You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Massimo Bottini | -- | 2714 | 2024-01-03 21:48:18 | | | |
| 2 | Catherine Yang | + 1 word(s) | 2715 | 2024-01-04 02:08:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mebarek, S.; Buchet, R.; Pikula, S.; Strzelecka-Kiliszek, A.; Brizuela, L.; Corti, G.; Collacchi, F.; Anghieri, G.; Magrini, A.; Ciancaglini, P.; et al. Matrix Vesicles and Media Vesicles. Encyclopedia. Available online: https://encyclopedia.pub/entry/53394 (accessed on 26 December 2025).
Mebarek S, Buchet R, Pikula S, Strzelecka-Kiliszek A, Brizuela L, Corti G, et al. Matrix Vesicles and Media Vesicles. Encyclopedia. Available at: https://encyclopedia.pub/entry/53394. Accessed December 26, 2025.
Mebarek, Saida, Rene Buchet, Slawomir Pikula, Agnieszka Strzelecka-Kiliszek, Leyre Brizuela, Giada Corti, Federica Collacchi, Genevieve Anghieri, Andrea Magrini, Pietro Ciancaglini, et al. "Matrix Vesicles and Media Vesicles" Encyclopedia, https://encyclopedia.pub/entry/53394 (accessed December 26, 2025).
Mebarek, S., Buchet, R., Pikula, S., Strzelecka-Kiliszek, A., Brizuela, L., Corti, G., Collacchi, F., Anghieri, G., Magrini, A., Ciancaglini, P., Millan, J.L., Davies, O., & Bottini, M. (2024, January 03). Matrix Vesicles and Media Vesicles. In Encyclopedia. https://encyclopedia.pub/entry/53394
Mebarek, Saida, et al. "Matrix Vesicles and Media Vesicles." Encyclopedia. Web. 03 January, 2024.
Copy Citation
Mineralization-competent cells, including hypertrophic chondrocytes, mature osteoblasts, and osteogenic-differentiated smooth muscle cells secrete media extracellular vesicles (media vesicles) and extracellular vesicles bound to the extracellular matrix (matrix vesicles). Media vesicles are purified directly from the extracellular medium. On the other hand, matrix vesicles are purified after discarding the extracellular medium and subjecting the cells embedded in the extracellular matrix or bone or cartilage tissues to an enzymatic treatment.
media extracellular vesicles
matrix vesicles
cell-cell communication
1. Discovery of Matrix Vesicles
Lipid components in the mineralizing front of cartilage were revealed by Sudan Black B staining of growth plate cartilage [1][2][3]. Later, electron microscopy indicated the presence of 100–300 nm in diameter vesicular structures at the site of epiphyseal cartilage in mice [4][5]. Cartilage at an early stage of calcification of 1-month-old guinea pigs and at proximal tibial-distal femoral epiphyses of 3-day-old rats showed the presence of roundish bodies, which gradually become filled with crystallites [6]. The first extraction of mineralizing vesicles was carried out using bovine fetal or rabbit epiphyseal cartilages [7][8]. A collagenase digestion of the epiphyseal cartilage, followed by several differential centrifugations, was performed [7][8][9]. An enriched amount of cholesterol, phosphatidylserine, and sphingomyelin was found in mineralizing vesicles, as compared to the composition of plasma membranes [10][11][12]. The isolated vesicles had high tissue-nonspecific alkaline phosphatase (TNAP) activity [9][13][14]. The first mineralizing vesicles were isolated directly from cartilage tissues and were released after collagenase digestion. They were not extracted from either the extracellular medium or biological fluids. At that time, mineralizing vesicles were referred to as matrix vesicles or collagenase-released vesicles [15]. Since their initial discovery in the growth plate cartilage, other mineralizing vesicles have been found at the first mineral deposit site during intramembranous bone formation [16], in fracture callus [17], in developing dentin [18], in pathological calcification of valves [19], and in osteosarcoma [20].
2. Discovery of Media Vesicles
The discovery of media (extracellular) vesicles has been reviewed elsewhere [21][22][23][24]. Here, the researchers briefly communicate work describing the occurrence of extracellular vesicles not bound to the extracellular matrix. Early work on the clotting factors in human plasma indicated the presence of blood corpuscles, in addition to the thromboplastic agent, sedimented at 31,000× g [25]. The material extracted from plasma and separated by ultracentrifugation was enriched in phospholipids and showed coagulant properties resembling those of platelet factor 3 [26]. Around 1960, several pieces of evidence suggesting the occurrence of extracellular vesicles in platelets [26] secreted by mammalian cells [27], as well as non-mammalian phagocytic cells [28][29] were collected by means of electron microscopy. Extracellular synaptic vesicles at sites of the periaxonal space within the mouse atrium were evidenced by electron microscopy [30][31]. It was also discovered that extracellular vesicles can contribute to neuronal signaling [32][33]. Addition of the A23187 cation ionophore to human red blood cells induced a discocyte to echinocyte morphological change and the release of extracellular vesicles enriched in 1,2-diacylglycerol [34]. Extracellular vesicles released from Ochromonas danica were evidenced by electron microscopy in the early 1970s [35]. It was not a fixation artifact since extracellular vesicles could be isolated [35]. Similarly, other microorganisms such as Candida [36], Corynebacterium [37], Acinetobacter [38], and trypanosoma cruzi [39] can release extracellular vesicles. The first indication that these particles could mediate functional biological effects was indicated by the discovery that major histocompatibility complex (MHC) class II-containing extracellular vesicles from B lymphocytes could regulate the activity of T cells [40]. Later, horizontal RNA transfer was reported between extracellular vesicles and recipient cells [41][42]. Extracellular vesicles were referred to by several names around this time by groups working in different fields and it was often unclear exactly what these particles were [21]. In this respect, it is important to emphasize that exosomes are generated via the endocytic pathway, which correspond to one subcategory of extracellular vesicles [43][44]. Another category of extracellular vesicles which shed directly from plasma membranes are called ectosomes [21]. Since the sizes of ectosomes and exosomes may overlap and sometimes no specific markers have been identified, they are collectively referred as extracellular vesicles [21].
3. How to Differentiate Matrix Vesicles and Media Vesicles
There is no perfect extraction method to isolate extracellular vesicles, and therefore the isolation step should be accompanied by a biochemical analysis to fully characterize the extracellular vesicles and their functions [45][46]. Several pieces of evidence from studying mineralizing cells support the model that media vesicles are distinct from matrix vesicles [15][47].
Media vesicles have been isolated by using a variety of published protocols [48] (Figure 1A). Ultracentrifugation, polyethylene glycol precipitation, total exosome isolation reagent, and an aqueous two-phase system with and without repeat washes or size exclusion chromatography have been assessed for their ability to extract extracellular vesicles [49]. Among these methods, size exclusion chromatography and ultracentrifugation were favored for overall efficiency [49]. Although there are variations in sample purity between isolation methods, scalability, and yield, ultracentrifugation represents the most common method but remains not specific. Further specificity could be gained after ultracentrifugation by using affinity chromatography or a combination of size exclusion chromatography with ultrafiltration to maximize both yield and purity [50]. One of the hallmarks of the mineralization process is the presence of tissue non-specific phosphatase (TNAP) activity of the cells and of extracellular vesicles [15]. The expression of TNAP in media vesicles and in matrix vesicles could be compared to further substantiate their differences. Briefly, extracellular medium is harvested and centrifuged at 1000× g for 30 min at 4 °C to remove cells and bigger debris. A second centrifugation, which is optional, can be performed at 20,000× g for 30 min to isolate large-sized media vesicles along with smaller debris (smaller debris can be removed by a successive step based, for instance, on chromatography). The final centrifugation step is performed at 100,000× g for a time ranging from 30 min to 2 h. The pellet obtained at this stage contain small- and medium-sized media vesicles. The pellet is resuspended preferably in ice-cold synthetic cartilage lymph, which is a buffer matching the electrolyte composition of the extracellular milieu in cartilage and bone tissues. This is not the case for most media vesicle studies. However, buffer composition has been optimized to obtain the most stable matrix vesicles [51]. To compare media vesicles and matrix vesicles, the same buffer should be used. In this respect, most of the findings concerning the comparisons of properties of media vesicles and matrix vesicles are from mineral-competent cells. The synthetic cartilage lymph contains 1.42 mM Na2HPO4, 1.83 mM NaHCO2, 12.7 mM KCl, 0.57 mM MgCl2, 100 mM NaCl, 0.57 mM Na2SO4, 5.55 mM glucose, 63.5 mM sucrose, and 16.5 mM 2-{2-hydroxy-1,1-bis (hydroxymethyl) ethyl} amino)-propanesulfonic acid (pH 7.4) [51][52].
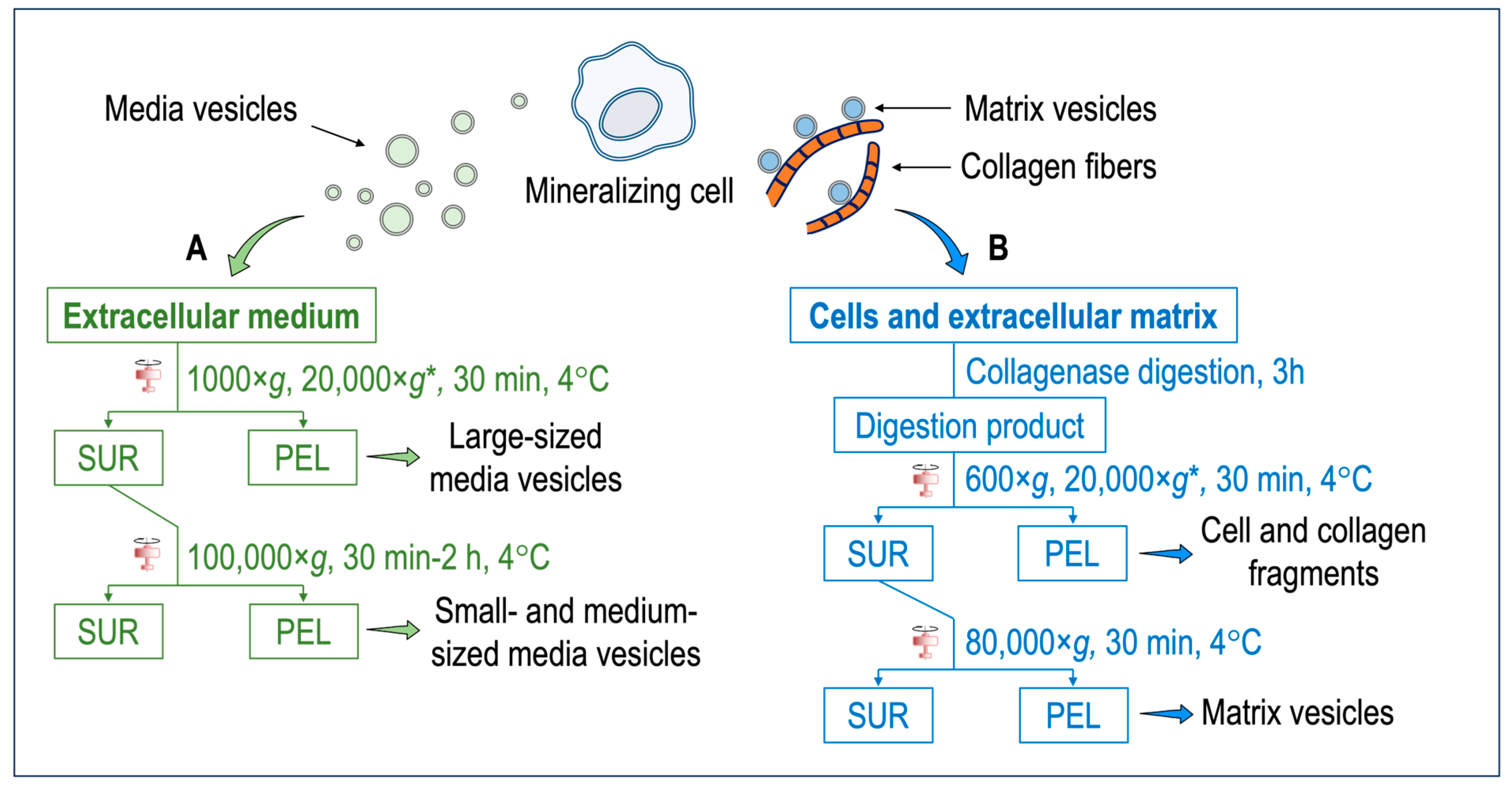
Figure 1. (A) Isolation of media vesicles. Cells and the extracellular matrix are discarded. The extracellular medium is subjected to two–three centrifugation steps to obtain either the large-sized vesicles or small- and medium-sized vesicles. Extracellular vesicles anchored to the collagenous matrix (i.e., matrix vesicles) remain mostly attached to the extracellular matrix and only a small population can be extracted. (B) Isolation of matrix vesicles. The extracellular medium is discarded. Cells and the extracellular matrix are washed with synthetic cartilage lymph [51][52]. An enzymatic digestion, for instance, with collagenase, is performed to degrade the collagen fibers and release matrix vesicles. After several centrifugation steps, matrix vesicles are obtained. * = optional step. Abbreviations: SUR = supernatant; PEL = pellet.
Most matrix vesicles with high TNAP activity are released from fully differentiated and mineral competent cells, while undifferentiated cells release extracellular vesicles with little TNAP activity [47]. Matrix vesicles are released from hypertrophic chondrocytes [5][5][53][54][55][56]. Hypertrophic chondrocyte differentiation is associated with high TNAP activity, and the synthesis and secretion of type X collagen followed by type II collagen by proliferating and pre-hypertrophic chondrocytes [5][53][54][55]. Expression of type I collagen by hypertrophic chondrocytes might be associated with differentiation into osteoblast-like cells [56][57][58]. Matrix vesicles correspond to mineralizing cartilage, where the growing cartilage is replaced by bone, while articular cartilage matrix vesicles originate from normal cartilage that does not undergo matrix mineralization except in pathologic conditions such as osteoarthritis [59]. Articular matrix vesicles from normal cartilage show low TNAP activity, however during osteoarthritis chondrocytes can become hypertrophic and fully mineralized. They release articular matrix vesicles which can induce pathologic calcium crystal deposition in articular cartilage matrix [60]. The release of matrix vesicles by osteoblasts is stimulated in osteogenic medium containing ascorbic acid and beta-glycerophosphate [61]. TNAP activity of Saos-2 cells increased with the duration of the treatment with osteogenic factors which coincided with the amount of released matrix vesicles [62]. Matrix vesicles have been isolated by the collagenase-digestion method [15][63][64] (Figure 1B). Briefly, tissues (for instance, growth plates and epiphyseal cartilage from 17-day-old chicken embryos) are cut into 1–3 mm thick slices and washed with synthetic cartilage lymph. In the case of cells (for instance, osteoblast and chondrocyte cultures), the extracellular medium is discarded, and the cells embedded in the collagenous matrix are washed with synthetic cartilage lymph. The osseous and cartilage tissues or cells are vortexed in synthetic cartilage lymph containing 1 mM CaCl2 and 100–200 U of type-I collagenase from Clostritidium histolyticum at 37 °C for 180 min. Since the quality of the commercial collagenase is variable, the amount of the collagenase to be adjusted can be determined by measuring TNAP activity of the matrix vesicles [63]. Indeed, scientists have taken advantage of this to define matrix vesicle purity as having TNAP activity that is a minimum of two-fold greater than that of the plasma membrane fraction [65]. From this stage, either a three-step centrifugation [66] or a two-step centrifugation [67] is usually performed. The collagenase-digested sample is filtered through a nylon filter and centrifuged at 600× g for 30 min at 4 °C to remove cells and fragments of the extracellular matrix. The pellet is discarded, and the supernatant is subjected to an optional centrifugation step at 20,000× g for 30 min at 4 °C to remove membrane debris. Then, the supernatant is ultracentrifuged at 80,000× g for 60 min at 4 °C. The final supernatant is removed, while the pellet, containing matrix vesicles, is washed three times with synthetic cartilage lymph to remove collagenase and calcium. Matrix vesicles shall not be frozen—however they can be stored at 4 °C up to five days [63] to preserve their original enzymatic activity, including those of TNAP, Pi and Ca transporters, and other membrane proteins. The freezing thaw process can induce membranous defects which can leak ions and/or other soluble particles. Cryoprotectant, as sucrose or trehalose, can be added [68] to preserve the integrity of extracellular vesicles [68]. The collagenase-digestion method provides a relatively high amount of matrix vesicles displaying a TNAP specific activity of around 15 to 30 μmole min−1 mg−1 [63]. Alternatively, trypsin [69], trypsin/collagenase [70], liberase/blendzyme-1 [71], hyaluronidase [59][64], and hyaluronidase/collagenase [70] can be used to release matrix vesicles from the extracellular matrix (Table 1). Collagenase digestion is among the most used digestion method to isolate matrix vesicles which can yield a high ratio of alkaline phosphatase activity in matrix vesicles compared to that in media vesicles or plasma membrane (Table 1). The collagenase and hyaluronidase digestion, which removed the surface collagens on the matrix vesicles, induced a loss of calcium uptake [70] (Table 1). In contrast collagenase and trypsin digestion, which maintained a part of surface collagens on matrix vesicles, preserved the calcium uptake [70] (Table 1). The process of trypsin digestion resulted in the release of matrix vesicles from the extracellular matrix, exhibiting an alkaline phosphatase activity six times greater than that of the plasma membrane. [72] (Table 1). Liberase and blendzyme1, which is gentle digestion, was the less efficient method to release matrix vesicles than the other digestion methods, as indicated by the lower ratio of alkaline phosphatase in matrix vesicles as compared to that in media vesicles [71] (Table 1).
Table 1. Properties, ratio of TNAP activity of matrix vesicles (MVs): Media extracellular vesicles (media EVs), mineralization assay, and determination of apatite in the lumen of matrix vesicles subjected to different types of enzymatic digestion. * refers to the ratio of TNAP activity of matrix vesicles:basolateral membranes and ** refers to the ratio of TNAP activity of matrix vesicles: plasma membranes. IR = infrared; ND = not determined.
| Digestion | Properties | Samples | Ratio TNAP | Mineralization | Apatite in | References |
|---|---|---|---|---|---|---|
| Process | Activity | Assay | Lumen | |||
| MVs: Media EVs | ||||||
| Growth plate | From 4 to 6 | YES | YES (IR) | [67] | ||
| cartilage chicken | ||||||
| Collagenase | Widely used | Primary | From 8 to 12 | YES | ND | [66] |
| Chondrocytes | ||||||
| Saos2 cells | Around 16 * | YES | YES (IR) | [62] | ||
| Collagenase and | MVs without | Growth plate | ND | Calcium uptake | Calcium uptake | [70] |
| hyaluronidase | surface collagens | cartilage chicken | was impaired | was impaired | ||
| Collagenase and | MVs with surface | Growth plate | ND | Calcium uptake | Calcium uptake | [70] |
| trypsin | attached collagens | cartilage chicken | was optimum | was optimum | ||
| Hyaluronidase | Used for | Non mineralizing | ND | ND | ND | [59] |
| articular | articular | |||||
| chondrocytes | chondrocytes | |||||
| Trypsin | MG-63 cells | Around 6 ** | ND | ND | [72] | |
| Liberase and | Gentle digestion | MC3T3-E1 | From 0.7 to 0.8 | ND | ND | [71] |
Although collagenase digestion is often used to release the cells from the extracellular matrix, enzymatic digestion may alter/damage surface protein expression of the matrix vesicles. For instance, the main collagen identified in matrix vesicles from growth plate cartilage is collagen type VI, which is consistent with the resistance of this type of collagen to the collagenase digestion [73]. Only three peptides corresponding to cartilage specific collagen type X are identified from proteomic analysis [73]. Generally, the quality of matrix vesicles is assessed by TNAP activity, an ability to form apatites inside MVs, turbidity measurements, and morphological assessment by electron microscopy [15][63]. As a result of matrix vesicles not being lysed during isolation, their native conformation remains intact. Thus, they remain right side out, with the ectoenzyme TNAP facing outward [64]. Each digestion method displays a different efficiency to release matrix vesicles and/or maintain their physical and biochemical properties [70].
One essential function of matrix vesicles is to initiate apatite formation and to deposit it onto collagen fibers [15][74][75]. Several pieces of evidence support that matrix vesicles are strongly bound to the extracellular matrix. Northern blot and immunohistochemical analyses on matrix vesicles from chondrocytes revealed an increase in annexin A5 and type I collagen [55]. Annexin A5 is highly enriched in matrix vesicles and was found to bind to native type I, II, and X collagens [76][77][78][79]. Neutral metalloproteases 2, 9, and 13 [80][81] were found in matrix vesicles, which would suggest that the initiation of matrix vesicle-induced mineralization is coupled with the degradation of the inhibitory proteoglycan matrix [15]. Matrix vesicles are anchored within the extracellular matrix via integrin binding to type II collagen [82].
4. Matrix Vesicles and Media Vesicles from Smooth Muscle Cells
Vascular calcification occurs when vascular smooth muscle cells or circulating cells differentiate to an osteogenic-like phenotype, synthesize an extracellular matrix, and form apatite [83]. Histologic, ultrastructural, and cytochemical techniques indicated that extracellular vesicles are involved in arterial medial calcification [84] but they are distinct from their bone counterparts [85]. Extracellular vesicles released by vascular smooth muscle cells are at the sites of medial vascular calcification [86][87][88][89][90][91][92] and can be involved in atherosclerosis-related vascular calcification [85][93]. Extracellular vesicles can communicate with cells and organs to regulate vascular calcification and may serve as therapeutic methods in vascular calcification [94]. In this respect, it is essential to make a clear distinction between matrix vesicles and media vesicles. Matrix vesicles extracted after a collagenase digestion [95][96][97][98][99] and media vesicles isolated without collagenase digestion, both from vascular smooth muscle cells cultured in an osteogenic medium [87][95][100][101][102][103][104][105], have distinct characteristics both at molecular and functional levels. Proteomic profiles of matrix vesicles and media vesicles from MOVAS cell line are distinct [106]. Matrix vesicles from vascular smooth muscle cells are enriched in endosomal CD63 as compared to media vesicles, which are enriched in CD81 and CD9 [95]. Matrix vesicles induced calcification of recipient vascular muscle cells, while media vesicles appear to be less efficient [95] probably due to the enriched amount of fetuin (which inhibits calcification) in media vesicles. Media vesicles from vascular smooth muscle cells are of exosomal origin [107]. This is supported by the presence of phosphatidylserine on the outside layer leaflet of media vesicles [100]. It was proposed that phosphatidylserine exposure on the external surface of extracellular vesicles together with annexin A6 could drive the mineralization process [100], although direct experimental evidence of apatite formation induced by media vesicles released by vascular smooth muscle cells is lacking. TNAP-enriched matrix vesicles released from vascular smooth muscle cells subjected to collagenase contained more minerals with higher Ca/P ratio than the less TNAP-enriched vesicles isolated without collagenase treatment. These findings suggest a role for collagen in promoting calcification induced by TNAP in atherosclerotic plaques [108]. To explain why osteoporosis can contribute to vascular calcification—a calcification paradox—it was reported that extracellular vesicles from aged bone matrix during bone resorption can favour the adipogenesis of bone marrow mesenchymal stem/stromal cells and increase vascular calcification [109]. So far it is still unclear if such extracellular vesicles could originate from osteoclasts within the aged bone matrix. This opens the possibility that osteoclasts can release matrix-vesicles and media vesicles with distinct properties than those released from osteoblasts and from smooth muscle cells.
References
- Irving, J.T. A Histological Staining Method for Sites of Calcification in Teeth and Bone. Arch. Oral. Biol. 1959, 1, 89–96.
- Irving, J.T. The Sudanophil Material at Sites of Calcification. Arch. Oral. Biol. 1963, 8, 735–745.
- Irving, J.T.; Wuthier, R.E. Further Observations on the Sudan Black Stain for Calcification. Arch. Oral. Biol. 1961, 5, 323–324.
- Anderson, H.C. Electron Microscopic Studies of Induced Cartilage Development and Calcification. J. Cell Biol. 1967, 35, 81–101.
- Anderson, H.C. Vesicles Associated with Calcification in the Matrix of Epiphyseal Cartilage. J. Cell Biol. 1969, 41, 59–72.
- Bonucci, E. Fine Structure of Early Cartilage Calcification. J. Ultrastruct. Res. 1967, 20, 33–50.
- Ali, S.Y.; Anderson, H.C.; Sajdera, S.W. Enzymic and Electron-Microscopic Analysis of Extracellular Matrix Vesicles Associated with Calcification in Cartilage. Biochem. J. 1971, 122, 56P.
- Ali, S.Y.; Sajdera, S.W.; Anderson, H.C. Isolation and Characterization of Calcifying Matrix Vesicles from Epiphyseal Cartilage. Proc. Natl. Acad. Sci. USA 1970, 67, 1513–1520.
- Majeska, R.J.; Wuthier, R.E. Studies on Matrix Vesicles Isolated from Chick Epiphyseal Cartilage. Association of Pyrophosphatase and ATPase Activities with Alkaline Phosphatase. Biochim. Biophys. Acta 1975, 391, 51–60.
- Peress, N.S.; Anderson, H.C.; Sajdera, S.W. The Lipids of Matrix Vesicles from Bovine Fetal Epiphyseal Cartilage. Calcif. Tissue Res. 1974, 14, 275–281.
- Wuthier, R.E. Lipid Composition of Isolated Epiphyseal Cartilage Cells, Membranes and Matrix Vesicles. Biochim. Biophys. Acta 1975, 409, 128–143.
- Wuthier, R.E. Lipids of Matrix Vesicles. Fed. Proc. 1976, 35, 117–121.
- Ketenjian, A.Y.; Arsenis, C. Morphological and Biochemical Studies during Differentiation and Calcification of Fracture Callus Cartilage. Clin. Orthop. Relat. Res. 1975, 107, 266–273.
- Kahn, S.E.; Jafri, A.M.; Lewis, N.J.; Arsenis, C. Purification of Alkaline Phosphatase from Extracellular Vesicles of Fracture Callus Cartilage. Calcif. Tissue Res. 1978, 25, 85–92.
- Wuthier, R.E.; Lipscomb, G.F. Matrix Vesicles: Structure, Composition, Formation and Function in Calcification. Front. Biosci. 2011, 16, 2812–2902.
- Bernard, G.W.; Pease, D.C. An Electron Microscopic Study of Initial Intramembranous Osteogenesis. Am. J. Anat. 1969, 125, 271–290.
- Ketenjian, A.Y.; Jafri, A.M.; Arsenis, C. Studies on the Mechanism of Callus Cartilage Differentiation and Calcification during Fracture Healing. Orthop. Clin. N. Am. 1978, 9, 43–65.
- Sisca, R.F.; Provenza, D.V. Initial Dentin Formation in Human Deciduous Teeth. An Electron Microscope Study. Calcif. Tissue Res. 1972, 9, 1–16.
- Kim, K.M. Calcification of Matrix Vesicles in Human Aortic Valve and Aortic Media. Fed. Proc. 1976, 35, 156–162.
- Sela, J.; Bab, I.A. The Relationship between Extracellular Matrix Vesicles and Calcospherities in Primary Mineralization of Neoplastic Bone Tissue. TEM and SEM Studies on Osteosarcoma. Virchows Arch. A Pathol. Anat. Histol. 1979, 382, 1–9.
- Witwer, K.W.; Théry, C. Extracellular Vesicles or Exosomes? On Primacy, Precision, and Popularity Influencing a Choice of Nomenclature. J. Extracell. Vesicles 2019, 8, 1648167.
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A Brief History of Nearly EV-Erything—The Rise and Rise of Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12144.
- Veziroglu, E.M.; Mias, G.I. Characterizing Extracellular Vesicles and Their Diverse RNA Contents. Front. Genet. 2020, 11, 700.
- Davies, O.G. Extracellular Vesicles: From Bone Development to Regenerative Orthopedics. Mol. Ther. 2023, 31, 1251–1274.
- Chargaff, E.; West, R. The Biological Significance of the Thromboplastic Protein of Blood. J. Biol. Chem. 1946, 166, 189–197.
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288.
- Sun, C.N. Lattice Structures and Osmiophilic Bodies in the Developing Respiratory Tissue of Rats. J. Ultrastruct. Res. 1966, 15, 380–388.
- Mercer, E.H.; Shaffer, B.M. Electron Microscopy of Solitary and Aggregated Slime Mould Cells. J. Biophys. Biochem. Cytol. 1960, 7, 353–356.
- Vickerman, K. Patterns of Cellular Organisation in Limax Amoebae. An Electron Microscope Study. Exp. Cell Res. 1962, 26, 497–519.
- Grillo, M.A. Extracellular Synaptic Vesicles in the Mouse Heart. J. Cell Biol. 1970, 47, 547–553.
- Dermietzel, R.; Venjakob, K.; Brettschneider, H. Occurrence of Extracellular Synaptic Vesicles in the Autonomic Nervous System. Naturwissenschaften 1972, 59, 125–126.
- Basso, M.; Bonetto, V. Extracellular Vesicles and a Novel Form of Communication in the Brain. Front. Neurosci. 2016, 10, 127.
- Budnik, V.; Ruiz-Cañada, C.; Wendler, F. Extracellular Vesicles Round off Communication in the Nervous System. Nat. Rev. Neurosci. 2016, 17, 160–172.
- Allan, D.; Billah, M.M.; Finean, J.B.; Michell, R.H. Release of Diacylglycerol-Enriched Vesicles from Erythrocytes with Increased Intracellular (Ca2+). Nature 1976, 261, 58–60.
- Aaronson, S.; Behrens, U.; Orner, R.; Haines, T.H. Ultrastructure of Intracellular and Extracellular Vesicles, Membranes, and Myelin Figures Produced by Ochromonas Danica. J. Ultrastruct. Res. 1971, 35, 418–430.
- Chigaleĭchik, A.G.; Belova, L.A.; Grishchenko, V.M.; Rylkin, S.S. Several properties of the extracellular vesicles of Candida tropicalis yeasts grown on n-alkanes. Mikrobiologiia 1977, 46, 467–471.
- Vysotskiĭ, V.V.; Mazurova, I.K.; Shmeleva, E.A. Extracellular material of some representatives of the genus Corynebacterium (the electron microscopic aspect). Zh. Mikrobiol. Epidemiol. Immunobiol. 1977, 8, 90–95.
- Käppeli, O.; Finnerty, W.R. Partition of Alkane by an Extracellular Vesicle Derived from Hexadecane-Grown Acinetobacter. J. Bacteriol. 1979, 140, 707–712.
- da Silveira, J.F.; Abrahamsohn, P.A.; Colli, W. Plasma Membrane Vesicles Isolated from Epimastigote Forms of Trypanosoma Cruzi. Biochim. Biophys. Acta 1979, 550, 222–232.
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172.
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic Stem Cell-Derived Microvesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of mRNA and Protein Delivery. Leukemia 2006, 20, 847–856.
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of mRNAs and microRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659.
- Trams, E.G.; Lauter, C.J.; Salem, N.; Heine, U. Exfoliation of Membrane Ecto-Enzymes in the Form of Micro-Vesicles. Biochim. Biophys. Acta 1981, 645, 63–70.
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Shekari, F.; Alibhai, F.J.; Baharvand, H.; Börger, V.; Bruno, S.; Davies, O.; Giebel, B.; Gimona, M.; Salekdeh, G.H.; Martin-Jaular, L.; et al. Cell Culture-Derived Extracellular Vesicles: Considerations for Reporting Cell Culturing Parameters. J. Extracell. Biol. 2023, 2, e115.
- Bottini, M.; Mebarek, S.; Anderson, K.L.; Strzelecka-Kiliszek, A.; Bozycki, L.; Simão, A.M.S.; Bolean, M.; Ciancaglini, P.; Pikula, J.B.; Pikula, S.; et al. Matrix Vesicles from Chondrocytes and Osteoblasts: Their Biogenesis, Properties, Functions and Biomimetic Models. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 532–546.
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3.22.1–3.22.29.
- Williams, S.; Fernandez-Rhodes, M.; Law, A.; Peacock, B.; Lewis, M.P.; Davies, O.G. Comparison of Extracellular Vesicle Isolation Processes for Therapeutic Applications. J. Tissue Eng. 2023, 14, 20417314231174609.
- Fernández-Rhodes, M.; Adlou, B.; Williams, S.; Lees, R.; Peacock, B.; Aubert, D.; Jalal, A.R.; Lewis, M.P.; Davies, O.G. Defining the Influence of Size-Exclusion Chromatography Fraction Window and Ultrafiltration Column Choice on Extracellular Vesicle Recovery in a Skeletal Muscle Model. J. Extracell. Biol. 2023, 2, e85.
- Wu, L.N.; Genge, B.R.; Dunkelberger, D.G.; LeGeros, R.Z.; Concannon, B.; Wuthier, R.E. Physicochemical Characterization of the Nucleational Core of Matrix Vesicles. J. Biol. Chem. 1997, 272, 4404–4411.
- Wuthier, R.E. Electrolytes of Isolated Epiphyseal Chondrocytes, Matrix Vesicles, and Extracellular Fluid. Calcif. Tissue Res. 1977, 23, 125–133.
- Garimella, R.; Bi, X.; Camacho, N.; Sipe, J.B.; Anderson, H.C. Primary Culture of Rat Growth Plate Chondrocytes: An in Vitro Model of Growth Plate Histotype, Matrix Vesicle Biogenesis and Mineralization. Bone 2004, 34, 961–970.
- Kirsch, T.; von der Mark, K. Remodelling of Collagen Types I, II and X and Calcification of Human Fetal Cartilage. Bone Miner. 1992, 18, 107–117.
- Kirsch, T.; Nah, H.D.; Shapiro, I.M.; Pacifici, M. Regulated Production of Mineralization-Competent Matrix Vesicles in Hypertrophic Chondrocytes. J. Cell Biol. 1997, 137, 1149–1160.
- Descalzi Cancedda, F.; Gentili, C.; Manduca, P.; Cancedda, R. Hypertrophic Chondrocytes Undergo Further Differentiation in Culture. J. Cell Biol. 1992, 117, 427–435.
- Gentili, C.; Bianco, P.; Neri, M.; Malpeli, M.; Campanile, G.; Castagnola, P.; Cancedda, R.; Cancedda, F.D. Cell Proliferation, Extracellular Matrix Mineralization, and Ovotransferrin Transient Expression during in Vitro Differentiation of Chick Hypertrophic Chondrocytes into Osteoblast-like Cells. J. Cell Biol. 1993, 122, 703–712.
- Roach, H.I.; Erenpreisa, J.; Aigner, T. Osteogenic Differentiation of Hypertrophic Chondrocytes Involves Asymmetric Cell Divisions and Apoptosis. J. Cell Biol. 1995, 131, 483–494.
- Rosenthal, A.K.; Gohr, C.M.; Ninomiya, J.; Wakim, B.T. Proteomic Analysis of Articular Cartilage Vesicles from Normal and Osteoarthritic Cartilage. Arthritis Rheum. 2011, 63, 401–411.
- Ak, R. Articular Cartilage Vesicles and Calcium Crystal Deposition Diseases. Curr. Opin. Rheumatol. 2016, 28, 127.
- Dean, D.D.; Schwartz, Z.; Bonewald, L.; Muniz, O.E.; Morales, S.; Gomez, R.; Brooks, B.P.; Qiao, M.; Howell, D.S.; Boyan, B.D. Matrix Vesicles Produced by Osteoblast-like Cells in Culture Become Significantly Enriched in Proteoglycan-Degrading Metalloproteinases after Addition of Beta-Glycerophosphate and Ascorbic Acid. Calcif. Tissue Int. 1994, 54, 399–408.
- Thouverey, C.; Strzelecka-Kiliszek, A.; Balcerzak, M.; Buchet, R.; Pikula, S. Matrix Vesicles Originate from Apical Membrane Microvilli of Mineralizing Osteoblast-like Saos-2 Cells. J. Cell Biochem. 2009, 106, 127–138.
- Buchet, R.; Pikula, S.; Magne, D.; Mebarek, S. Isolation and Characteristics of Matrix Vesicles. Methods Mol. Biol. 2013, 1053, 115–124.
- Boyan, B.D.; Asmussen, N.C.; Lin, Z.; Schwartz, Z. The Role of Matrix-Bound Extracellular Vesicles in the Regulation of Endochondral Bone Formation. Cells 2022, 11, 1619.
- Schwartz, Z.; Knight, G.; Swain, L.D.; Boyan, B.D. Localization of Vitamin D3-Responsive Alkaline Phosphatase in Cultured Chondrocytes. J. Biol. Chem. 1988, 263, 6023–6026.
- Bessueille, L.; Briolay, A.; Como, J.; Mebarek, S.; Mansouri, C.; Gleizes, M.; El Jamal, A.; Buchet, R.; Dumontet, C.; Matera, E.L.; et al. Tissue-Nonspecific Alkaline Phosphatase Is an Anti-Inflammatory Nucleotidase. Bone 2020, 133, 115262.
- Balcerzak, M.; Radisson, J.; Azzar, G.; Farlay, D.; Boivin, G.; Pikula, S.; Buchet, R. A Comparative Analysis of Strategies for Isolation of Matrix Vesicles. Anal. Biochem. 2007, 361, 176–182.
- Walker, S.A.; Davidovich, I.; Yang, Y.; Lai, A.; Goncalves, J.P.; Deliwala, V.; Busatto, S.; Shapiro, S.; Koifman, N.; Salomon, C.; et al. Sucrose-Based Cryoprotective Storage of Extracellular Vesicles. Extracell. Vesicle 2022, 1, 100016.
- Boyan, B.D.; Schwartz, Z.; Swain, L.D.; Carnes, D.L.; Zislis, T. Differential Expression of Phenotype by Resting Zone and Growth Region Costochondral Chondrocytes In Vitro. Bone 1988, 9, 185–194.
- Kirsch, T.; Ishikawa, Y.; Mwale, F.; Wuthier, R.E. Roles of the Nucleational Core Complex and Collagens (Types II and X) in Calcification of Growth Plate Cartilage Matrix Vesicles. J. Biol. Chem. 1994, 269, 20103–20109.
- Xiao, Z.; Camalier, C.E.; Nagashima, K.; Chan, K.C.; Lucas, D.A.; de la Cruz, M.J.; Gignac, M.; Lockett, S.; Issaq, H.J.; Veenstra, T.D.; et al. Analysis of the Extracellular Matrix Vesicle Proteome in Mineralizing Osteoblasts. J. Cell Physiol. 2007, 210, 325–335.
- Skelton, A.M.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Osteoblast-Derived Matrix Vesicles Exhibit Exosomal Traits and a Unique Subset of microRNA: Their Caveolae-Dependent Endocytosis Results in Reduced Osteogenic Differentiation. Int. J. Mol. Sci. 2023, 24, 12770.
- Balcerzak, M.; Malinowska, A.; Thouverey, C.; Sekrecka, A.; Dadlez, M.; Buchet, R.; Pikula, S. Proteome Analysis of Matrix Vesicles Isolated from Femurs of Chicken Embryo. Proteomics 2008, 8, 192–205.
- Golub, E.E. Role of Matrix Vesicles in Biomineralization. Biochim. Biophys. Acta 2009, 1790, 1592–1598.
- Anderson, H.C. Molecular Biology of Matrix Vesicles. Clin. Orthop. Relat. Res. 1995, 314, 266–280.
- Kirsch, T.; Pfäffle, M. Selective Binding of Anchorin CII (Annexin V) to Type II and X Collagen and to Chondrocalcin (C-Propeptide of Type II Collagen). Implications for Anchoring Function between Matrix Vesicles and Matrix Proteins. FEBS Lett. 1992, 310, 143–147.
- Kirsch, T.; Harrison, G.; Golub, E.E.; Nah, H.D. The Roles of Annexins and Types II and X Collagen in Matrix Vesicle-Mediated Mineralization of Growth Plate Cartilage. J. Biol. Chem. 2000, 275, 35577–35583.
- von der Mark, K.; Mollenhauer, J. Annexin V Interactions with Collagen. Cell Mol. Life Sci. 1997, 53, 539–545.
- Wu, L.N.; Genge, B.R.; Lloyd, G.C.; Wuthier, R.E. Collagen-Binding Proteins in Collagenase-Released Matrix Vesicles from Cartilage. Interaction between Matrix Vesicle Proteins and Different Types of Collagen. J. Biol. Chem. 1991, 266, 1195–1203.
- D’Angelo, M.; Billings, P.C.; Pacifici, M.; Leboy, P.S.; Kirsch, T. Authentic Matrix Vesicles Contain Active Metalloproteases (MMP). a Role for Matrix Vesicle-Associated MMP-13 in Activation of Transforming Growth Factor-Beta. J. Biol. Chem. 2001, 276, 11347–11353.
- Katsura, N.; Yamada, K. Isolation and Characterization of a Metalloprotease Associated with Chicken Epiphyseal Cartilage Matrix Vesicles. Bone 1986, 7, 137–143.
- Anderson, H.C. Matrix Vesicles and Calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226.
- Chen, N.X.; Moe, S.M. Pathophysiology of Vascular Calcification. Curr. Osteoporos. Rep. 2015, 13, 372–380.
- Tanimura, A.; McGregor, D.H.; Anderson, H.C. Matrix Vesicles in Atherosclerotic Calcification. Proc. Soc. Exp. Biol. Med. 1983, 172, 173–177.
- Schurgers, L.J.; Akbulut, A.C.; Kaczor, D.M.; Halder, M.; Koenen, R.R.; Kramann, R. Initiation and Propagation of Vascular Calcification Is Regulated by a Concert of Platelet- and Smooth Muscle Cell-Derived Extracellular Vesicles. Front. Cardiovasc. Med. 2018, 5, 36.
- Bakhshian Nik, A.; Hutcheson, J.D.; Aikawa, E. Extracellular Vesicles As Mediators of Cardiovascular Calcification. Front. Cardiovasc. Med. 2017, 4, 78.
- Kapustin, A.N.; Davies, J.D.; Reynolds, J.L.; McNair, R.; Jones, G.T.; Sidibe, A.; Schurgers, L.J.; Skepper, J.N.; Proudfoot, D.; Mayr, M.; et al. Calcium Regulates Key Components of Vascular Smooth Muscle Cell-Derived Matrix Vesicles to Enhance Mineralization. Circ. Res. 2011, 109, e1–e12.
- Kapustin, A.N.; Shanahan, C.M. Emerging Roles for Vascular Smooth Muscle Cell Exosomes in Calcification and Coagulation. J. Physiol. 2016, 594, 2905–2914.
- Zazzeroni, L.; Faggioli, G.; Pasquinelli, G. Mechanisms of Arterial Calcification: The Role of Matrix Vesicles. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 425–432.
- Aikawa, E.; Blaser, M.C. 2020 Jeffrey M. Hoeg Award Lecture: Calcifying Extracellular Vesicles as Building Blocks of Microcalcifications in Cardiovascular Disorders. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 117–127.
- Li, T.; Yu, H.; Zhang, D.; Feng, T.; Miao, M.; Li, J.; Liu, X. Matrix Vesicles as a Therapeutic Target for Vascular Calcification. Front. Cell Dev. Biol. 2022, 10, 825622.
- Krohn, J.B.; Hutcheson, J.D.; Martínez-Martínez, E.; Aikawa, E. Extracellular Vesicles in Cardiovascular Calcification: Expanding Current Paradigms. J. Physiol. 2016, 594, 2895–2903.
- Hutcheson, J.D.; Goettsch, C.; Bertazzo, S.; Maldonado, N.; Ruiz, J.L.; Goh, W.; Yabusaki, K.; Faits, T.; Bouten, C.; Franck, G.; et al. Genesis and Growth of Extracellular-Vesicle-Derived Microcalcification in Atherosclerotic Plaques. Nat. Mater. 2016, 15, 335–343.
- Yang, S.; Zeng, Z.; Yuan, Q.; Chen, Q.; Wang, Z.; Xie, H.; Liu, J. Vascular Calcification: From the Perspective of Crosstalk. Mol. Biomed. 2023, 4, 35.
- Chen, N.X.; O’Neill, K.D.; Moe, S.M. Matrix Vesicles Induce Calcification of Recipient Vascular Smooth Muscle Cells through Multiple Signaling Pathways. Kidney Int. 2018, 93, 343–354.
- Chaudhary, S.C.; Kuzynski, M.; Bottini, M.; Beniash, E.; Dokland, T.; Mobley, C.G.; Yadav, M.C.; Poliard, A.; Kellermann, O.; Millán, J.L.; et al. Phosphate Induces Formation of Matrix Vesicles during Odontoblast-Initiated Mineralization in Vitro. Matrix Biol. 2016, 52–54, 284–300.
- Chen, N.X.; Chen, X.; O’Neill, K.D.; Atkinson, S.J.; Moe, S.M. RhoA/Rho Kinase (ROCK) Alters Fetuin-A Uptake and Regulates Calcification in Bovine Vascular Smooth Muscle Cells (BVSMC). Am. J. Physiol. Renal Physiol. 2010, 299, F674–F680.
- Chaturvedi, P.; Chen, N.X.; O’Neill, K.; McClintick, J.N.; Moe, S.M.; Janga, S.C. Differential miRNA Expression in Cells and Matrix Vesicles in Vascular Smooth Muscle Cells from Rats with Kidney Disease. PLoS ONE 2015, 10, e0131589.
- Chen, N.X.; O’Neill, K.D.; Dominguez, J.M.; Moe, S.M. Regulation of Reactive Oxygen Species in the Pathogenesis of Matrix Vesicles Induced Calcification of Recipient Vascular Smooth Muscle Cells. Vasc. Med. 2021, 26, 585–594.
- Reynolds, J.L.; Joannides, A.J.; Skepper, J.N.; McNair, R.; Schurgers, L.J.; Proudfoot, D.; Jahnen-Dechent, W.; Weissberg, P.L.; Shanahan, C.M. Human Vascular Smooth Muscle Cells Undergo Vesicle-Mediated Calcification in Response to Changes in Extracellular Calcium and Phosphate Concentrations: A Potential Mechanism for Accelerated Vascular Calcification in ESRD. J. Am. Soc. Nephrol. 2004, 15, 2857–2867.
- Shroff, R.C.; McNair, R.; Skepper, J.N.; Figg, N.; Schurgers, L.J.; Deanfield, J.; Rees, L.; Shanahan, C.M. Chronic Mineral Dysregulation Promotes Vascular Smooth Muscle Cell Adaptation and Extracellular Matrix Calcification. J. Am. Soc. Nephrol. 2010, 21, 103–112.
- Kapustin, A.N.; Chatrou, M.L.L.; Drozdov, I.; Zheng, Y.; Davidson, S.M.; Soong, D.; Furmanik, M.; Sanchis, P.; De Rosales, R.T.M.; Alvarez-Hernandez, D.; et al. Vascular Smooth Muscle Cell Calcification Is Mediated by Regulated Exosome Secretion. Circ. Res. 2015, 116, 1312–1323.
- Kapustin, A.N.; Schoppet, M.; Schurgers, L.J.; Reynolds, J.L.; McNair, R.; Heiss, A.; Jahnen-Dechent, W.; Hackeng, T.M.; Schlieper, G.; Harrison, P.; et al. Prothrombin Loading of Vascular Smooth Muscle Cell-Derived Exosomes Regulates Coagulation and Calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e22–e32.
- New, S.E.P.; Goettsch, C.; Aikawa, M.; Marchini, J.F.; Shibasaki, M.; Yabusaki, K.; Libby, P.; Shanahan, C.M.; Croce, K.; Aikawa, E. Macrophage-Derived Matrix Vesicles: An Alternative Novel Mechanism for Microcalcification in Atherosclerotic Plaques. Circ. Res. 2013, 113, 72–77.
- Cui, L.; Rashdan, N.A.; Zhu, D.; Milne, E.M.; Ajuh, P.; Milne, G.; Helfrich, M.H.; Lim, K.; Prasad, S.; Lerman, D.A.; et al. End Stage Renal Disease-Induced Hypercalcemia May Promote Aortic Valve Calcification via Annexin VI Enrichment of Valve Interstitial Cell Derived-Matrix Vesicles. J. Cell Physiol. 2017, 232, 2985–2995.
- Chaudhary, S.C.; Khalid, S.; Smethurst, V.; Monier, D.; Mobley, J.; Huet, A.; Conway, J.F.; Napierala, D. Proteomic Profiling of Extracellular Vesicles Released from Vascular Smooth Muscle Cells during Initiation of Phosphate-Induced Mineralization. Connect. Tissue Res. 2018, 59, 55–61.
- Karpman, D.; Ståhl, A.-L.; Arvidsson, I. Extracellular Vesicles in Renal Disease. Nat. Rev. Nephrol. 2017, 13, 545–562.
- Roszkowska, M.; Strzelecka-Kiliszek, A.; Bessueille, L.; Buchet, R.; Magne, D.; Pikula, S. Collagen Promotes Matrix Vesicle-Mediated Mineralization by Vascular Smooth Muscle Cells. J. Inorg. Biochem. 2018, 186, 1–9.
- Wang, Z.-X.; Luo, Z.-W.; Li, F.-X.-Z.; Cao, J.; Rao, S.-S.; Liu, Y.-W.; Wang, Y.-Y.; Zhu, G.-Q.; Gong, J.-S.; Zou, J.-T.; et al. Aged Bone Matrix-Derived Extracellular Vesicles as a Messenger for Calcification Paradox. Nat Commun 2022, 13, 1453.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
551
Revisions:
2 times
(View History)
Update Date:
04 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




