Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesco Izzo | -- | 1198 | 2024-01-02 15:18:47 | | | |
| 2 | Sirius Huang | Meta information modification | 1198 | 2024-01-03 02:25:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marrone, G.; Di Lauro, M.; Izzo, F.; Cornali, K.; Masci, C.; Vita, C.; Occhiuto, F.; Di Daniele, N.; De Lorenzo, A.; Noce, A. Hydrolyzable Tannins' (Castanea Sativa) Mechanism of Action. Encyclopedia. Available online: https://encyclopedia.pub/entry/53326 (accessed on 01 March 2026).
Marrone G, Di Lauro M, Izzo F, Cornali K, Masci C, Vita C, et al. Hydrolyzable Tannins' (Castanea Sativa) Mechanism of Action. Encyclopedia. Available at: https://encyclopedia.pub/entry/53326. Accessed March 01, 2026.
Marrone, Giulia, Manuela Di Lauro, Francesco Izzo, Kevin Cornali, Claudia Masci, Chiara Vita, Francesco Occhiuto, Nicola Di Daniele, Antonino De Lorenzo, Annalisa Noce. "Hydrolyzable Tannins' (Castanea Sativa) Mechanism of Action" Encyclopedia, https://encyclopedia.pub/entry/53326 (accessed March 01, 2026).
Marrone, G., Di Lauro, M., Izzo, F., Cornali, K., Masci, C., Vita, C., Occhiuto, F., Di Daniele, N., De Lorenzo, A., & Noce, A. (2024, January 02). Hydrolyzable Tannins' (Castanea Sativa) Mechanism of Action. In Encyclopedia. https://encyclopedia.pub/entry/53326
Marrone, Giulia, et al. "Hydrolyzable Tannins' (Castanea Sativa) Mechanism of Action." Encyclopedia. Web. 02 January, 2024.
Copy Citation
Hydrolyzable tannins (HTs) deriving from chestnuts have demonstrated, through numerous studies, the ability to exert multiple beneficial effects, including antioxidant and antimicrobial effects, on the lipid metabolism and cancer cells. The latter effect is very fascinating, since different polyphenols deriving from chestnuts were able to synergistically induce the inhibition of cancerous cells through multiple pathways. Moreover, the main mechanisms by which tannins induce antioxidant functions include: the reduction in oxidative stress, the ability to scavenge free radicals, and the modulation of specific enzymes, such as superoxide dismutase.
chestnuts
hydrolyzable tannins
antioxidant effects
anticancer effects
cardioprotective effects
nutraceuticals
lipid metabolism
1. Introduction
Tannins are water-soluble polyphenols commonly found within many sources in nature. These compounds include oligomers or polymers of flavanol and phenolic acid monomers, and have different sensory/chemical properties and functions compared to their monomers. Tannins are encountered as plant secondary metabolites and can be divided into two main categories: condensed and hydrolyzable [1]. The distinction between the two groups relies on chemical aspects, such as the resistance to hydrolysis and the chemical stability [2][3].
Tannin condensates are oligomers (from 2 to 10 monomers) or polymers (>10 monomers) of flavan-3-ol units that, based on the positioning of their -OH and -H groups, can give rise to procyanidins, prodelphinidins, prorobinetinidins, and prodelfinidins (Figure 1). For instance, the procyanidins contain catechin or epicatechin, while the prodelfinidins contain gallocatechin or epigallocatechin [2].
Figure 1. Chemical structure of prodelphinidin B-2 3′-O-gallate.
The hydrolyzable tannins (HTs) are hydrolyzed by acids into glucose and ellagic acid or into glucose and gallic acid (GA). The HT group includes ellagitannins (ETs) (Figure 2) and gallotannins (Figure 3).
Figure 2. Chemical structure of chebulic acid, which pertains to the group of ellagitannins.
Figure 3. Complex gallotannin, named tannic acid, also known as Chinese tannin, is highly present in chestnuts.
The HTs deriving from Castanea sativa L. have shown to induce multiple beneficial effects on both human and animal health [4]. The genus Castanea consists of twelve species of deciduous trees, all native of the temperate regions of the northern hemisphere. Among them, Castanea sativa, also known as European or sweet chestnut, is the most emblematic species present in the southern area of Europe. Italy is the country which produces the highest quantity of chestnuts [5].
The fruit of the chestnut is enclosed in a thorny shell and is composed of many elements, among which are the leathery pericarp and two thin integuments covering two large fleshy cotyledons. All the components of the chestnut, including the waste and byproducts, are rich in bioactive compounds, with several beneficial properties. For this reason, the chestnut represents an ideal candidate in various fields, like in the pharmaceutical and cosmetic industries [5].
The health effects of these compounds (Figure 4) lie in the fact that the chestnut and its derivatives are rich in HTs. The latter have been shown to exert antioxidant and antimicrobial actions, antineoplastic effects, to favor the lipid metabolism [6], and to exert cardioprotective actions [7].
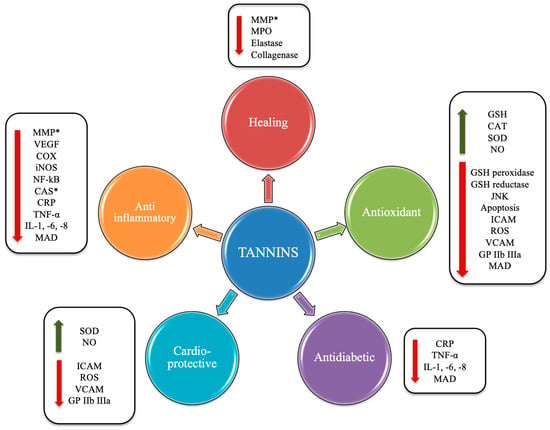
Figure 4. Potential beneficial effects induced by tannins. Abbreviations: *, antioxidant capacity; VEGF, vascular endothelial growth factor; MMP, matrix metalloproteinase; COX, cyclooxygenase; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; CAS, caspase; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; IL, interleukin; MAD, malondialdehyde; GP IIb IIIa, glycoprotein IIb/IIIa; NO, nitric oxide; VCAM, vascular cell adhesion protein; ROS, reactive oxygen species; ICAM, intercellular adhesion molecule; SOD, superoxide dismutase; CAT, catalase; JNK, C-Jun N-terminal kinase.
2. Tannins’ Mechanism of Action
As previous described, HTs deriving from chestnuts have demonstrated the exertion of multiple beneficial effects both in human and animal studies.
The accumulation of reactive oxygen species (ROS) is commonly implicated with the occurrence of chronic degenerative non-communicable diseases, namely, pathological conditions related to systemic and chronic inflammation. Examples include: atherosclerosis, chronic kidney disease, cardiovascular disease, diabetes mellitus, and neurodegenerative pathologies, such as Parkinson’s and Alzheimer’s diseases [8].
The administration of natural antioxidants may help or serve as a supplement against oxidative mechanisms [8]. One possible mechanism, which induces antioxidant functions, is the ability to directly scavenge free radicals thanks to the donation of hydrogen atoms. Another possible antioxidant mechanism, induced by the administration of chestnut extracts, is the modulation of different enzymes normally involved in antioxidant functions. These enzymes include superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) [9]. The interaction between tannins and free radicals aims at creating resonance-stabilized phenix radicals, which confer important antioxidant properties.
In recent years, the antimicrobial activity induced by some phenolic molecules has been a prominent element of research. This effect has been substantially associated to the hydroxyl groups present in these molecules. In fact, both the number and the position of hydroxyl groups present within phenolic molecules appear to be related to the level of their inhibitory capacity of bacterial growth [10]. The proposal of possible substitutes to combat bacterial strains resistant to conventional antibiotics has become fundamental [11]. Lima et al. [12] demonstrated how the interaction of phenolic compounds with host-gut microbiota results in a positive effect by increasing the beneficial bacterial population. According to Chanwitheesuk et al. [13], Staphylococcus aureus and Salmonella reported susceptibility to GA. Furthermore, the antimicrobial activity of phenolic molecules encountered within the chestnut extracts is exerted on different components of the bacterial cell, including some inactive essential enzymes and the cell membrane. These molecules are also able to modify specific functions of the genetic material of the bacteria. The Gram-negative bacterial cell wall is the principal obstacle for the entrance of the phenolic molecules into the cytoplasm because of the repulsion created between lipopolysaccharides and phenols [14].
Both hydrolyzable and condensed tannins have demonstrated the ability to inhibit the growth of several bacterial strains which are potentially pathogenic, such as Escherichia coli, without having any effects on the physiological growth of other gut beneficial bacteria [15].
Tannins have the ability to bind reversibly to some amino acids and dietary and endogenous proteins [16][17][18]. In this regard, a positive effect of both the HTs and the condensed ones on Campylobacter jejuni was demonstrated in vitro. Interestingly, it was also evaluated whether the amino acid supplementation would limit the tannin extracts’ activity. This experiment was performed to evaluate if the abundance of amino acids and soluble proteins within the gastrointestinal tract could interfere with the tannins’ antimicrobial action [19].
Moreover, polyphenolic molecules, including HTs, have also shown to exert antineoplastic properties. In fact, these molecules counteract different cancerous cell development [20], modulating several mechanisms, such as the regulation of cell signaling cascades. Among these are the transcription factors and various kinase enzymes that modulate the expression of genes involved in apoptosis. In addition, they are also able to check the interaction between growth factors and target receptors involved in specific signaling effects [20]. HTs used as antineoplastic agents have various advantages, including the specificity of the biological response and low toxicity [21].
ETs have received increased attention on this matter due to their chemopreventive and chemotherapeutic activities [22]. These effects seem to be induced by their high antioxidant capacity. The latter can vary according to the degree of tannin hydroxylation and it depends on iron chelation activity and on capacity of the radical scavenging [23].
ETs have also demonstrated the ability to inhibit angiogenesis in both in vitro and in vivo prostate cancer models. They exert this action by binding directly to VEGF receptors and by indirectly reducing endothelial growth [24].
References
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582.
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464.
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649.
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78.
- Formato, M.; Vastolo, A.; Piccolella, S.; Calabro, S.; Cutrignelli, M.I.; Zidorn, C.; Pacifico, S. Castanea sativa Mill. Leaf: UHPLC-HR MS/MS Analysis and Effects on In Vitro Rumen Fermentation and Methanogenesis. Molecules 2022, 27, 8662.
- Sieniawska, E. Activities of Tannins—From In Vitro Studies to Clinical Trials. Nat. Prod. Commun. 2015, 10, 1877–1884.
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Battino, M.; et al. The Effect of Dietary Polyphenols on Vascular Health and Hypertension: Current Evidence and Mechanisms of Action. Nutrients 2022, 14, 545.
- Dinis, L.T.; Oliveira, M.M.; Almeida, J.; Costa, R.; Gomes-Laranjo, J.; Peixoto, F. Antioxidant activities of chestnut nut of Castanea sativa Mill. (cultivar ‘Judia’) as function of origin ecosystem. Food Chem. 2012, 132, 1–8.
- Liu, H.W.; Zhou, D.W.; Li, K. Effects of chestnut tannins on performance and antioxidative status of transition dairy cows. J. Dairy Sci. 2013, 96, 5901–5907.
- Sanhueza, L.; Melo, R.; Montero, R.; Maisey, K.; Mendoza, L.; Wilkens, M. Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli. PLoS ONE 2017, 12, e0172273.
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of the Phenolic Profile of Castanea sativa Mill. By-Products and Their Antioxidant and Antimicrobial Activity against Multiresistant Bacteria. Antioxidants 2020, 9, 87.
- Lima, M.C.; Paiva de Sousa, C.; Fernandez-Prada, C.; Harel, J.; Dubreuil, J.D.; de Souza, E.L. A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb. Pathog. 2019, 130, 259–270.
- Chanwitheesuk, A.; Teerawutgulrag, A.; Kilburn, J.D.; Rakariyatham, N. Antimicrobial gallic acid from Caesalpinia mimosoides Lamk. Food Chem. 2007, 100, 1044–1048.
- Sara, M.; Javier, R.-P.; Manuel, C.-B.; Daniel, M.-V.; Jonathan, D.-A. Application of Phenolic Compounds for Food Preservation: Food Additive and Active Packaging. In Phenolic Compounds; Marcos, S.-H., Mariana, P.-T., Maria del Rosario, G.-M., Eds.; IntechOpen: Rijeka, Croatia, 2017; p. 3.
- Girard, M.; Bee, G. Invited review: Tannins as a potential alternative to antibiotics to prevent coliform diarrhea in weaned pigs. Animal 2020, 14, 95–107.
- Serrano, J.; Puupponen-Pimia, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S310–S329.
- He, Q.; Shi, B.; Yao, K. Interactions of gallotannins with proteins, amino acids, phospholipids and sugars. Food Chem. 2006, 95, 250–254.
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037.
- Anderson, R.C.; Vodovnik, M.; Min, B.R.; Pinchak, W.E.; Krueger, N.A.; Harvey, R.B.; Nisbet, D.J. Bactericidal effect of hydrolysable and condensed tannin extracts on Campylobacter jejuni in vitro. Folia Microbiol. 2012, 57, 253–258.
- Way, T.D.; Kao, M.C.; Lin, J.K. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 2004, 279, 4479–4489.
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278.
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621.
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160.
- Sartippour, M.R.; Seeram, N.P.; Rao, J.Y.; Moro, A.; Harris, D.M.; Henning, S.M.; Firouzi, A.; Rettig, M.B.; Aronson, W.J.; Pantuck, A.J.; et al. Ellagitannin-rich pomegranate extract inhibits angiogenesis in prostate cancer in vitro and in vivo. Int. J. Oncol. 2008, 32, 475–480.
More
Information
Subjects:
Medicine, General & Internal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
03 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





