease undergoing non-cardiac surgery. Br. J. Anaesth. 2002, 89, 747–759.
- Cao, D.; Chandiramani, R.; Capodanno, D.; Berger, J.S.; Levin, M.A.; Hawn, M.T.; Angiolillo, D.J.; Mehran, R. Non-cardiac surgery in patients with coronary artery disease: Risk evaluation and periprocedural management. Nat. Rev. Cardiol. 2021, 18, 37–57.
- Vincenti, F. Immunosuppression minimization: Current and future trends in transplant immunosuppression. J. Am. Soc. Nephrol. 2003, 14, 1940–1948.
- Chaikriangkrai, K.; Jyothula, S.; Jhun, H.Y.; Estep, J.; Loebe, M.; Scheinin, S.; Torre-Amione, G. Impact of pre-operative coronary artery disease on cardiovascular events following lung transplantation. J. Heart Lung Transplant. 2016, 35, 115–121.
- Halloran, K.; Hirji, A.; Li, D.; Jackson, K.; Kapasi, A.; Meyer, S.; Mullen, J.; Lien, D.; Weinkauf, J. Coronary Artery Disease and Coronary Artery Bypass Grafting at the Time of Lung Transplantation Do Not Impact Overall Survival. Transplantation 2019, 103, 2190–2195.
- Choong, C.K.; Meyers, B.F.; Guthrie, T.J.; Trulock, E.P.; Patterson, G.A.; Moazami, N. Does the presence of preoperative mild or moderate coronary artery disease affect the outcomes of lung transplantation? Ann. Thorac. Surg. 2006, 82, 1038–1042.
- Zanotti, G.; Hartwig, M.G.; Castleberry, A.W.; Martin, J.T.; Shaw, L.K.; Williams, J.B.; Lin, S.S.; Davis, R.D. Preoperative mild-to-moderate coronary artery disease does not affect long-term outcomes of lung transplantation. Transplantation 2014, 97, 1079–1085.
- Jones, M.; Patel, S.; Tefera, L.; Borrebach, J.; Bermudez, C.; Bhama, J.; Kormos, R.; Shigemura, N.; D’Cunha, J.; Mulukutla, S. The impact of coronary artery disease on lung transplantation outcomes. J. Heart Lung Transplant. 2014, 33, S80.
- Sherman, W.; Rabkin, D.G.; Ross, D.; Saggar, R.; Lynch, J.P., 3rd; Belperio, J.; Saggar, R.; Hamilton, M.; Ardehali, A. Lung transplantation and coronary artery disease. Ann. Thorac. Surg. 2011, 92, 303–308.
- Castleberry, A.W.; Martin, J.T.; Osho, A.A.; Hartwig, M.G.; Hashmi, Z.A.; Zanotti, G.; Shaw, L.K.; Williams, J.B.; Lin, S.S.; Davis, R.D. Coronary revascularization in lung transplant recipients with concomitant coronary artery disease. Am. J. Transplant. 2013, 13, 2978–2988.
- Mateen, F.J.; Dierkhising, R.A.; Rabinstein, A.A.; Van De Beek, D.; Wijdicks, E.F.M. Neurological complications following adult lung transplantation. Am. J. Transplant. 2010, 10, 908–914.
- Shigemura, N.; Sclabassi, R.J.; Bhama, J.K.; Gries, C.J.; Crespo, M.M.; Johnson, B.; Pilewski, J.M.; Bermudez, C.A. Early major neurologic complications after lung transplantation: Incidence, risk factors, and outcome. Transplantation 2013, 95, 866–871.
- Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; Meade, T.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996, 348, 1329–1339.
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143.
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681.
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722.
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107.
- Cannon, C.P.; Blazing, M.A.; Braunwald, E. Ezetimibe plus a Statin after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 373, 1473–1477.
- Wright, J.T., Jr.; Williamson, J.D.; Whelton, P.K.; Snyder, J.K.; Sink, K.M.; Rocco, M.V.; Reboussin, D.M.; Rahman, M.; Oparil, S.; Lewis, C.E.; et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015, 373, 2103–2116.
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324.
- Arnold, S.V.; Bhatt, D.L.; Barsness, G.W.; Beatty, A.L.; Deedwania, P.C.; Inzucchi, S.E.; Kosiborod, M.; Leiter, L.A.; Lipska, K.J.; Newman, J.D.; et al. Clinical Management of Stable Coronary Artery Disease in Patients With Type 2 Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e779–e806.
- Sorbets, E.; Steg, P.G.; Young, R.; Danchin, N.; Greenlaw, N.; Ford, I.; Tendera, M.; Ferrari, R.; Merkely, B.; Parkhomenko, A.; et al. β-blockers, calcium antagonists, and mortality in stable coronary artery disease: An international cohort study. Eur. Heart J. 2019, 40, 1399–1407.
- Belsey, J.; Savelieva, I.; Mugelli, A.; Camm, A.J. Relative efficacy of antianginal drugs used as add-on therapy in patients with stable angina: A systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2015, 22, 837–848.
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131.
- International guidelines for the selection of lung transplant candidates. The American Society for Transplant Physicians (ASTP)/American Thoracic Society(ATS)/European Respiratory Society(ERS)/International Society for Heart and Lung Transplantation(ISHLT). Am. J. Respir. Crit. Care Med. 1998, 158, 335–339.
- Weill, D.; Benden, C.; Corris, P.A.; Dark, J.H.; Davis, R.D.; Keshavjee, S.; Lederer, D.J.; Mulligan, M.J.; Patterson, G.A.; Singer, L.G.; et al. A consensus document for the selection of lung transplant candidates: 2014—An update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2015, 34, 1–15.
- Bossone, E.; Martinez, F.J.; Whyte, R.I.; Iannettoni, M.D.; Armstrong, W.F.; Bach, D.S. Dobutamine stress echocardiography for the preoperative evaluation of patients undergoing lung volume reduction surgery. J. Thorac. Cardiovasc. Surg. 1999, 118, 542–546.
- Manoushagian, S.; Meshkov, A. Evaluation of solid organ transplant candidates for coronary artery disease. Am. J. Transplant. 2014, 14, 2228–2234.
- Henzlova, M.J.; Padilla, M.L.; Freilich, A.; Gass, A.L.; Courtney, M.C.; Diamond, J.A.; Machac, J. Dobutamine thallium 201 perfusion imaging in candidates for lung transplantation. J. Heart Lung Transplant. 1995, 14, 251–256.
- Schiopu, S.R.I.; Zacherl, M.; Todica, A.; Bartenstein, P.; Milger, K.; Leuschner, G.; Munker, D.; Bauer, M.; Massberg, S.; Behr, J.; et al. Feasibility and accuracy of SPECT myocardial perfusion imaging in end-stage lung disease. J. Nucl. Cardiol. 2020, 27, 903–911.
- Schindler, T.H.; Bateman, T.M.; Berman, D.S.; Chareonthaitawee, P.; De Blanche, L.E.; Dilsizian, V.; Dorbala, S.; Gropler, R.J.; Shaw, L.; Soman, P.; et al. Appropriate Use Criteria for PET Myocardial Perfusion Imaging. J. Nucl. Med. 2020, 61, 1221–1265.
- Jaarsma, C.; Leiner, T.; Bekkers, S.C.; Crijns, H.J.; Wildberger, J.E.; Nagel, E.; Nelemans, P.J.; Schalla, S. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: A meta-analysis. J. Am. Coll. Cardiol. 2012, 59, 1719–1728.
- Koshy, A.N.; Ha, F.J.; Gow, P.J.; Han, H.C.; Amirul-Islam, F.M.; Lim, H.S.; Teh, A.W.; Farouque, O. Computed tomographic coronary angiography in risk stratification prior to non-cardiac surgery: A systematic review and meta-analysis. Heart 2019, 105, 1335–1342.
- Sinha, N.; Balayla, G.; Braghiroli, J. Coronary artery disease in lung transplant patients. Clin. Transplant. 2020, 34, e14078.
- Gotberg, M.; Christiansen, E.H.; Gudmundsdottir, I.J.; Sandhall, L.; Danielewicz, M.; Jakobsen, L.; Olsson, S.E.; Ohagen, P.; Olsson, H.; Omerovic, E.; et al. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N. Engl. J. Med. 2017, 376, 1813–1823.
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; de Laval, I.; Geisler, T.; Hinterbuchner, L.; et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur. Heart J. 2022, 43, 3826–3924.
- McFalls, E.O.; Ward, H.B.; Moritz, T.E.; Goldman, S.; Krupski, W.C.; Littooy, F.; Pierpont, G.; Santilli, S.; Rapp, J.; Hattler, B.; et al. Coronary-artery revascularization before elective major vascular surgery. N. Engl. J. Med. 2004, 351, 2795–2804.
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2014, 130, e278–e333.
- Kanaparthi, J.; Kashem, M.A.; Suryapalam, M.; Zhao, H.; Brann, S.; Leotta, E.; Minakata, K.; Keshavamurthy, S.; Shigemura, N.; Toyoda, Y. Prior and Perioperative Revascularization Does Not Affect Survival in Lung Transplant Patients. Ann. Thorac. Surg. 2020, 109, 1677–1683.
More
Information
Subjects:
Transplantation
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
576
Revisions:
2 times
(View History)
Update Date:
10 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





