Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrés París-Muñoz | -- | 3459 | 2024-01-03 12:40:27 | | | |
| 2 | Rita Xu | Meta information modification | 3459 | 2024-01-04 03:15:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
París-Muñoz, A.; León-Triana, O.; Pérez Martínez, A.; Barber, D.F. Helios in the Immune System. Encyclopedia. Available online: https://encyclopedia.pub/entry/53373 (accessed on 08 February 2026).
París-Muñoz A, León-Triana O, Pérez Martínez A, Barber DF. Helios in the Immune System. Encyclopedia. Available at: https://encyclopedia.pub/entry/53373. Accessed February 08, 2026.
París-Muñoz, Andrés, Odelaisy León-Triana, Antonio Pérez Martínez, Domingo F. Barber. "Helios in the Immune System" Encyclopedia, https://encyclopedia.pub/entry/53373 (accessed February 08, 2026).
París-Muñoz, A., León-Triana, O., Pérez Martínez, A., & Barber, D.F. (2024, January 03). Helios in the Immune System. In Encyclopedia. https://encyclopedia.pub/entry/53373
París-Muñoz, Andrés, et al. "Helios in the Immune System." Encyclopedia. Web. 03 January, 2024.
Copy Citation
The Helios protein (encoded by the IKZF2 gene) is a member of the Ikaros transcription family and it has recently been proposed as a promising biomarker for systemic lupus erythematosus (SLE) disease progression in both mouse models and patients. Helios is beginning to be studied extensively for its influence on the T regulatory (Treg) compartment, both CD4+ Tregs and KIR+/Ly49+ CD8+ Tregs, with alterations to the number and function of these cells correlated to the autoimmune phenomenon.
Helios
biomarkers
systemic lupus erythematosus
autoimmunity
1. General Considerations on the Immune System
The immune system of complex organisms like mammals is a coordinated network of innate and adaptive cells, molecules, tissues, and organs that has evolved to fight against the foreign pathogens causing infectious disease. In parallel, the immune system also has the means to protect host self-antigens, part of a dynamic homeostatic equilibrium aimed at preventing any potential side effects due to an overzealous immunogenic response.
Unlike innate cells, one of the essential features of adaptive cells is their ability to recognize a wide range of different antigens (endogenous and exogenous). Adaptive immunity is driven by cellular (helper or CD4+ T cells, and cytotoxic or CD8+ T cells) and humoral immunity (via B cells). From a functional and perhaps simplified perspective, each immune response (innate and adaptive) can be classified as immunogenic (e.g., the immune response directed against pathogenic microorganisms) or tolerogenic (for example, the immunosuppression of autoreactive processes). Overall, the immune system aims to establish a dynamic and continuously regulated balance between these two opposing forces: reactivity to foreign molecules and self-antigen tolerance [1][2]. Interestingly, the same cell type may be involved in both activities, depending on factors like the molecular environment, their activation, and the patterns of gene expression [3]. Most of the known innate and adaptive immune cell populations are presented in Figure 1, showing their immunogenic and tolerogenic counterparts. Finding elements common to both sides of the balance, and that influence the general status of the immune system, will be interesting to better understand pathologies with a predominantly immune component, such as cancer and autoimmunity.
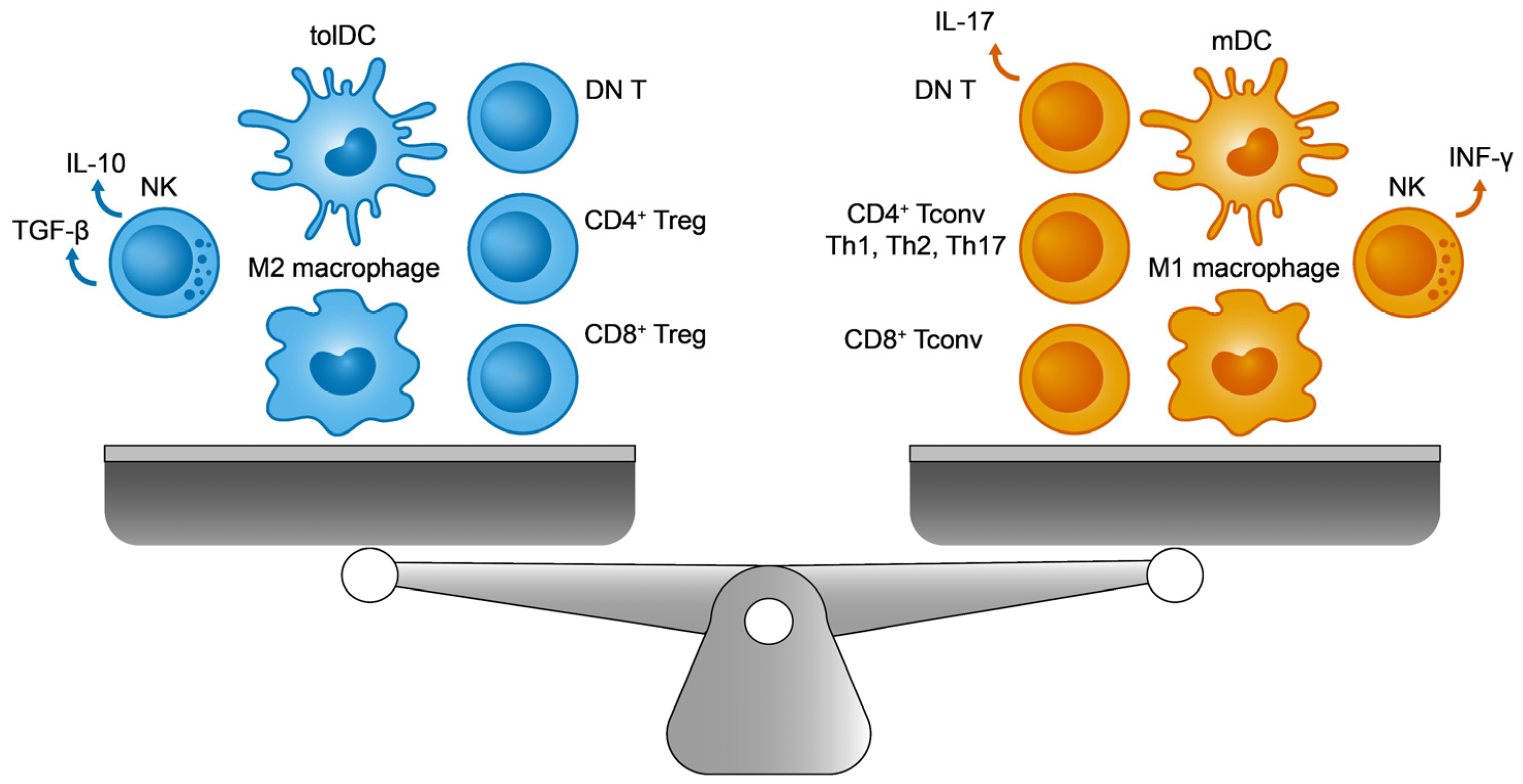
Figure 1. The immune balance. On the (left), the immunosuppressive cells include M2 macrophages, regulatory T cells (Tregs), tolerogenic dendritic cells (tolDCs), double negative T cells (DN T), and TGF-β producing natural killer (NK) cells. On the (right), their immunogenic counterparts are M1 macrophages, conventional T lymphocytes (Tconv), immunogenic mature DCs (mDCs), IL-17 producing DN T cells, and INF-γ producing NK cells.
1.1. The Adaptive Immune Equilibrium
In addition to T cells, dendritic cells (DCs) play a crucial role in lymphoid organs (thymus, lymph nodes, and spleen), orchestrating the adaptive immune system.
1.1.1. Conventional CD4+ and CD8+ T Cells
After their generation in bone marrow from hematopoietic progenitors, in both humans and mice, immature T cells move to the thymus to complete their development. Once there, and during their maturation, thymocytes reorganize their TCR by genetic recombination to generate a specific repertoire of immune cells with distinct affinities for the MHC molecules associated to their peptides. Subsequently, after positive and negative selection, the remaining fraction of TCRαβ+CD4+CD8+ cells silence one of their co-receptors, CD8 or CD4 depending on the interaction with antigen presenting cells (APCs), through their MHCI or MHCII molecules, respectively [4][5]. Thus, naive TCRαβ+CD4+CD8− (restricted MHCII cells) and TCRαβ+CD4−CD8+ (restricted MHCI cells) T lymphocytes arise in the thymus, exhibiting tolerance for self-antigens towards peripheral lymphoid tissues to be activated by APCs (mainly, DCs), and they then trigger the cellular adaptive response.
Globally, these mature T lymphocytes are defined as conventional T cells (Tconv), and they are responsible for the positive and immunogenic responses to foreign antigens. Yet what are the homeostatic mechanisms that avoid the potentially autoreactive reactions of Tconv cells that escape the process of negative selection in the thymus? Other subpopulations of immune cells play a pivotal role in readdressing the immune equilibrium through immunosuppression, cells known as T regulatory cells (Tregs). Among these cells, most research has focused on CD4+ Tregs, and in particular, on a CD4+ Treg population derived from the thymus (CD4+ tTreg). These cells survive the process of negative selection and they exhibit high affinity for MHCII-self-peptide complexes, expressing higher levels of CD25 and FoxP3 [6]. Nevertheless, incipient CD8+ Tregs that express the receptors of natural killer (NK) cells are currently generating much interest [7].
1.1.2. Regulatory CD4+ T Cells
The discovery of the FoxP3 transcription factor as a marker and an essential regulator that maintains CD4+ tTreg’s immunosuppressive activity implied a paradigm shift in the field of immunology [8][9]. After years of intense research in this topic, a CD4+ tTreg population (CD25+ FoxP3+) has been clearly shown to maintain the peripheral immune equilibrium by controlling aberrant and exacerbated autoreactive responses against self-antigens, microorganisms, and environmental antigens [10][11]. In this regard, several allergies and autoimmune pathologies are associated with certain alterations to CD4+ tTregs, revealing the true importance of their correct function [12]. In addition, some roles of CD4+ tTregs in other body systems beyond the immune system have also been reported. For example, the close links between these lymphocytes and tissue regeneration [13], or other metabolic diseases like obesity [14], have been studied.
Although CD4+ tTreg lymphocytes represent the most abundant fraction of Tregs in the periphery, there are also other regulatory CD4+ cell types of different origins that reliably express FoxP3 and that participate in establishing tolerance. For example, peripheral CD4+ Tregs (CD4+ pTregs) are generated from CD4+ Tconvs outside the thymus in tolerogenic contexts, such as in the intestinal mucosa. Moreover, CD4+ iTregs can be induced in vitro from CD4+ Tconvs under specific culture conditions and these cells are currently being tested in clinical trials as potential autologous cell therapy for patients affected by several autoimmune diseases [15]. Despite their differences, these CD4+ tTregs, pTregs, and iTregs employ some common immunosuppressive mechanisms.
Given the intrinsic molecular and cellular complexity of the immune system, CD4+ Treg lymphocytes need to act on several elements and processes, of both the innate and adaptive responses, in order to ensure effective peripheral tolerance in a coordinated manner [16][17][18] (see Figure 2 for a summary of the main immunosuppressive strategies identified in CD4+ Tregs). Among these events, the overexpression of CD25 (the α subunit of the interleukin 2 receptor or IL-2R) facilitates the capture and sequestering of the available IL-2. The reduced availability of IL-2 specifically dampens the activation and proliferation of CD8+ Tconv cells that depends on this cytokine [19]. From a metabolic perspective, the surface ectoenzymes CD39 and CD73 transform extracellular ATP into adenosine molecules, the latter potentially inhibiting effector T and myeloid cells (DCs and macrophages) [20][21]. Furthermore, these Tregs produce tolerogenic cytokines like IL-10 and TGF-β that exert multiple effects on immune cells. By contrast, IL-10 and TGF-β inhibit T and B lymphocyte proliferation and stimulation [22]. Moreover, these cytokines would polarize DCs towards a more tolerogenic phenotype, which promotes the induction of pTregs [23]. Finally, Tregs and DCs interact directly through two different signaling systems that could induce a tolerogenic state in DCs: PD-1 (Treg)—PDL-1 (DCs) [24], and CTLA-4 (Treg)—CD80/86 (DCs) [25].
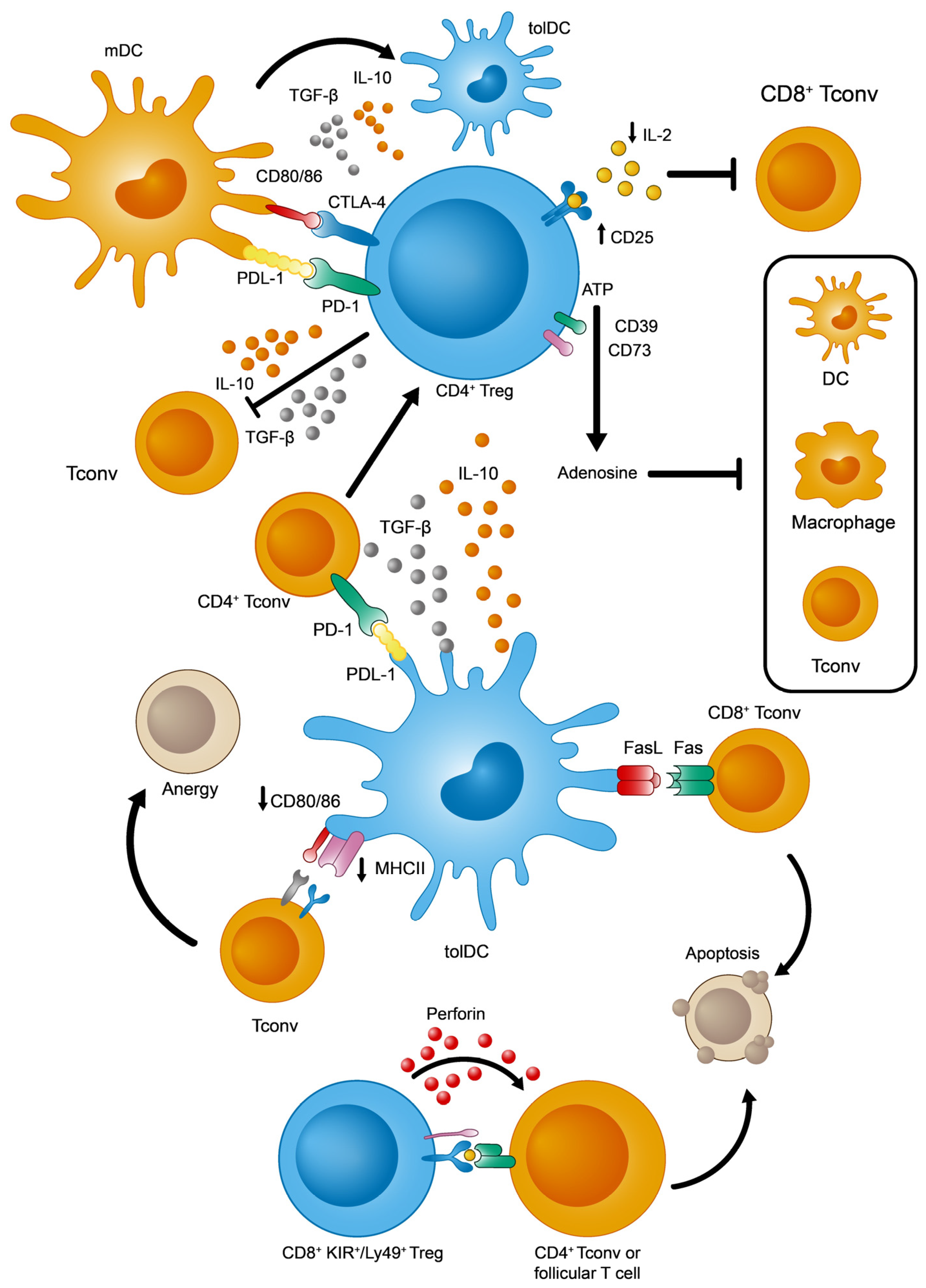
Figure 2. Main immunosuppressive mechanisms adopted by tolDCs, CD4+, and KIR+/Ly49+ CD8+ Tregs.
1.1.3. Regulatory CD8+ Tregs
Globally, although most biomedical research into Treg cells has focused on the CD4+ Treg compartment, a recently described subset of CD8+ Treg lymphocytes that expresses inhibitory markers from NK cells is being investigated intensively in mice and humans. Previously, a wide range of different CD8+ T cells exhibiting immunomodulatory properties were classified as CD8+ Tregs, yet with heterogeneous phenotypes: CD8+ FoxP3+ [26], CD8+ CD28− [27], CD8+ CD103+ [28], and CD8+ CD122+ CD49dlow [29]. However, unlike CD4+ tTreg cells, these populations were found peripherally in very small proportions or in experimental contexts of antigenic exposure in mice. In other words, these subsets were never found naturally in significant numbers in healthy and young mice without prior manipulation [30][31].
This situation changed with the discovery of a new regulatory subpopulation of CD8+ T cells in naïve mice without any immune disruption, reliably defined by the expression of three markers: CD44+, CD122+, and Ly49+ (Ly49+ CD8+ Tregs from here on). This population has the ability to modulate the adaptive immune response by eliminating autoreactive CD4+ Tconv and follicular T lymphocytes [32][33][34]. Since then, other properties of this population have been clarified, such as (1) the absence of intranuclear FoxP3 expression; (2) high levels of surface CD127 (in contrast to CD4+ Treg cells) [34]; (3) homeostatic regulation, mainly through IL-15 and TGF-β [35][36][37]; (4) phenotypic acquisition induced peripherally after thymic maturation [38]; and (5) finally, the fact that these cells have a non-redundant role in controlling autoreactive antibody titers by acting in germinal centers from lymphoid organs [33][37], thereby regulating autoimmune phenomena [36][37][39]. In terms of their functionality, the best-defined effector mechanism used by CD8+ Ly49+ Tregs is the cytotoxic killing of autoreactive CD4+ T cells, regardless of IL-10 production [32] (Figure 2).
1.1.4. Tolerogenic Dendritic Cells (tolDCs)
As previously stated, DCs are the primary APCs that initiate the adaptive immune responses of T lymphocytes in response to foreign antigens in the periphery. However, in addition to this crucial role, they also contribute to immune tolerance in the organism in two other ways. Firstly, DCs mediate the negative selection and differentiation of tTregs in the thymus (central tolerance), while they also maintain peripheral tolerance by elimination, anergic induction, or conversion of autoreactive T cells to pTregs [40]. While immature DCs (imDCs) were simply believed to perform a tolerogenic function and mature DCs (mDCs) an immunogenic function, increasing experimental evidence suggests that this functional divide extends beyond the maturation state of DCs. In fact, maturation is necessary for their optimal tolerogenic function, even under basal conditions with no exogenous stimulation. Thus, researchers will refer to them functionally as imDCs, mDCs, and tolerogenic mature DCs (tolDCs) [41][42].
Apart from these states of maturation, distinct DC subpopulations fulfill specific roles in the immune response. These subpopulations are well-defined in both mouse models and humans, and include conventional (cDCs) and plasmacytoid dendritic cells (pDCs) [43]. Type 1 (cDC1s) and type 2 (cDC2s) DCs are defined by their developmental transcriptional program, presenting MHCI antigens for CD8+ T cells or MHCII for CD4+ T cells, respectively. Furthermore, pDCs specialize in the production of type I interferons upon exposure to viral antigens [44], as well as helping to establish immune tolerance [45]. In line with this, it seems that cDC1 cells, with their inherent capacity to present antigens via MHCI molecules, also play a crucial role in ensuring peripheral tolerance to self-antigens [40][42][46][47][48][49].
Focusing on their tolerogenic functions, tolDCs capture and process self-antigens, whether soluble or derived from apoptotic bodies, for their presentation to peripheral T cells [50]. Among the numerous mechanisms described, some are worthy of particular attention (Figure 2): (1) tolDCs promote the generation of CD4+ pTregs from CD4+ Tconvs in two ways, by secreting immunosuppressive cytokines (IL-10 and TGF-β) or by signaling through PDL-1 molecules [51][52]; (2) tolDCs eliminate autoreactive CD8+ T cells via the Fas/FasL apoptotic system [53]; and (3) tolDCs favor an anergic state in T cells due to the weak expression of MHCII and CD80/CD86 [54].
Also, as for iTreg lymphocytes, it is also possible to induce an immunosuppressive state in mouse and human DCs in vitro for their use in cell-based immunotherapies. This polarization towards tolerance is achieved using different immunomodulators, including glucocorticoids (e.g., dexamethasone), cytokines (e.g., IL-10 and TGF-β), rapamycin, or vitamin D3 [50].
1.2. Autoimmune Alterations in the Adaptive Immune Balance
In the case of cancer, the immune balance is inclined in favor of immunosuppression as a strategy to help the tumor evade active immune responses. By contrast, in the case of autoimmunity, the immune equilibrium is oriented towards immunogenicity against self-antigens. In this respect, there is considerable evidence of defects and alterations in number, frequency, and immunosuppressive function of CD4+ Tregs from patients with allergies, and other inflammatory or autoimmune diseases [11][12][55]. Although some inconsistencies have been noted depending on the patient cohort, the pathological state, and the different flow cytometry panels employed to define and phenotype Treg subsets, some common defects have been reported globally in CD4+ T lymphocytes in autoimmune contexts: (1) the conversion of CD4+ Tregs into effector CD4+ Tconvs; (2) the reduced ability of CD4+ Tregs to promote tolerogenic effects; and (3) the resistance to immunosuppression observed in some activated CD4+ Tconvs. By contrast, in type I diabetes, autoreactive CD8+ Tconv cells have been extensively described as an important inducer of cytotoxicity of pancreatic β cell death [56], and implicated in other autoimmune pathologies [57]. There is an increase in the number of CD8+ KIR+ Tregs in both blood and at inflammatory sites in autoimmune patients, and it was proposed that these lymphocytes eliminate pathogenic CD4+ T cells to avoid their exacerbated response [7].
Finally, the role of DCs in autoimmune progression has also been analyzed in depth [58][59], establishing that they promote an immunogenic environment due to inflammatory cytokine production. For example, IL-6, IL-12, and IL-23 secretion by immunogenic mDCs facilitates the polarization of self-reactive T cells. In the case of SLE, type-I interferons (IFN-I) mainly produced by pDCs deserve a special mention because they represent one of the most important molecular markers for this disease.
2. Helios in the Immune System
One element in this delicate immune balance is the transcription factor Helios (Ikzf2 in mice and IKZF2 in humans), a member of the Ikaros family of transcription factors that appears to fulfill a critical role in maintaining a regulatory profile in the T-cell lineage. Helios deficiency, either in mice lacking Helios in all cells [34] or specifically in FoxP3+ cells [60] (mostly CD4+ Tregs), leads to an autoimmune phenotype at 5–6 months of age that is associated with distinct symptoms, reproducing some canonical immunological manifestations of lupus: hypergammaglobulinemia against nuclear antigens, splenomegaly, glomerulonephritis, an enhanced presence of activated Tconvs, and an expansion of follicular T lymphocytes (see below). Indeed, a germline mutation in IKZF2 was recently described in an SLE patient that was responsible for severe immune dysregulation [61]. This raises the question as to what the role of Helios is in each immune cell population.
2.1. Helios in CD4+ T Cells
Initially, Helios was identified in mouse models as a specific marker of CD4+ tTregs through a string of discoveries. (1) Until the first week of life, nearly all Tregs (CD4+ FoxP3+ T lymphocytes) that migrate from the thymus express Helios. (2) FoxP3+ Helios− CD4+ T cells only appear from the second week after weaning. (3) In adult animals, around 70% of the CD4+ Treg compartment expresses Helios. (4) Helios is not expressed in either iTregs or pTreg lymphocytes in an experimental tolerance model [62][63]. However, the validity of this latter assumption has become increasingly controversial due to the more recent detection of Helios in CD4+ pTregs [64], in activated and exhausted CD4+ Tconvs [65][66][67], and in iTregs [68]. Nonetheless, few studies have assessed the relative expression of Helios in specific subpopulations of immune cells beyond simply distinguishing between Helios+ and Helios− cells. In this sense, the merits of distinguishing up to three biologically relevant levels of Helios expression is currently being explored (Helioshigh, Heliosmid, and Helioslow) [69][70].
Beyond its proposed function as a unique molecular marker for tTregs, it is apparent that Helios+ and Helios− CD4+ Treg lymphocytes have notable phenotypic, genetic, and functional differences [71][72]. CD4+ Tregs expressing Helios exhibit a more activated phenotype, with a higher percentage of effector (CD44+ CD62L−) cells. Moreover, in vitro studies indicate that these Tregs have a stronger immunosuppressive capacity relative to CD4+ Tregs lacking Helios [73]. Furthermore, experiments on lymphopenic animals have shown that CD4+ Tregs expressing Helios in vivo express FoxP3 more stably. Indeed, the combined use of FoxP3 and Helios has been proposed to identify bona fide CD4+ Tregs in humans [74].
Interestingly, the deletion of Helios in mice, both in all cells [34] and specifically in FoxP3+ cells [60], provokes an autoimmune phenotype at 6 months of age, demonstrating the role of this transcription factor in stabilizing regulatory activity. However, the absence of Helios in the entire CD4+ T cell repertoire, including both Tconvs and Tregs, does not apparently evoke autoreactivity [34][62]. This curiosity may suggest that Helios is involved in both sides of the immune balance and it leads researchers to draw two hypotheses. (1) Helios expression in CD4+ Tconvs plays a significant role in the autoimmune reaction; and (2) other cell types with Helios-dependent regulatory activity impede autoreactive immune responses.
2.2. Helios in KIR+/Ly49+ CD8+
If there is one thing that CD4+ Tregs and CD8+ Ly49+ Tregs have in common, it is the expression of Helios as a maker of their regulatory identity. Nevertheless, research on CD8+ Ly49+ Tregs is still in its infancy compared to that on CD4+ Tregs. Evidence for the physiological relevance of Helios in CD8+ Ly49+ Tregs and their immunosuppressive function comes from the inability of CD8+ Ly49+ Helios− T lymphocytes to inhibit the follicular T cell response relative to CD8+ Ly49+ Helios+ cells. Moreover, unlike their Helios+ counterparts, CD8+ Ly49+ Helios− Treg lymphocytes exert a stronger effector phenotype (CD127low) and worse survival under inflammatory conditions [34]. Independently, weaker Helios expression has also been found in the CD8+ Ly49+ Tregs, correlating with autoimmune progression in a murine model that replicates the typical SLE symptomatology from the age of 5 months (including splenomegaly and germinal center reactivity). From this model, with deficient TGF-β signaling, expression of this cytokine may influence the Helios expressed by CD8+ T cells [37]. In addition, researchers found that both the reduction in the proportion of Ly49+ CD8+ T cells and the weaker Helios expression by these cells was correlated to disease progression in two mouse models of lupus (MRL/MPJ and MRL/LPR) [70].
A recent article in Science characterized the function and phenotype of the human equivalent of Ly49+ CD8+ Tregs in infectious and autoimmune contexts [7]. Although the murine family of Ly49 receptors lacks a genetic human homologue in terms of sequence, the members of the human Killer cell Immunoglobulin-like Receptor (KIR) family are functionally equivalent to these. Both populations, KIR+ CD8+ and their murine Ly49+ CD8+ T cell counterparts, can eliminate autoreactive CD4+ T cells in autoimmune and viral contexts, and they are characterized by their Helios+ phenotype.
As demonstrated, altered Helios expression in the T lymphocyte pool is linked to an autoimmune phenotype that replicates the typical symptoms observed in SLE, an archetypal condition of systemic autoimmunity. Thus, while the “mystery” surrounding CD8+ regulatory T cells is slowly lifting [30], many questions remain, such as what the origin of these KIR+/Ly49+ CD8+ T lymphocytes is, and what is their relationship to other cells in the immune system, like DCs.
2.3. Helios in Other Immune Cells: Double Negative (DN) T and NK Cells
2.3.1. Helios in DN T Cells
In addition to CD4+ and CD8+ T cells, there is a specific subpopulation of T lymphocytes with a TCRαβ+ profile that expresses neither CD4 nor the CD8 co-receptor. In terms of their possible origin, the model that currently seems to attract the most interest, according to recent studies, suggests that double negative (DN) T cells are derived from autoreactive CD8+ T cells that have lost their CD8 co-receptor [75][76]. In this context, DN T lymphocytes would be characterized by the expression of PD-1 at their surface and by the transient presence of Helios in their nucleus [75]. The pathological role of these PD-1+ DN T cells is witnessed by their proinflammatory effector phenotype (CD127low) and by the production of the IL-17 cytokine, which is tightly correlated with the autoimmune phenomenon [77][78]. However, the regulatory properties of the DN T repertoire cannot be ignored, and they have also been reported on the other side of the immunological equilibrium, in non-autoimmune contexts and in organ transplantation [79][80][81]. Again, this dual behavior of DN T cells would reflect the complexity of the immune system, the existence of specific subpopulations characterized by the expression of markers yet to be defined, and the influence of the molecular context (autoimmune or non-autoimmune).
The transcriptional profile of DN T cells from mice was recently analyzed [82], revealing different DN T subpopulations to be distinguished by the Helios gene (Ikzf2). Indeed, activated and non-activated DN T lymphocytes could be identified through this biomarker. Helios expression is enhanced in naïve DN T cells, whereas activated DN T cells express this transcription factor weakly.
2.3.2. Helios in NK Cells
In humans, after CD4+ Tregs and mucosal-associated invariant T (MAIT) lymphocytes, NK cells are the immune cell population with the strongest Helios expression (supplemental Figure S3 from [61]). Nevertheless, there are few data regarding Helios expression in the NK compartment. Helios has been proposed to regulate the activity of hyperreactive NK cells in mice resistant to viral infection [83]. Moreover, Helios downregulation was observed in some subsets of “memory-like” NK cells from cytomegalovirus-infected individuals [84], and finally, the proportion of CD16+ CD56dim NK cells was reduced in a lupus patient due to germline mutation of the IKZF2 gene [61]. Together, Helios appears to be an important transcription factor that controls the activity and homeostasis of NK cells, which are involved in autoimmunity [85] and lupus [86]. Further studies will be needed to examine the expression of Helios in autoimmune contexts and in different immune cells.
References
- Eberl, G. Immunity by equilibrium. Nat. Rev. Immunol. 2016, 16, 524–532.
- Eberl, G.; Pradeu, T. Towards a General Theory of Immunity? Trends Immunol. 2018, 39, 261–263.
- Horwitz, D.A.; Fahmy, T.M.; Piccirillo, C.A.; La Cava, A. Rebalancing Immune Homeostasis to Treat Autoimmune Diseases. Trends Immunol. 2019, 40, 888–908.
- Germain, R.N. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2002, 2, 309–322.
- Taniuchi, I. CD4 Helper and CD8 Cytotoxic T Cell Differentiation. Annu. Rev. Immunol. 2018, 36, 579–601.
- Savage, P.A.; Klawon, D.E.J.; Miller, C.H. Regulatory T Cell Development. Annu. Rev. Immunol. 2020, 38, 421–453.
- Li, J.; Zaslavsky, M.; Su, Y.; Guo, J.; Sikora, M.J.; van Unen, V.; Christophersen, A.; Chiou, S.H.; Chen, L.; Li, J.; et al. KIR(+)CD8(+) T cells suppress pathogenic T cells and are active in autoimmune diseases and COVID-19. Science 2022, 376, eabi9591.
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061.
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336.
- Josefowicz, S.Z.; Lu, L.F.; Rudensky, A.Y. Regulatory T cells: Mechanisms of differentiation and function. Annu. Rev. Immunol. 2012, 30, 531–564.
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566.
- Grant, C.R.; Liberal, R.; Mieli-Vergani, G.; Vergani, D.; Longhi, M.S. Regulatory T-cells in autoimmune diseases: Challenges, controversies and--yet--unanswered questions. Autoimmun. Rev. 2015, 14, 105–116.
- Burzyn, D.; Benoist, C.; Mathis, D. Regulatory T cells in nonlymphoid tissues. Nat. Immunol. 2013, 14, 1007–1013.
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S.; et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939.
- Shevach, E.M. Foxp3(+) T Regulatory Cells: Still Many Unanswered Questions-A Perspective After 20 Years of Study. Front. Immunol. 2018, 9, 1048.
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532.
- Plitas, G.; Rudensky, A.Y. Regulatory T Cells: Differentiation and Function. Cancer Immunol. Res. 2016, 4, 721–725.
- Shevyrev, D.; Tereshchenko, V. Treg Heterogeneity, Function, and Homeostasis. Front. Immunol. 2019, 10, 3100.
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 2016, 17, 1322–1333.
- Ernst, P.B.; Garrison, J.C.; Thompson, L.F. Much ado about adenosine: Adenosine synthesis and function in regulatory T cell biology. J. Immunol. 2010, 185, 1993–1998.
- Whiteside, T.L.; Jackson, E.K. Adenosine and prostaglandin e2 production by human inducible regulatory T cells in health and disease. Front. Immunol. 2013, 4, 212.
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular mechanisms of treg-mediated T cell suppression. Front. Immunol. 2012, 3, 51.
- Wallet, M.A.; Sen, P.; Tisch, R. Immunoregulation of dendritic cells. Clin. Med. Res. 2005, 3, 166–175.
- Gianchecchi, E.; Fierabracci, A. Inhibitory Receptors and Pathways of Lymphocytes: The Role of PD-1 in Treg Development and Their Involvement in Autoimmunity Onset and Cancer Progression. Front. Immunol. 2018, 9, 2374.
- Onishi, Y.; Fehervari, Z.; Yamaguchi, T.; Sakaguchi, S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA 2008, 105, 10113–10118.
- Mayer, C.T.; Floess, S.; Baru, A.M.; Lahl, K.; Huehn, J.; Sparwasser, T. CD8+ Foxp3+ T cells share developmental and phenotypic features with classical CD4+ Foxp3+ regulatory T cells but lack potent suppressive activity. Eur. J. Immunol. 2011, 41, 716–725.
- Vuddamalay, Y.; van Meerwijk, J.P. CD28(−) and CD28(low)CD8(+) Regulatory T Cells: Of Mice and Men. Front. Immunol. 2017, 8, 31.
- Liu, Y.; Deng, W.; Meng, Q.; Qiu, X.; Sun, D.; Dai, C. CD8+iTregs attenuate glomerular endothelial cell injury in lupus-prone mice through blocking the activation of p38 MAPK and NF-kappaB. Mol. Immunol. 2018, 103, 133–143.
- Akane, K.; Kojima, S.; Mak, T.W.; Shiku, H.; Suzuki, H. CD8+CD122+CD49dlow regulatory T cells maintain T-cell homeostasis by killing activated T cells via Fas/FasL-mediated cytotoxicity. Proc. Natl. Acad. Sci. USA 2016, 113, 2460–2465.
- Mishra, S.; Srinivasan, S.; Ma, C.; Zhang, N. CD8(+) Regulatory T Cell—A Mystery to Be Revealed. Front. Immunol. 2021, 12, 708874.
- Niederlova, V.; Tsyklauri, O.; Chadimova, T.; Stepanek, O. CD8(+) Tregs revisited: A heterogeneous population with different phenotypes and properties. Eur. J. Immunol. 2021, 51, 512–530.
- Kim, H.J.; Verbinnen, B.; Tang, X.; Lu, L.; Cantor, H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature 2010, 467, 328–332.
- Kim, H.J.; Wang, X.; Radfar, S.; Sproule, T.J.; Roopenian, D.C.; Cantor, H. CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc. Natl. Acad. Sci. USA 2011, 108, 2010–2015.
- Kim, H.J.; Barnitz, R.A.; Kreslavsky, T.; Brown, F.D.; Moffett, H.; Lemieux, M.E.; Kaygusuz, Y.; Meissner, T.; Holderried, T.A.; Chan, S.; et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 2015, 350, 334–339.
- Satooka, H.; Nagakubo, D.; Sato, T.; Hirata, T. The ERM Protein Moesin Regulates CD8(+) Regulatory T Cell Homeostasis and Self-Tolerance. J. Immunol. 2017, 199, 3418–3426.
- Stocks, B.T.; Wilson, C.S.; Marshall, A.F.; Hoopes, E.M.; Moore, D.J. Regulation of Diabetogenic Immunity by IL-15-Activated Regulatory CD8 T Cells in Type 1 Diabetes. J. Immunol. 2019, 203, 158–166.
- Mishra, S.; Liao, W.; Liu, Y.; Yang, M.; Ma, C.; Wu, H.; Zhao, M.; Zhang, X.; Qiu, Y.; Lu, Q.; et al. TGF-beta and Eomes control the homeostasis of CD8+ regulatory T cells. J. Exp. Med. 2021, 218, e20200030.
- Shytikov, D.; Rohila, D.; Li, D.; Wang, P.; Jiang, M.; Zhang, M.; Xu, Q.; Lu, L. Functional Characterization of Ly49(+)CD8 T-Cells in Both Normal Condition and During Anti-Viral Response. Front. Immunol. 2020, 11, 602783.
- Saligrama, N.; Zhao, F.; Sikora, M.J.; Serratelli, W.S.; Fernandes, R.A.; Louis, D.M.; Yao, W.; Ji, X.; Idoyaga, J.; Mahajan, V.B.; et al. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature 2019, 572, 481–487.
- Iberg, C.A.; Jones, A.; Hawiger, D. Dendritic Cells As Inducers of Peripheral Tolerance. Trends Immunol. 2017, 38, 793–804.
- Lutz, M.B.; Schuler, G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002, 23, 445–449.
- Iberg, C.A.; Hawiger, D. Natural and Induced Tolerogenic Dendritic Cells. J. Immunol. 2020, 204, 733–744.
- Verheye, E.; Bravo Melgar, J.; Deschoemaeker, S.; Raes, G.; Maes, A.; De Bruyne, E.; Menu, E.; Vanderkerken, K.; Laoui, D.; De Veirman, K. Dendritic Cell-Based Immunotherapy in Multiple Myeloma: Challenges, Opportunities, and Future Directions. Int. J. Mol. Sci. 2022, 23, 904.
- Li, S.; Wu, J.; Zhu, S.; Liu, Y.J.; Chen, J. Disease-Associated Plasmacytoid Dendritic Cells. Front. Immunol. 2017, 8, 1268.
- Uto, T.; Takagi, H.; Fukaya, T.; Nasu, J.; Fukui, T.; Miyanaga, N.; Arimura, K.; Nakamura, T.; Choijookhuu, N.; Hishikawa, Y.; et al. Critical role of plasmacytoid dendritic cells in induction of oral tolerance. J. Allergy Clin. Immunol. 2018, 141, 2156–2167.e9.
- Bonifaz, L.; Bonnyay, D.; Mahnke, K.; Rivera, M.; Nussenzweig, M.C.; Steinman, R.M. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002, 196, 1627–1638.
- Belz, G.T.; Behrens, G.M.; Smith, C.M.; Miller, J.F.; Jones, C.; Lejon, K.; Fathman, C.G.; Mueller, S.N.; Shortman, K.; Carbone, F.R.; et al. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J. Exp. Med. 2002, 196, 1099–1104.
- Lutz, M.B.; Kurts, C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur. J. Immunol. 2009, 39, 2325–2330.
- Joeris, T.; Gomez-Casado, C.; Holmkvist, P.; Tavernier, S.J.; Silva-Sanchez, A.; Klotz, L.; Randall, T.D.; Mowat, A.M.; Kotarsky, K.; Malissen, B.; et al. Intestinal cDC1 drive cross-tolerance to epithelial-derived antigen via induction of FoxP3(+)CD8(+) T(regs). Sci. Immunol. 2021, 6, eabd3774.
- Domogalla, M.P.; Rostan, P.V.; Raker, V.K.; Steinbrink, K. Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity. Front. Immunol. 2017, 8, 1764.
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029.
- Bourque, J.; Hawiger, D. Immunomodulatory Bonds of the Partnership between Dendritic Cells and T Cells. Crit. Rev. Immunol. 2018, 38, 379–401.
- Kurts, C.; Heath, W.R.; Kosaka, H.; Miller, J.F.; Carbone, F.R. The peripheral deletion of autoreactive CD8+ T cells induced by cross-presentation of self-antigens involves signaling through CD95 (Fas, Apo-1). J. Exp. Med. 1998, 188, 415–420.
- Steinbrink, K.; Graulich, E.; Kubsch, S.; Knop, J.; Enk, A.H. CD4(+) and CD8(+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood 2002, 99, 2468–2476.
- Martin-Orozco, E.; Norte-Munoz, M.; Martinez-Garcia, J. Regulatory T Cells in Allergy and Asthma. Front. Pediatr. 2017, 5, 117.
- Burrack, A.L.; Martinov, T.; Fife, B.T. T Cell-Mediated Beta Cell Destruction: Autoimmunity and Alloimmunity in the Context of Type 1 Diabetes. Front Endocrinol. 2017, 8, 343.
- Deng, Q.; Luo, Y.; Chang, C.; Wu, H.; Ding, Y.; Xiao, R. The Emerging Epigenetic Role of CD8+T Cells in Autoimmune Diseases: A Systematic Review. Front. Immunol. 2019, 10, 856.
- Ganguly, D.; Haak, S.; Sisirak, V.; Reizis, B. The role of dendritic cells in autoimmunity. Nat. Rev. Immunol. 2013, 13, 566–577.
- Liu, Y.; Wang, X.; Yang, F.; Zheng, Y.; Ye, T.; Yang, L. Immunomodulatory Role and Therapeutic Potential of Non-Coding RNAs Mediated by Dendritic Cells in Autoimmune and Immune Tolerance-Related Diseases. Front. Immunol. 2021, 12, 678918.
- Sebastian, M.; Lopez-Ocasio, M.; Metidji, A.; Rieder, S.A.; Shevach, E.M.; Thornton, A.M. Helios Controls a Limited Subset of Regulatory T Cell Functions. J. Immunol. 2016, 196, 144–155.
- Shahin, T.; Mayr, D.; Shoeb, M.R.; Kuehn, H.S.; Hoeger, B.; Giuliani, S.; Gawriyski, L.M.; Petronczki, O.Y.; Hadjadj, J.; Bal, S.K.; et al. Identification of germline monoallelic mutations in IKZF2 in patients with immune dysregulation. Blood Adv. 2022, 6, 2444–2451.
- Thornton, A.M.; Korty, P.E.; Tran, D.Q.; Wohlfert, E.A.; Murray, P.E.; Belkaid, Y.; Shevach, E.M. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J. Immunol. 2010, 184, 3433–3441.
- Thornton, A.M.; Shevach, E.M. Helios: Still behind the clouds. Immunology 2019, 158, 161–170.
- Gottschalk, R.A.; Corse, E.; Allison, J.P. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J. Immunol. 2012, 188, 976–980.
- Akimova, T.; Beier, U.H.; Wang, L.; Levine, M.H.; Hancock, W.W. Helios expression is a marker of T cell activation and proliferation. PLoS ONE 2011, 6, e24226.
- Crawford, A.; Angelosanto, J.M.; Kao, C.; Doering, T.A.; Odorizzi, P.M.; Barnett, B.E.; Wherry, E.J. Molecular and transcriptional basis of CD4(+) T cell dysfunction during chronic infection. Immunity 2014, 40, 289–302.
- Ross, E.M.; Bourges, D.; Hogan, T.V.; Gleeson, P.A.; van Driel, I.R. Helios defines T cells being driven to tolerance in the periphery and thymus. Eur. J. Immunol. 2014, 44, 2048–2058.
- Verhagen, J.; Wraith, D.C. Comment on “Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells”. J. Immunol. 2010, 185, 7129.
- Lam, A.J.; Uday, P.; Gillies, J.K.; Levings, M.K. Helios is a marker, not a driver, of human Treg stability. Eur. J. Immunol. 2022, 52, 75–84.
- Paris-Munoz, A.; Aizpurua, G.; Barber, D.F. Helios Expression Is Downregulated on CD8(+) Treg in Two Mouse Models of Lupus During Disease Progression. Front. Immunol. 2022, 13, 922958.
- Fuhrman, C.A.; Yeh, W.I.; Seay, H.R.; Saikumar Lakshmi, P.; Chopra, G.; Zhang, L.; Perry, D.J.; McClymont, S.A.; Yadav, M.; Lopez, M.C.; et al. Divergent Phenotypes of Human Regulatory T Cells Expressing the Receptors TIGIT and CD226. J. Immunol. 2015, 195, 145–155.
- Thornton, A.M.; Lu, J.; Korty, P.E.; Kim, Y.C.; Martens, C.; Sun, P.D.; Shevach, E.M. Helios(+) and Helios(-) Treg subpopulations are phenotypically and functionally distinct and express dissimilar TCR repertoires. Eur. J. Immunol. 2019, 49, 398–412.
- Sugita, K.; Hanakawa, S.; Honda, T.; Kondoh, G.; Miyachi, Y.; Kabashima, K.; Nomura, T. Generation of Helios reporter mice and an evaluation of the suppressive capacity of Helios(+) regulatory T cells in vitro. Exp. Dermatol. 2015, 24, 554–556.
- Morina, L.; Jones, M.E.; Oguz, C.; Kaplan, M.J.; Gangaplara, A.; Fitzhugh, C.D.; Kanakry, C.G.; Shevach, E.M.; Buszko, M. Co-expression of Foxp3 and Helios facilitates the identification of human T regulatory cells in health and disease. Front. Immunol. 2023, 14, 1114780.
- Rodriguez-Rodriguez, N.; Apostolidis, S.A.; Penaloza-MacMaster, P.; Martin Villa, J.M.; Barouch, D.H.; Tsokos, G.C.; Crispin, J.C. Programmed cell death 1 and Helios distinguish TCR-alphabeta+ double-negative (CD4−CD8−) T cells that derive from self-reactive CD8 T cells. J. Immunol. 2015, 194, 4207–4214.
- Rodriguez-Rodriguez, N.; Flores-Mendoza, G.; Apostolidis, S.A.; Rosetti, F.; Tsokos, G.C.; Crispin, J.C. TCR-alpha/beta CD4(−) CD8(-) double negative T cells arise from CD8(+) T cells. J. Leukoc. Biol. 2020, 108, 851–857.
- Rodriguez-Rodriguez, N.; Apostolidis, S.A.; Fitzgerald, L.; Meehan, B.S.; Corbett, A.J.; Martin-Villa, J.M.; McCluskey, J.; Tsokos, G.C.; Crispin, J.C. Pro-inflammatory self-reactive T cells are found within murine TCR-alphabeta(+) CD4(−) CD8(−) PD-1(+) cells. Eur. J. Immunol. 2016, 46, 1383–1391.
- Li, H.; Adamopoulos, I.E.; Moulton, V.R.; Stillman, I.E.; Herbert, Z.; Moon, J.J.; Sharabi, A.; Krishfield, S.; Tsokos, M.G.; Tsokos, G.C. Systemic lupus erythematosus favors the generation of IL-17 producing double negative T cells. Nat. Commun. 2020, 11, 2859.
- Fischer, K.; Voelkl, S.; Heymann, J.; Przybylski, G.K.; Mondal, K.; Laumer, M.; Kunz-Schughart, L.; Schmidt, C.A.; Andreesen, R.; Mackensen, A. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(−)CD8- double-negative regulatory T cells. Blood 2005, 105, 2828–2835.
- Voelkl, S.; Gary, R.; Mackensen, A. Characterization of the immunoregulatory function of human TCR-alphabeta+ CD4- CD8- double-negative T cells. Eur. J. Immunol. 2011, 41, 739–748.
- Juvet, S.C.; Zhang, L. Double negative regulatory T cells in transplantation and autoimmunity: Recent progress and future directions. J. Mol. Cell Biol. 2012, 4, 48–58.
- Yang, L.; Zhu, Y.; Tian, D.; Wang, S.; Guo, J.; Sun, G.; Jin, H.; Zhang, C.; Shi, W.; Gershwin, M.E.; et al. Transcriptome landscape of double negative T cells by single-cell RNA sequencing. J. Autoimmun. 2021, 121, 102653.
- Narni-Mancinelli, E.; Jaeger, B.N.; Bernat, C.; Fenis, A.; Kung, S.; De Gassart, A.; Mahmood, S.; Gut, M.; Heath, S.C.; Estelle, J.; et al. Tuning of natural killer cell reactivity by NKp46 and Helios calibrates T cell responses. Science 2012, 335, 344–348.
- Lee, J.; Zhang, T.; Hwang, I.; Kim, A.; Nitschke, L.; Kim, M.; Scott, J.M.; Kamimura, Y.; Lanier, L.L.; Kim, S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015, 42, 431–442.
- Kucuksezer, U.C.; Aktas Cetin, E.; Esen, F.; Tahrali, I.; Akdeniz, N.; Gelmez, M.Y.; Deniz, G. The Role of Natural Killer Cells in Autoimmune Diseases. Front. Immunol. 2021, 12, 622306.
- Spada, R.; Rojas, J.M.; Perez-Yague, S.; Mulens, V.; Cannata-Ortiz, P.; Bragado, R.; Barber, D.F. NKG2D ligand overexpression in lupus nephritis correlates with increased NK cell activity and differentiation in kidneys but not in the periphery. J. Leukoc. Biol. 2015, 97, 583–598.
More
Information
Subjects:
Immunology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
615
Revisions:
2 times
(View History)
Update Date:
04 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




