Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Matěj Malík | -- | 2875 | 2023-12-28 11:42:06 | | | |
| 2 | Jason Zhu | Meta information modification | 2875 | 2023-12-29 03:45:45 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Malík, M.; Mika, O.J.; Navrátilová, Z.; Killi, U.K.; Tlustoš, P.; Patočka, J. Pharmacological Activity and Toxicity of Bracken Fern. Encyclopedia. Available online: https://encyclopedia.pub/entry/53220 (accessed on 07 February 2026).
Malík M, Mika OJ, Navrátilová Z, Killi UK, Tlustoš P, Patočka J. Pharmacological Activity and Toxicity of Bracken Fern. Encyclopedia. Available at: https://encyclopedia.pub/entry/53220. Accessed February 07, 2026.
Malík, Matěj, Otakar Jiří Mika, Zdeňka Navrátilová, Uday Kumar Killi, Pavel Tlustoš, Jiří Patočka. "Pharmacological Activity and Toxicity of Bracken Fern" Encyclopedia, https://encyclopedia.pub/entry/53220 (accessed February 07, 2026).
Malík, M., Mika, O.J., Navrátilová, Z., Killi, U.K., Tlustoš, P., & Patočka, J. (2023, December 28). Pharmacological Activity and Toxicity of Bracken Fern. In Encyclopedia. https://encyclopedia.pub/entry/53220
Malík, Matěj, et al. "Pharmacological Activity and Toxicity of Bracken Fern." Encyclopedia. Web. 28 December, 2023.
Copy Citation
Bracken fern (Pteridium aquilinum (L.) Kuhn) is ubiquitous and acts as a cosmopolitan weed in pastures and similar environments. Despite its historical uses, it presents risks due to toxicity.
ptaquiloside
weed
bracken fern
fiddleheads
bioactive compounds
1. Antimicrobial Potency: In Vitro Studies
Phenolic acids, known for their antimicrobial properties even at concentrations as low as 100 to 150 ppm w/v [1][2], are present in bracken fern sporophytes (0.25–0.75%, phenolic acids/g dry weight) and gametophytes (0.15–0.20%, phenolic acids/g dry weight) at sufficiently high levels to act as significant antimicrobial compounds. The concentrations of phenolic acids in P. aquilinum leaves vary seasonally, influenced by plant age and various environmental and biological factors. These phenolic acids differentially impede the growth of fern pathogens and other bacteria in vitro, persisting in plant tissues throughout most of the growing season and effectively inhibiting the proliferation of pathogenic microorganisms [3].
The minimum inhibitory concentration (MIC) for the n-hexane extract of leaves, determined via the tube dilution method, demonstrated inhibition at 300 to 350 mg/mL for Staphylococcus aureus, Enterobacter aerogenes, and Pseudomonas aeruginosa, and at 350 mg/mL for Escherichia coli. In contrast, there was no inhibition of the ethanolic extract for any of the organisms. The minimum bactericidal concentration (MBC) of the n-hexane extract for P. aeruginosa, E. coli, and E. aerogenes indicated susceptibility at 300 to 350 mg/mL [4].
The MIC test of the ethanolic and petroleum ether extract of P. aquilinum against bacterial pathogens (Bacillus subtilis and S. aureus) was observed as 1 mg/mL. For E. coli and Proteus vulgaris, it was found to be 0.8 mg/mL.
2. Antioxidant Properties: In Vitro Insights and Prospects for In Vivo Exploration
The antioxidant activity of P. aquilinum leaves was slightly lower when compared with ascorbic acid (% inhibition in mg/mL). The scavenging activities of 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radicals were 84 and 73.3%, respectively, for P. aquilinum leaves, while for ascorbic acid, it was 88.2% and 83%, respectively [5]. In a comparison of extracts from selected ferns (Onoclea struthiopteris, Onoclea orientalis, Osmunda japonica, and P. aquilinum), all fern extracts exhibited antioxidant effects in vitro. Notably, the roots of O. japonica and the fronds of O. orientalis were the most efficient [6].
Utilizing a ferric-reducing antioxidant power assay (FRAP), it was demonstrated that the purified polysaccharide obtained from P. aquilinum possessed strong reductive power (FRAP value: 827.6 μmol/L), comparable to that of vitamin C. Additionally, it exhibited moderate scavenging activities against DPPH radicals (83.1%) at an 800 μg/mL dose. These results suggest that the purified polysaccharide had a noticeable effect on scavenging free radicals, particularly at higher concentrations. However, its activity was not more potent than vitamin C at the same dose. The percentage inhibition of superoxide generation via 800 μg/mL doses of the crude polysaccharides, the purified polysaccharide, and vitamin C was found to be 42.2%, 60.5%, and 84.2%, respectively. The scavenging effects of the crude polysaccharides, the purified polysaccharide, and vitamin C on the self-oxidation of 1,2,3-phentriol concentration were dependently increasing. At 100 μg/mL doses, they were 23.3%, 52.4%, and 90.7%, respectively [7].
Similar results were also obtained by Zhao et al. [8] in their study when another new polysaccharide (named PAP-3) was derived from P. aquilinum. PAP-3 exhibited strong free-radical scavenging activity on DPPH and ABTS radicals in vitro, albeit slightly lower than that of vitamin C. These studies collectively demonstrated the robust antioxidant capacity of bracken fern compounds in in vitro antioxidation tests. Future research endeavors should focus on investigating the antioxidant activity of this plant through in vivo experiments.
3. Immunomodulatory and Anticancer Potential: Insights into Cellular Responses and Therapeutic Prospects
Immunostimulation is considered a crucial defense mechanism against various diseases. The immunomodulatory effect of PAP-3 on RAW264.7 cells, a macrophage cell line derived from a male mouse tumor induced via the Abelson murine leukemia virus, was examined across a concentration range of 0 to 200 μg/mL. PAP-3 demonstrated the ability to induce RAW264.7 cell proliferation at concentrations ranging from 12.5 to 200 μg/mL, with no detectable cytotoxic effects up to 200 μg/mL. Incubation with PAP-3 significantly increased nitric oxide (NO) production from RAW264.7 cells, reaching 17.31 μM at 25 μg/mL concentration, comparable to the positive control (lipopolysaccharide, 10.0 μg/mL) [8].
Immunomodulatory effects associated with prolonged bracken fern feeding and enzootic bovine hematuria (EBH) were assessed using various tests, including the tube agglutination test for Brucella abortus antibodies, dinitrochlorobenzene skin test, lymphocyte proliferation assay, histopathological examination of lymph nodes, and skin biopsies in cows. Cows subjected to prolonged bracken fern feeding and those affected by EBH exhibited a decrease in humoral and an increase in cell-mediated immune responses compared to controls [9].
Another study investigated the immunomodulatory effects of bracken fern through daily extract ingestion by murine hosts over 14 (or up to 30) days. In C57BL/6 mice administered the extract via gavage, histological analyses revealed a significant reduction in splenic white pulp area. Various immune response parameters/functions were assessed, demonstrating a reduction in delayed-type hypersensitivity and interferon-gamma production by natural killer cells during T helper 1 priming. The innate response, assessed via natural killer cell cytotoxic functionality, was also diminished. While bracken fern affected components of acquired and innate immune responses, not all responses were modified, as seen in the analyses of humoral response and macrophage activity. These results suggest the immunosuppressive effect of P. aquilinum in mice, potentially contributing to an increased risk of cancer formation in exposed hosts [10].
The study by Dion et al. [6] revealed decreased interleukin-1 beta (IL1-β) gene expression for P. aquilinum frond extracts. Indirect measurement of NO via inducible nitric oxide gene expression showed a 50% decrease with the extract of O. orientalis fronds at a low concentration (20 μg/mL) compared to P. aquilinum fronds (160 μg/mL) and leaves of O. japonica, with the latter showing a higher decrease at a high extract concentration (160 μg/mL).
Additionally, extracts from bracken fern have demonstrated the potential to inhibit the growth of specific cancer cells in in vitro studies [11][12]. Treatment of human cancer cell lines with bracken fern dichloromethane extracts induced DNA damage and apoptosis at high concentrations (200 μg/mL) and caused cell cycle arrest at milder concentrations (50 and 30 μg/mL), depending on the cell type and tumor origin [12]. Williams et al. [11] reported that ptaquiloside exhibited selective toxicity against cancer cells compared to noncancer retinal epithelial cells, indicating its potential as a therapeutic agent.
These findings underscore the potential of P. aquilinum as a source of natural compounds with therapeutic value. However, it is crucial to note that bracken fern also contains several toxic compounds and bleeding factors, especially thiaminases and ptaquiloside, found in high concentrations in the plant’s young parts (young fronds) and rhizomes [13][14].
4. Ptaquiloside: Carcinogenic Properties and Metabolic Fate
The norsesquiterpene glycoside ptaquiloside has been extensively investigated for its potential carcinogenic properties [15][16][17][18][19]. Ingesting large quantities or prolonged consumption of ptaquiloside can be toxic to humans, emphasizing the need for caution when considering the edibility of bracken fern. The prevalence of bracken fern in Japanese cuisine is posited as a potential explanation for the increased incidence of bladder, stomach, and esophageal cancers among young Japanese individuals [20][21][22].
Ptaquiloside is implicated as a major contributor to the observed toxicity in ruminant farm animals. Intravenous administration of ptaquiloside to sheep induces progressive retinal degeneration, highlighting its harmful effects [23]. Guinea pigs, unlike rats or mice, exhibit hemorrhagic cystitis and hematuria upon subcutaneous administration of ptaquiloside [24]. It has been suggested that ptaquiloside accounts for more than half of the mutagenic potency associated with bracken [15][16][19]. Although a comprehensive modern carcinogenicity bioassay of ptaquiloside is lacking, limited studies in rats have explored its carcinogenicity. Rats receiving an initial oral dose of 780 mg/kg bw followed by weekly doses of 100 to 200 mg/kg bw or twice-weekly doses of 100 to 150 mg/kg bw for 8½ weeks developed tumors of the mammary gland and ileum, along with other adverse effects [25][26]. In a separate study, rats fed a diet containing 0.027 to 0.08% ptaquiloside developed cancers of the ileum and/or bladder within 15 to 60 days [27]. While parenteral dosing showed no tumors in rats given weekly intravenous doses, adverse effects such as monocytosis and focal renal tubular necrosis were observed [28]. Oral dosing, although not resulting in tumors, led to adverse effects, including tubular necrosis, monocytosis, and elevated plasma tumor necrosis factor-alpha [29].
The mutagenicity of ptaquiloside was tested under different pH conditions. It was found to be nonmutagenic in strains of Salmonella typhimurium at pH 7.4 without metabolic activation but exhibited mutagenicity at pH 8.5 after pre-incubation [30][31]. Moreover, ptaquiloside induced chromatid exchange-type aberrations in Chinese hamster lung cells at various pH values, with higher pH values showing greater genotoxic potency [32][33]. In vitro, ptaquiloside produced DNA adducts and induced unscheduled DNA synthesis in a rat hepatocyte culture at pH 7.2 [34][35][36]. Cumulatively, the available evidence suggests that ptaquiloside likely contributes to bracken’s carcinogenicity and toxicity, with a genotoxic mode of action being implicated.
Ptaquiloside undergoes diverse decomposition processes influenced by environmental conditions, particularly pH and temperature [37][38]. Degradation can occur in both acidic and alkaline environments, posing a heightened risk of leaching when soil pH is slightly acidic to neutral, clay content is high, and microbiological degradation is slow [39][40]. Notably, the occurrence of ptaquiloside-induced bovine esophageal and bladder tumors correlates with pH values of 8.1 to 8.2 (saliva) and 7.5 to 8.5 (urine) [41][42].
Under normal physiological conditions, enzymatic degradation of ptaquiloside occurs in the gastrointestinal tract, especially in the stomach and small intestine, catalyzed by glycosidase enzymes. The stomach’s low pH facilitates the hydrolysis of ptaquiloside, leading to the formation of an aglycon called ptaquilosine and a dienone intermediate known as ptaquilodienone (Figure 1) [43][44][45]. Subsequently, two possible paths emerge.
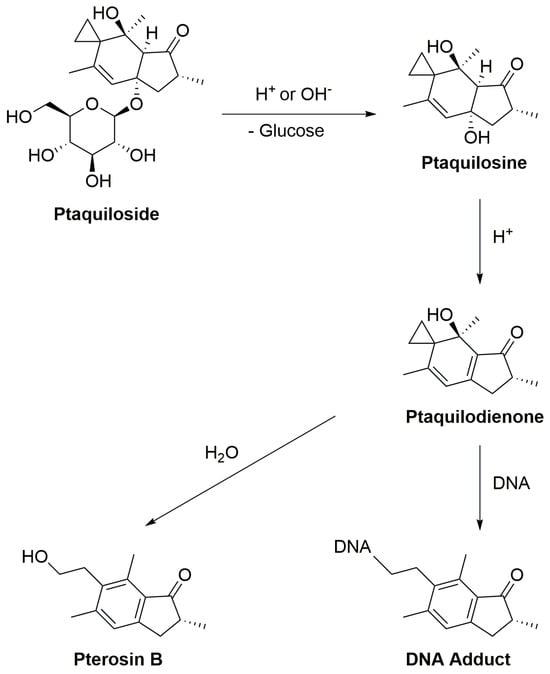
Figure 1. The scheme of ptaquiloside degradation reactions.
In one scenario, ptaquilodienone reacts with water to produce an aromatic compound, pterosin B, devoid of significant biological effects [32]. These pterosins enter the bloodstream and are transported to the liver, where a series of enzymatic reactions, involving glucosidases, cytochrome P450 enzymes, sulfotransferases, and glucuronosyltransferases, metabolize the breakdown products. This process enhances water solubility, facilitating excretion, typically through urine [16][46][47][48].
Alternatively, ptaquilodienone may covalently bind to DNA molecules [49]. This dienone primarily alkylates amino acids such as cysteine, glutathione, and methionine at their thiol groups. Additionally, minor alkylation occurs at the carboxylate groups, forming corresponding esters [32][50][51]. The dienone also reacts with DNA, targeting adenine (predominantly at N-3) and guanine (primarily at N-7) residues, resulting in the formation of DNA adducts. This alkylation induces spontaneous depurination and DNA cleavage at adenine base sites [32][52][53]. These alkylated DNA by-products play a crucial role in carcinogenesis, as they introduce mutations and aberrations, leading to the development of cancers. Importantly, if the ptaquiloside molecule degrades to pterosin B, these harmful consequences are averted [16][53][54].
5. Thiaminase, Caffeic Acid, and Cyanogenic Glycosides: Assessing Bracken Fern’s Metabolic Challenges in Animals
Thiaminases, enzymes with antithiamine properties, are implicated in the short- to medium-term symptoms of bracken fern poisoning in monogastric animals. Unlike ruminants that produce their own thiamine, monogastric animals rely on dietary sources, rendering them more susceptible to thiaminase impact from various origins, including bracken [55]. Thiamine (vitamin B1) is sourced by humans from yeast, whole grains (e.g., brown rice, whole wheat), legumes (e.g., lentils, soybeans), nuts and seeds (e.g., sunflower seeds, macadamia nuts), and animal-derived options such as pork and organ meats (liver, kidney, and heart). Fortified foods, particularly cereals and bread, and thiamine-based supplements contribute to thiamine intake in case of dietary insufficiency [56]. Vitamin B1 plays a crucial role in maintaining myelin in the peripheral nervous system and overall organism functioning. Its deficiency, leading to beriberi in humans, manifests as peripheral or central neuropathy with symptoms including poor health, weight loss, and loss of coordination, with reported cases suggestive of heart failure [57][58][59].
Monogastric animals acquire vitamin B1 through forage diets, with common feed components such as cereal grains (e.g., corn, wheat), oilseed meals (e.g., soybean meal), and specific animal by-products serving as thiamine sources. Animal diets are meticulously formulated to meet thiamine requirements for optimal growth and health [60].
Thiaminase enzymes degrade thiamine into thiazole and pyrimidine. Thiamine deficiency restricts reactions dependent on thiamin diphosphate, leading to substrate accumulation (pyruvate, pentoses, etc.,) associated with these reactions [13][14][61][62]. While thiaminases are heat-stable, autoclaving effectively destroys them [62]. Within P. aquilinum, thiaminases contribute to thiamine deficiency, with the highest activity observed in rhizomes and very young fronds, the former having concentrations 10–30 times greater than mature fronds [63][64].
Rats fed on bracken displaying thiaminase activity developed nervous system lesions typical of antivitaminosis B1, curable with thiamine therapy [65]. Similar effects were observed in other monogastric animals, such as horses and pigs [62][66]. A 52-week feeding study in rats aimed to explore bracken’s antithiamine activity and its potential link to carcinogenicity. Control groups showed no tumors, while all surviving rats in bracken-fed groups developed multiple intestinal tumors. Bladder tumors occurred in 11% of males and 7% of females in the bracken-alone group, increasing to 53% of males and 67% of females when thiamine was supplemented. Intriguingly, thiamine supplements did not reduce tumor incidence, indicating thiaminase might not be the primary cause of bracken’s carcinogenicity [64][67].
P. aquilinum collected in November in the Pretoria district, South Africa, exhibited mean thiaminase activities of 3.1 and 3.5 µg thiamine destroyed/g plant material/hour for types 1 and 2, respectively, with a thiamine content of 0.04 µg thiamine/g plant material [13][14][68]. No reports of thiamine deficiency in human populations consuming bracken have been found, potentially attributed to humans having more varied diets and consuming significantly less bracken than animals [55][69]. Another heat-stable antithiamine factor, potentially caffeic acid, has been linked to bracken-induced thiamine deficiency in horses [70][71]. Caffeic acid, a phenolic substance with antioxidant properties found in many plants [72], has shown diverse effects in animal studies, including stomach papillomas in rats and a reduction in colon tumor growth in the same rats [73].
Another potentially hazardous compound occasionally found in specific bracken fern varieties is the cyanogenic glycoside prunasin. While generally present in harmless quantities in bracken fronds, instances of sudden animal death due to suspected hydrogen cyanide (HCN) poisoning from young bracken fronds have been documented [71][74]. Cyanogenesis occurs in both gametophytic and sporophyte generations of ferns, with young fronds being more cyanogenic than older ones [75][76], a typical defense strategy against herbivores [77]. Cyanogenic glycosides, such as prunasin, become toxic through conversion into HCN by β-glycosidase enzymes released upon tissue damage. When bracken is crushed or chewed, this conversion occurs, leading to the release of the unstable α-cyanohydrin mandelonitrile, which undergoes mandelonitrile β-elimination catalyzed via oxynitrilase to produce HCN and benzaldehyde [78][79]. Notably, polymorphism exists in bracken, with some plants lacking either prunasin or the required enzymes, rendering them noncyanogenic [80]. No reports exist of cyanide poisoning in people consuming foods from animals grazing on bracken, indicating animals are more affected by direct prunasin and cyanide exposure.
6. Ptaquiloside Accumulation and Health Risks: Impact on Animals and the Food Chain
P. aquilinum is a widespread plant thriving in diverse environments and posing challenges for pasture control [81]. Documented cases of intoxication after bracken fern ingestion are prevalent in various animals, particularly ruminants such as cattle, sheep, and wild deer [82]. In cattle, ingestion leads to enzootic hematuria, characterized by bleeding into the lower urinary tract and the development of epithelial and mesenchymal neoplasms. Sheep, on the other hand, may experience acute hemorrhagic syndrome, bone marrow aplasia, retinal atrophy, and polioencephalomalacia [83]. While relatively poorly documented in monogastric species, horses, for instance, can suffer from poisoning when exposed to bracken fern through low-quality hay or grazing. This leads to a decrease in thiamine content and an increase in pyruvate and lactate levels in the blood [13][84].
Upon ingestion, ptaquiloside, a key compound in bracken fern, is absorbed into the bloodstream from the stomach and intestines without significant metabolic alterations. Ptaquiloside has the potential to accumulate in various tissues, including the mammary glands, a phenomenon observed during lactation [85]. Since the mammary glands actively produce milk, compounds present in the bloodstream, including ptaquiloside, can contaminate the milk and meat of these animals [47][48][86][87]. The quantity of bracken fern consumed directly correlates with the potential for ptaquiloside accumulation in the animal’s tissues, milk, or meat. Animals engaging in prolonged grazing on P. aquilinum are prone to accumulating higher levels of ptaquiloside in their systems. Research indicates that ptaquiloside is excreted in milk at a concentration of 8.6 ± 1.2% of the ingested amount by the cow from the fern, and this excretion is linearly dependent on the dose. Ptaquiloside was detected in milk 38 h after the initial feeding of cows with this plant [87]. Milk from cows fed on bracken has been associated with various adverse effects. These include leukopenia in calves, bladder cancer in mice, and the development of intestinal, bladder, and kidney cancers in rats [25].
Estimates suggest that infants consuming milk from cows exposed to bracken could potentially receive up to 22.8 mg/person/day of ptaquiloside, with elderly individuals and toddlers having the highest per capita chronic milk intake. These estimations, ranging from 2.9 to 22.1 mg/person/day (0.047–0.36 mg/kg bw/day) for toddlers and 2.8 to 21.6 mg/person/day (0.19–1.49 mg/kg bw/day) for the elderly, consider the maximum intake from nontoxic doses of ptaquiloside in milk. However, the actual exposure may be higher when considering milk from poisoned cows or other sources such as drinking water and bracken-exposed animal-derived foods [88][89][90].
Various animal species and individuals metabolize and eliminate ptaquiloside at different rates, influencing susceptibility to its toxicity. Importantly, the consumption of milk or meat from animals exposed to bracken fern poses health risks to humans. Therefore, preventing animal exposure to P. aquilinum or its contaminated products is crucial to mitigate the potential transfer of ptaquiloside to the food chain [45][71][91].
References
- Raccach, M. The antimicrobial activity of phenolic antioxidants in foods: A review. J. Food Saf. 1984, 6, 141–170.
- Ijabadeniyi, O.A.; Govender, A.; Olagunju, O.F.; Oyedeji, A.B. The antimicrobial activity of two phenolic acids against foodborne Escherichia coli and Listeria monocytogenes and their effectiveness in a meat system. Ital. J. Food Sci. 2021, 33, 39–45.
- San Francisco, M.; Cooper-Driver, G. Anti-Microbial Activity of Phenolic Acids in Pteridium aquilinum. Am. Fern J. 1984, 74, 87–96.
- Awe, S.; Amobi, O.O. Antibacterial, phytochemical and proximate analysis of Pteridium aquilinum. Int. J. Res. Pharm. Biomed. Sci. 2015, 2, 1–7.
- Kardong, D.; Upadhyaya, S.; Saikia, L.R. Screening of phytochemicals, antioxidant and antibacterial activity of crude extract of Pteridium aquilinum Kuhn. J. Pharm. Res. 2013, 6, 179–182.
- Dion, C.; Haug, C.; Guan, H.; Ripoll, C.; Spiteller, P.; Coussaert, A.; Boulet, E.; Schmidt, D.; Wei, J.; Zhou, Y. Evaluation of the anti-inflammatory and antioxidative potential of four fern species from China intended for use as food supplements. Nat. Prod. Commun. 2015, 10, 597–603.
- Xu, W.; Zhang, F.; Luo, Y.; Ma, L.; Kou, X.; Huang, K. Antioxidant activity of a water-soluble polysaccharide purified from Pteridium aquilinum. Carbohydr. Res. 2009, 344, 217–222.
- Zhao, Z.-H.; Ju, X.-Y.; Wang, K.-W.; Chen, X.-J.; Sun, H.-X.; Cheng, K.-J. Structure Characterization, Antioxidant and Immunomodulatory Activities of Polysaccharide from Pteridium aquilinum (L.) Kuhn. Foods 2022, 11, 1834.
- Ravisankar, R.; Somvanshi, R.; Kataria, J.; Singh, D. Immunomodulation studies on chronic bracken fern (Pteridium aquilinum) toxicity and enzootic bovine haematuria affected hill cows. J. Immunol. Immunopathol. 2004, 6, 58–59.
- Latorre, A.O.; Furlan, M.S.; Sakai, M.; Fukumasu, H.; Hueza, I.M.; Haraguchi, M.; Górniak, S.L. Immunomodulatory effects of Pteridium aquilinum on natural killer cell activity and select aspects of the cellular immune response of mice. J. Immunotoxicol. 2009, 6, 104–114.
- Williams, C.; Allison, S.J.; Phillips, R.M.; Linley, P.A.; Wright, C.W. An Efficient Method for the Isolation of Toxins from Pteridium aquilinum and Evaluation of Ptaquiloside Against Cancer and Non-cancer Cells. Planta Med. 2021, 87, 892–895.
- Roudsari, M.T.; Bahrami, A.R.; Dehghani, H.; Iranshahi, M.; Matin, M.M.; Mahmoudi, M. Bracken-fern extracts induce cell cycle arrest and apoptosis in certain cancer cell lines. Asian Pac. J. Cancer Prev. 2012, 13, 6047–6053.
- Vetter, J. Toxicological and Medicinal Aspects of the Most Frequent Fern Species, Pteridium aquilinum (L.) Kuhn. In Working with Ferns: Issues and Applications; Kumar, A., Fernández, H., Revilla, M.A., Eds.; Springer New York: New York, NY, USA, 2010; pp. 361–375.
- Vetter, J. A biological hazard of our age: Bracken fern —A review. Acta Vet. Hung. 2009, 57, 183–196.
- Rasmussen, L.H. Presence of the carcinogen ptaquiloside in fern-based food products and traditional medicine: Four cases of human exposure. Curr. Res. Food Sci. 2021, 4, 557–564.
- Vetter, J. The Norsesquiterpene Glycoside Ptaquiloside as a Poisonous, Carcinogenic Component of Certain Ferns. Molecules 2022, 27, 6662.
- Skrbic, N.; Pedersen, A.-K.; Christensen, S.C.B.; Hansen, H.C.B.; Rasmussen, L.H. A Novel Method for Determination of the Natural Toxin Ptaquiloside in Ground and Drinking Water. Water 2020, 12, 2852.
- Gil da Costa, R.M.; Neto, T.; Estêvão, D.; Moutinho, M.; Félix, A.; Medeiros, R.; Lopes, C.; Bastos, M.M.S.M.; Oliveira, P.A. Ptaquiloside from bracken (Pteridium spp.) promotes oral carcinogenesis initiated by HPV16 in transgenic mice. Food Funct. 2020, 11, 3298–3305.
- Silva, F.; Garcês, A.; Magalhães, C.; Pires, I. Diseases in Ruminants Associated with Pteridium aquilinum Ingestion. Biol. Life Sci. Forum 2023, 24, 8.
- George, E.S.; Sood, S.; Broughton, A.; Cogan, G.; Hickey, M.; Chan, W.S.; Sudan, S.; Nicoll, A.J. The Association between Diet and Hepatocellular Carcinoma: A Systematic Review. Nutrients 2021, 13, 172.
- Hirono, I.; Shibuya, C.; Fushimi, K.; Haga, M. Studies on carcinogenic properties of bracken, Pteridium aquilinum. J. Natl. Cancer Inst. 1970, 45, 179–188.
- Sugimura, T. Nutrition and dietary carcinogens. Carcinogenesis 2000, 21, 387–395.
- Hirono, I.; Ito, M.; Yagyu, S.; Haga, M.; Wakamatsu, K.; Kishikawa, T.; Nishikawa, O.; Yamada, K.; Ojika, M.; Kigoshi, H. Reproduction of progressive retinal degeneration (bright blindness) in sheep by administration of ptaquiloside contained in bracken. J. Vet. Med. Sci. 1993, 55, 979–983.
- Yoshida, M.; Saito, T. Acute toxicity of braxin C, a bracken toxin, in guinea pigs. J. Toxicol. Sci. 1994, 19, 17–23.
- O’Connor, P.J.; Alonso-Amelot, M.E.; Roberts, S.A.; Povey, A.C. The role of bracken fern illudanes in bracken fern-induced toxicities. Mutat. Res. Rev. Mutat. Res. 2019, 782, 108276.
- Hirono, I.; Aiso, S.; Yamaji, T.; Mori, H.; Yamada, K.; Niwa, H.; Ojika, M.; Wakamatsu, K.; Kigoshi, H.; Niiyama, K. Carcinogenicity in rats of ptaquiloside isolated from bracken. Jpn. J. Cancer Res. 1984, 75, 833–836.
- Hirono, I.; Ogino, H.; Fujimoto, M.; Yamada, K.; Yoshida, Y.; Ikagawa, M.; Okumura, M. Induction of Tumors in ACI Rats Given a Diet Containing Ptaquiloside, a Bracken Carcinogen. J. Natl. Cancer Inst. 1987, 79, 1143–1149.
- Shahin, M.; Moore, M.R.; Smith, B.L.; Seawright, A.A.; Prakash, A.S. Acute and Chronic Toxicity Induced by Activated Ptaquiloside in Rats: Comparison of Pathogenesis due to Oral and Intravenous Administrations in Toxic Plants and Other Natural Toxicants; Garland, T., Barr, A.C., Eds.; CAB International: New York, NY, USA, 1998; pp. 255–259.
- Shahin, M.; Moore, M.R.; Worrall, S.; Smith, B.L.; Seawright, A.A.; Prakash, A.S. H-ras Activation Is an Early Event in the Ptaquiloside-Induced Carcinogenesis: Comparison of Acute and Chronic Toxicity in Rats. Biochem. Biophys. Res. Commun. 1998, 250, 491–497.
- Smith, B.L. The toxicity of bracken fern (genus Pteridium) to animals and its relevance to man. In Handbook of Plant and Fungal Toxicants; CRC Press: Boca Raton, FL, USA, 2020; pp. 63–76.
- Nagao, T.; Saito, K.; Hirayama, E.; Uchikoshi, K.; Koyama, K.; Natori, S.; Morisaki, N.; Iwasaki, S.; Matsushima, T. Mutagenicity of ptaquiloside, the carcinogen in bracken, and its related illudane-type sesquiterpenes I. Mutagenicity in Salmonella typhimurium. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1989, 215, 173–178.
- Yamada, K.; Ojika, M.; Kigoshi, H. Ptaquiloside, the major toxin of bracken, and related terpene glycosides: Chemistry, biology and ecology. Nat. Prod. Rep. 2007, 24, 798–813.
- Matsuoka, A.; Hirosawa, A.; Natori, S.; Iwasaki, S.; Toshio, S.; Motoi, I., Jr. Mutagenicity of ptaquiloside, the carcinogen in bracken, and its related illudane-type sesquiterpenes: II. Chromosomal aberration tests with cultured mammalian cells. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1989, 215, 179–185.
- Fernández, H.; Sierra, L.M. Pteridium aquilinum: A Threat to Biodiversity and Human and Animal Health. In Ferns: Biotechnology, Propagation, Medicinal Uses and Environmental Regulation; Marimuthu, J., Fernández, H., Kumar, A., Thangaiah, S., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 697–713.
- Hemeryck, L.Y.; Vanhaecke, L. Diet-related DNA adduct formation in relation to carcinogenesis. Nutr. Rev. 2016, 74, 475–489.
- Mori, H.; Sugie, S.; Hirono, I.; Yamada, K.; Niwa, H.; Ojika, M. Genotoxicity of ptaquiloside, a bracken carcinogen, in the hepatocyte primary culture/DNA-repair test. Mutat. Res. Lett. 1985, 143, 75–78.
- Ayala-Luis, K.B.; Hansen, P.B.; Rasmussen, L.H.; Hansen, H.C.B. Kinetics of ptaquiloside hydrolysis in aqueous solution. Environ. Toxicol. Chem. 2006, 25, 2623–2629.
- Saito, K.; Nagao, T.; Matoba, M.; Koyama, K.; Natori, S.; Murakami, T.; Saiki, Y. Chemical assay of ptaquiloside, the carcinogen of Pteridium aquilinum, and the distribution of related compounds in the Pteridaceae. Phytochemistry 1989, 28, 1605–1611.
- Rasmussen, L.H.; Hansen, H.C.B.; Lauren, D. Sorption, degradation and mobility of ptaquiloside, a carcinogenic Bracken (Pteridium sp.) constituent, in the soil environment. Chemosphere 2005, 58, 823–835.
- Zaccone, C.; Cavoski, I.; Costi, R.; Sarais, G.; Caboni, P.; Traversa, A.; Miano, T.M. Ptaquiloside in Pteridium aquilinum subsp. aquilinum and corresponding soils from the South of Italy: Influence of physical and chemical features of soils on its occurrence. Sci. Total Environ. 2014, 496, 365–372.
- Van der Hoeven, J.C.M.; Lagerweij, W.J.; Posthumus, M.A.; van Veldhuizen, A.; Holterman, H.A.J. Aquilide A, a new mutagenic compound isolated from bracken fern (Pteridium aquilinum (L.) Kuhn). Carcinogenesis 1983, 4, 1587–1590.
- Tobar, Á.C.; Sánchez, A.J.; Perera, L.M.S.; Dorvigny, B.M.; Faz, E.M. Risk by human health for invasion of Pteridium arachnoideum, in Bolívar, Ecuador Ptaquiloside´ s content in fronds and in milk. Int. J. Appl. Sci. Technol. 2014, 4, 84–94.
- Rathaur, P.; Kaid Johar, S.R. Metabolism and Pharmacokinetics of Phytochemicals in the Human Body. Curr. Drug Metab. 2019, 20, 1085–1102.
- Majak, W. Mammalian Metabolism of Toxic Glycosides from Plants. J. Toxicol. Toxin Rev. 1992, 11, 1–40.
- Virgilio, A.; Sinisi, A.; Russo, V.; Gerardo, S.; Santoro, A.; Galeone, A.; Taglialatela-Scafati, O.; Roperto, F. Ptaquiloside, the Major Carcinogen of Bracken Fern, in the Pooled Raw Milk of Healthy Sheep and Goats: An Underestimated, Global Concern of Food Safety. J. Agric. Food Chem. 2015, 63, 4886–4892.
- Wink, M.; Schimmer, O. Molecular Modes of Action of Defensive Secondary Metabolites. In Annual Plant Reviews Volume 39: Functions and Biotechnology of Plant Secondary Metabolites; Wink, M., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; Volume 39, pp. 21–161.
- Aranha, P.C.R.; Hansen, H.C.B.; Rasmussen, L.H.; Strobel, B.W.; Friis, C. Determination of ptaquiloside and pterosin B derived from bracken (Pteridium aquilinum) in cattle plasma, urine and milk. J. Chromatogr. B 2014, 951-952, 44–51.
- Aranha, P.C.d.R.; Rasmussen, L.H.; Wolf-Jäckel, G.A.; Jensen, H.M.E.; Hansen, H.C.B.; Friis, C. Fate of ptaquiloside—A bracken fern toxin—In cattle. PLoS ONE 2019, 14, e0218628.
- Yoshihira, K.; Fukuoka, M.; Kuroyanagi, M.; Natori, S.; Umeda, M.; Morohoshi, T.; Enomoto, M.; Saito, M. Chemical and toxicological studies on bracken fern, Pteridium aquilinum var. latiusculum. I. Introduction, extraction and fractionation of constituents, and toxicological studies including carcinogenicity tests. Chem. Pharm. Bull. 1978, 26, 2346–2364.
- Kim, M.K.; Kang, J.S.; Kundu, A.; Kim, H.S.; Lee, B.-M. Risk Assessment and Risk Reduction of Ptaquiloside in Bracken Fern. Toxics 2023, 11, 115.
- Chauhan, P.; Mahajan, S.; Enders, D. Organocatalytic Carbon–Sulfur Bond-Forming Reactions. Chem. Rev. 2014, 114, 8807–8864.
- Ojika, M.; Wakamatsu, K.; Niwa, H.; Yamada, K. Ptaquiloside, a potent carcinogen isolated from bracken fern pteridiumaquilinum var. latiusculum: Structure elucidation based on chemical and spectral evidence, and reactions with amino acids, nucleosides, and nucleotides. Tetrahedron 1987, 43, 5261–5274.
- Kushida, T.; Uesugi, M.; Sugiura, Y.; Kigoshi, H.; Tanaka, H.; Hirokawa, J.; Ojika, M.; Yamada, K. DNA damage by ptaquiloside, a potent bracken carcinogen: Detection of selective strand breaks and identification of DNA cleavage products. J. Am. Chem. Soc. 1994, 116, 479–486.
- Potter, D.M.; Baird, M.S. Carcinogenic effects of ptaquiloside in bracken fern and related compounds. Br. J. Cancer 2000, 83, 914–920.
- Shahin, M.; Smith, B.L.; Prakash, A.S. Bracken carcinogens in the human diet. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 443, 69–79.
- Alamgir, A.N.M. Vitamins, Nutraceuticals, Food Additives, Enzymes, Anesthetic Aids, and Cosmetics. In Therapeutic Use of Medicinal Plants and their Extracts: Volume 2: Phytochemistry and Bioactive Compounds; Springer International Publishing: Cham, Switzerland, 2018; Volume 74, pp. 407–534.
- Weiss, S.; Wilkins, R.W. The nature of the cardiovascular disturbances in nutritional deficiency states (beriberi). Ann. Intern. Med. 1937, 11, 104–148.
- Fattal-Valevski, A. Thiamine (Vitamin B1). J. Evid. Based Complementary Altern. Med. 2011, 16, 12–20.
- Carlström, B.; Myrbäck, K.; Holmin, N.; Larsson, A. Biochemical Studies on B1-avitaminosis in Animals and Man. Acta Med. Scand. 1939, 102, 175–213.
- Setyahadi, S. Animal Feed from Oil Producing Plants. In Biorefinery of Oil Producing Plants for Value-Added Products; Abd-Aziz, S., Gozan, M., Ibrahim, M.F., Phang, L.-Y., Eds.; Wiley: Hoboken, NJ, USA, 2022; Volume 2, pp. 631–651.
- Waret-Szkuta, A.; Jégou, L.; Lucas, M.N.; Gaide, N.; Morvan, H.; Martineau, G.-P. A case of eagle fern (Pteridium aquilinum) poisoning on a pig farm. Porc. Health Manag. 2021, 7, 2.
- Evans, W.C. Thiaminases and their effects on animals. Vitam. Horm. 1976, 33, 467–504.
- Bates, N. Bracken and horsetail poisoning. UK Vet. Equine 2023, 7, 58–62.
- Bates, N. Bracken poisoning. Livestock 2023, 28, 100–105.
- Hirono, I.; Weisburger, E.K. Carcinogenic Principles Isolated from Bracken Fern. CRC Crit. Rev. Toxicol. 1986, 17, 1–22.
- Medeiros-Fonseca, B.; Abreu-Silva, A.L.; Medeiros, R.; Oliveira, P.A.; Gil da Costa, R.M. Pteridium spp. and Bovine Papillomavirus: Partners in Cancer. Front. Vet. Sci. 2021, 8, 758720.
- Pamukcu, A.M.; Yalçiner, S.; Price, J.M.; Bryan, G.T. Effects of the Coadministration of Thiamine on the Incidence of Urinary Bladder Carcinomas in Rats Fed Bracken Fern. Cancer Res. 1970, 30, 2671–2674.
- Meyer, P. Thiaminase activities and thiamine content of Pteridium aquilinum, Equisetum ramosissimum, Malva parviflora, Pennisetum clandestinum and Medicago sativa. Onderstepoort J. Vet. Res. 1989, 56, 145–146.
- Wilson, D.; Donaldson, L.J.; Sepai, O. Should we be frightened of bracken? A review of the evidence. J. Epidemiol. Community Health 1998, 52, 812–817.
- Ghosh, T.; Mitra, P.K. Isolation of an Anti-Thiamine Compound from Bergenia ciliata leaves of Sikkim Himalaya. Saudi J. Biomed. Res. 2022, 7, 41–44.
- Gil da Costa, R.M.; Bastos, M.M.S.M.; Oliveira, P.A.; Lopes, C. Bracken-associated human and animal health hazards: Chemical, biological and pathological evidence. J. Hazard. Mater. 2012, 203–204, 1–12.
- Tajner-Czopek, A.; Gertchen, M.; Rytel, E.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A. Study of Antioxidant Activity of Some Medicinal Plants Having High Content of Caffeic Acid Derivatives. Antioxidants 2020, 9, 412.
- Hirose, M.; Takesada, Y.; Tanaka, H.; Tamano, S.; Kato, T.; Shirai, T. Carcinogenicity of antioxidants BHA, caffeic acid, sesamol, 4-methoxyphenol and catechol at low doses, either alone or in combination, and modulation of their effects in a rat medium-term multi-organ carcinogenesis model. Carcinogenesis 1998, 19, 207–212.
- Tourchi-Roudsari, M. Multiple effects of bracken fern under in vivo and in vitro conditions. Asian Pac. J. Cancer Prev. 2014, 15, 7505–7513.
- Schreiner, I.; Nafus, D.; Pimentel, D. Effects of cyanogenesis in bracken fern (Pteridium aquilinum) on associated insects. Ecol. Entomol. 1984, 9, 69–79.
- Jones, C.G.; Firn, R.D. Some allelochemicals of Pteridium aquilinum and their involvement in resistance to Pieris brassicae. Biochem. Syst. Ecol. 1979, 7, 187–192.
- Douglas, M.M. Defense of bracken fern by arthropods attracted to axillary nectaries. Psyche J. Entomol. 1983, 90, 313–320.
- Vetter, J. Secondary Metabolites of Ferns. In Current Advances in Fern Research; Fernández, H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 305–327.
- Castrejón-Varela, A.; Pérez-García, B.; Guerrero-Analco, J.A.; Mehltreter, K. A Brief Review of Phytochemical Defenses of Ferns against Herbivores. Am. Fern J. 2022, 112, 233–250.
- Oliveros-Bastidas, A.d.J.; Alonso-Amelot, M.E. Cyanogenic polimorphysm in brackens, Pteridium arachnoideum and P. caudatum, from the northern Andes. Quím. Nova 2010, 33, 1520–1524.
- Štefanić, E.; Japundžić-Palenkić, B.; Antunović, S.; Gantner, V.; Zima, D. Botanical characteristics, toxicity and control of bracken fern (Pteridium aquilinum (L.) Kuhn). Zbor Veleuč Rij. 2022, 10, 467–478.
- Scala, C.; Ortiz, K.; Catinaud, J.; Lemberger, K. Hematuria and urinary bladder lesions compatible with bracken fern (Pteridium aquilinum) intoxication in captive fallow deer (Dama dama). J. Zoo. Wildl. Med. 2014, 45, 380–385.
- Cortinovis, C.; Caloni, F. Epidemiology of intoxication of domestic animals by plants in Europe. Vet. J. 2013, 197, 163–168.
- Evans, W.C.; Patel, M.; Koohy, Y. Acute bracken poisoning in homogastric and ruminant animals. Proc. R. Soc. B Biol. Sci. 1982, 81, 29–64.
- Mossie, T.; Yirdaw, B. Documentation of Major Poisonous Plants and Their Toxic Effects on Livestock: A Review. Am. J. Biosci. Bioieng 2023, 11, 47–55.
- Nicholson, S.S. Southeastern plants toxic to ruminants. Vet. Clin. Food Anim. Pract. 2011, 27, 447–458.
- Alonso-Amelot, M.E.; Castillo, U.; Smith, B.L.; Lauren, D.R. Excretion, through milk, of ptaquiloside in bracken-fed cows. A quantitative assessment. Lait 1998, 78, 413–423.
- Ugochukwu, I.C.I. Bracken fern toxicity and its associated clinicopathological effects in humans and animals: A review. Comp. Clin. Pathol. 2019, 28, 593–597.
- Alonso-Amelot, M.E.; Castillo, U.; De Jongh, F. Passage of the bracken fern carcinogen ptaquiloside into bovine milk. Lait 1993, 73, 323–332.
- Kobets, T.; Smith, B.P.C.; Williams, G.M. Food-Borne Chemical Carcinogens and the Evidence for Human Cancer Risk. Foods 2022, 11, 2828.
- Park, H.; Cho, Y.; Lee, J.; Lee, K.M.; Kim, H.J.; Lee, J.; Bahn, Y.-S.; Son, J. Evaluation and Monitoring of the Natural Toxin Ptaquiloside in Bracken Fern, Meat, and Dairy Products. Toxins 2023, 15, 231.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Revisions:
2 times
(View History)
Update Date:
29 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




