Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Andrés París-Muñoz and Version 2 by Rita Xu.
The Helios protein (encoded by the
IKZF2
gene) is a member of the Ikaros transcription family and it has recently been proposed as a promising biomarker for systemic lupus erythematosus (SLE) disease progression in both mouse models and patients. Helios is beginning to be studied extensively for its influence on the T regulatory (Treg) compartment, both CD4
+
Tregs and KIR
+
/Ly49
+
CD8
+
Tregs, with alterations to the number and function of these cells correlated to the autoimmune phenomenon.
- Helios
- biomarkers
- systemic lupus erythematosus
- autoimmunity
1. General Considerations on the Immune System
The immune system of complex organisms like mammals is a coordinated network of innate and adaptive cells, molecules, tissues, and organs that has evolved to fight against the foreign pathogens causing infectious disease. In parallel, the immune system also has the means to protect host self-antigens, part of a dynamic homeostatic equilibrium aimed at preventing any potential side effects due to an overzealous immunogenic response.
Unlike innate cells, one of the essential features of adaptive cells is their ability to recognize a wide range of different antigens (endogenous and exogenous). Adaptive immunity is driven by cellular (helper or CD4+ T cells, and cytotoxic or CD8+ T cells) and humoral immunity (via B cells). From a functional and perhaps simplified perspective, each immune response (innate and adaptive) can be classified as immunogenic (e.g., the immune response directed against pathogenic microorganisms) or tolerogenic (for example, the immunosuppression of autoreactive processes). Overall, the immune system aims to establish a dynamic and continuously regulated balance between these two opposing forces: reactivity to foreign molecules and self-antigen tolerance [1][2][1,2]. Interestingly, the same cell type may be involved in both activities, depending on factors like the molecular environment, their activation, and the patterns of gene expression [3]. Most of the known innate and adaptive immune cell populations are presented in Figure 1, showing their immunogenic and tolerogenic counterparts. Finding elements common to both sides of the balance, and that influence the general status of the immune system, will be interesting to better understand pathologies with a predominantly immune component, such as cancer and autoimmunity.
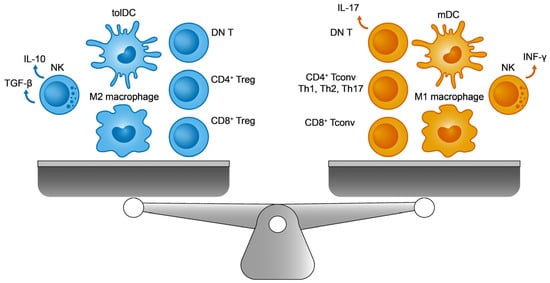
Figure 1. The immune balance. On the (left), the immunosuppressive cells include M2 macrophages, regulatory T cells (Tregs), tolerogenic dendritic cells (tolDCs), double negative T cells (DN T), and TGF-β producing natural killer (NK) cells. On the (right), their immunogenic counterparts are M1 macrophages, conventional T lymphocytes (Tconv), immunogenic mature DCs (mDCs), IL-17 producing DN T cells, and INF-γ producing NK cells.
1.1. The Adaptive Immune Equilibrium
In addition to T cells, dendritic cells (DCs) play a crucial role in lymphoid organs (thymus, lymph nodes, and spleen), orchestrating the adaptive immune system.
1.1.1. Conventional CD4+ and CD8+ T Cells
After their generation in bone marrow from hematopoietic progenitors, in both humans and mice, immature T cells move to the thymus to complete their development. Once there, and during their maturation, thymocytes reorganize their TCR by genetic recombination to generate a specific repertoire of immune cells with distinct affinities for the MHC molecules associated to their peptides. Subsequently, after positive and negative selection, the remaining fraction of TCRαβ+CD4+CD8+ cells silence one of their co-receptors, CD8 or CD4 depending on the interaction with antigen presenting cells (APCs), through their MHCI or MHCII molecules, respectively [4][5][4,5]. Thus, naive TCRαβ+CD4+CD8− (restricted MHCII cells) and TCRαβ+CD4−CD8+ (restricted MHCI cells) T lymphocytes arise in the thymus, exhibiting tolerance for self-antigens towards peripheral lymphoid tissues to be activated by APCs (mainly, DCs), and they then trigger the cellular adaptive response.
Globally, these mature T lymphocytes are defined as conventional T cells (Tconv), and they are responsible for the positive and immunogenic responses to foreign antigens. Yet what are the homeostatic mechanisms that avoid the potentially autoreactive reactions of Tconv cells that escape the process of negative selection in the thymus? Other subpopulations of immune cells play a pivotal role in readdressing the immune equilibrium through immunosuppression, cells known as T regulatory cells (Tregs). Among these cells, most research has focused on CD4+ Tregs, and in particular, on a CD4+ Treg population derived from the thymus (CD4+ tTreg). These cells survive the process of negative selection and they exhibit high affinity for MHCII-self-peptide complexes, expressing higher levels of CD25 and FoxP3 [6][8]. Nevertheless, incipient CD8+ Tregs that express the receptors of natural killer (NK) cells are currently generating much interest [7][9].
1.1.2. Regulatory CD4+ T Cells
The discovery of the FoxP3 transcription factor as a marker and an essential regulator that maintains CD4+ tTreg’s immunosuppressive activity implied a paradigm shift in the field of immunology [8][9][10,11]. After years of intense research in this topic, a CD4+ tTreg population (CD25+ FoxP3+) has been clearly shown to maintain the peripheral immune equilibrium by controlling aberrant and exacerbated autoreactive responses against self-antigens, microorganisms, and environmental antigens [10][11][12,13]. In this regard, several allergies and autoimmune pathologies are associated with certain alterations to CD4+ tTregs, revealing the true importance of their correct function [12][14]. In addition, some roles of CD4+ tTregs in other body systems beyond the immune system have also been reported. For example, the close links between these lymphocytes and tissue regeneration [13][15], or other metabolic diseases like obesity [14][16], have been studied.
Although CD4+ tTreg lymphocytes represent the most abundant fraction of Tregs in the periphery, there are also other regulatory CD4+ cell types of different origins that reliably express FoxP3 and that participate in establishing tolerance. For example, peripheral CD4+ Tregs (CD4+ pTregs) are generated from CD4+ Tconvs outside the thymus in tolerogenic contexts, such as in the intestinal mucosa. Moreover, CD4+ iTregs can be induced in vitro from CD4+ Tconvs under specific culture conditions and these cells are currently being tested in clinical trials as potential autologous cell therapy for patients affected by several autoimmune diseases [15][17]. Despite their differences, these CD4+ tTregs, pTregs, and iTregs employ some common immunosuppressive mechanisms.
Given the intrinsic molecular and cellular complexity of the immune system, CD4+ Treg lymphocytes need to act on several elements and processes, of both the innate and adaptive responses, in order to ensure effective peripheral tolerance in a coordinated manner [16][17][18][18,19,20] (see Figure 2 for a summary of the main immunosuppressive strategies identified in CD4+ Tregs). Among these events, the overexpression of CD25 (the α subunit of the interleukin 2 receptor or IL-2R) facilitates the capture and sequestering of the available IL-2. The reduced availability of IL-2 specifically dampens the activation and proliferation of CD8+ Tconv cells that depends on this cytokine [19][21]. From a metabolic perspective, the surface ectoenzymes CD39 and CD73 transform extracellular ATP into adenosine molecules, the latter potentially inhibiting effector T and myeloid cells (DCs and macrophages) [20][21][22,23]. Furthermore, these Tregs produce tolerogenic cytokines like IL-10 and TGF-β that exert multiple effects on immune cells. By contrast, IL-10 and TGF-β inhibit T and B lymphocyte proliferation and stimulation [22][24]. Moreover, these cytokines would polarize DCs towards a more tolerogenic phenotype, which promotes the induction of pTregs [23][25]. Finally, Tregs and DCs interact directly through two different signaling systems that could induce a tolerogenic state in DCs: PD-1 (Treg)—PDL-1 (DCs) [24][26], and CTLA-4 (Treg)—CD80/86 (DCs) [25][27].
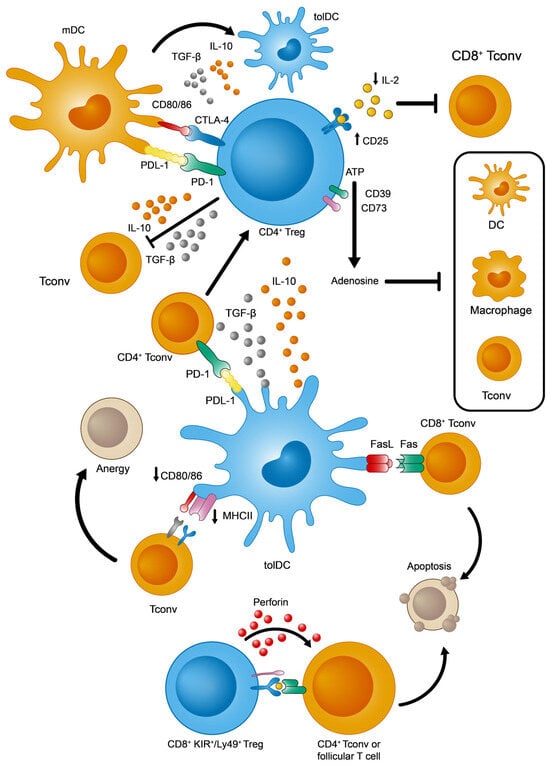

Figure 2. Main immunosuppressive mechanisms adopted by tolDCs, CD4+, and KIR+/Ly49+ CD8+ Tregs.
1.1.3. Regulatory CD8+ Tregs
Globally, although most biomedical research into Treg cells has focused on the CD4+ Treg compartment, a recently described subset of CD8+ Treg lymphocytes that expresses inhibitory markers from NK cells is being investigated intensively in mice and humans. Previously, a wide range of different CD8+ T cells exhibiting immunomodulatory properties were classified as CD8+ Tregs, yet with heterogeneous phenotypes: CD8+ FoxP3+ [26][30], CD8+ CD28− [27][31], CD8+ CD103+ [28][32], and CD8+ CD122+ CD49dlow [29][33]. However, unlike CD4+ tTreg cells, these populations were found peripherally in very small proportions or in experimental contexts of antigenic exposure in mice. In other words, these subsets were never found naturally in significant numbers in healthy and young mice without prior manipulation [30][31][34,35].
This situation changed with the discovery of a new regulatory subpopulation of CD8+ T cells in naïve mice without any immune disruption, reliably defined by the expression of three markers: CD44+, CD122+, and Ly49+ (Ly49+ CD8+ Tregs from here on). This population has the ability to modulate the adaptive immune response by eliminating autoreactive CD4+ Tconv and follicular T lymphocytes [32][33][34][36,37,38]. Since then, other properties of this population have been clarified, such as (1) the absence of intranuclear FoxP3 expression; (2) high levels of surface CD127 (in contrast to CD4+ Treg cells) [34][38]; (3) homeostatic regulation, mainly through IL-15 and TGF-β [35][36][37][39,40,41]; (4) phenotypic acquisition induced peripherally after thymic maturation [38][42]; and (5) finally, the fact that these cells have a non-redundant role in controlling autoreactive antibody titers by acting in germinal centers from lymphoid organs [33][37][37,41], thereby regulating autoimmune phenomena [36][37][39][40,41,43]. In terms of their functionality, the best-defined effector mechanism used by CD8+ Ly49+ Tregs is the cytotoxic killing of autoreactive CD4+ T cells, regardless of IL-10 production [32][36] (Figure 2).
1.1.4. Tolerogenic Dendritic Cells (tolDCs)
As previously stated, DCs are the primary APCs that initiate the adaptive immune responses of T lymphocytes in response to foreign antigens in the periphery. However, in addition to this crucial role, they also contribute to immune tolerance in the organism in two other ways. Firstly, DCs mediate the negative selection and differentiation of tTregs in the thymus (central tolerance), while they also maintain peripheral tolerance by elimination, anergic induction, or conversion of autoreactive T cells to pTregs [40][44]. While immature DCs (imDCs) were simply believed to perform a tolerogenic function and mature DCs (mDCs) an immunogenic function, increasing experimental evidence suggests that this functional divide extends beyond the maturation state of DCs. In fact, maturation is necessary for their optimal tolerogenic function, even under basal conditions with no exogenous stimulation. Thus, reswearchers will refer to them functionally as imDCs, mDCs, and tolerogenic mature DCs (tolDCs) [41][42][45,46].
Apart from these states of maturation, distinct DC subpopulations fulfill specific roles in the immune response. These subpopulations are well-defined in both mouse models and humans, and include conventional (cDCs) and plasmacytoid dendritic cells (pDCs) [43][47]. Type 1 (cDC1s) and type 2 (cDC2s) DCs are defined by their developmental transcriptional program, presenting MHCI antigens for CD8+ T cells or MHCII for CD4+ T cells, respectively. Furthermore, pDCs specialize in the production of type I interferons upon exposure to viral antigens [44][48], as well as helping to establish immune tolerance [45][49]. In line with this, it seems that cDC1 cells, with their inherent capacity to present antigens via MHCI molecules, also play a crucial role in ensuring peripheral tolerance to self-antigens [40][42][46][47][48][49][44,46,50,51,52,53].
Focusing on their tolerogenic functions, tolDCs capture and process self-antigens, whether soluble or derived from apoptotic bodies, for their presentation to peripheral T cells [50][28]. Among the numerous mechanisms described, some are worthy of particular attention (Figure 2): (1) tolDCs promote the generation of CD4+ pTregs from CD4+ Tconvs in two ways, by secreting immunosuppressive cytokines (IL-10 and TGF-β) or by signaling through PDL-1 molecules [51][52][54,55]; (2) tolDCs eliminate autoreactive CD8+ T cells via the Fas/FasL apoptotic system [53][56]; and (3) tolDCs favor an anergic state in T cells due to the weak expression of MHCII and CD80/CD86 [54][57].
Also, as for iTreg lymphocytes, it is also possible to induce an immunosuppressive state in mouse and human DCs in vitro for their use in cell-based immunotherapies. This polarization towards tolerance is achieved using different immunomodulators, including glucocorticoids (e.g., dexamethasone), cytokines (e.g., IL-10 and TGF-β), rapamycin, or vitamin D3 [50][28].
1.2. Autoimmune Alterations in the Adaptive Immune Balance
In the case of cancer, the immune balance is inclined in favor of immunosuppression as a strategy to help the tumor evade active immune responses. By contrast, in the case of autoimmunity, the immune equilibrium is oriented towards immunogenicity against self-antigens. In this respect, there is considerable evidence of defects and alterations in number, frequency, and immunosuppressive function of CD4+ Tregs from patients with allergies, and other inflammatory or autoimmune diseases [11][12][55][13,14,58]. Although some inconsistencies have been noted depending on the patient cohort, the pathological state, and the different flow cytometry panels employed to define and phenotype Treg subsets, some common defects have been reported globally in CD4+ T lymphocytes in autoimmune contexts: (1) the conversion of CD4+ Tregs into effector CD4+ Tconvs; (2) the reduced ability of CD4+ Tregs to promote tolerogenic effects; and (3) the resistance to immunosuppression observed in some activated CD4+ Tconvs. By contrast, in type I diabetes, autoreactive CD8+ Tconv cells have been extensively described as an important inducer of cytotoxicity of pancreatic β cell death [56][59], and implicated in other autoimmune pathologies [57][60]. There is an increase in the number of CD8+ KIR+ Tregs in both blood and at inflammatory sites in autoimmune patients, and it was proposed that these lymphocytes eliminate pathogenic CD4+ T cells to avoid their exacerbated response [7][9].
Finally, the role of DCs in autoimmune progression has also been analyzed in depth [58][59][61,62], establishing that they promote an immunogenic environment due to inflammatory cytokine production. For example, IL-6, IL-12, and IL-23 secretion by immunogenic mDCs facilitates the polarization of self-reactive T cells. In the case of SLE, type-I interferons (IFN-I) mainly produced by pDCs deserve a special mention because they represent one of the most important molecular markers for this disease.
