Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shery Jacob | -- | 1847 | 2023-12-28 10:43:54 | | | |
| 2 | Rita Xu | -3 word(s) | 1844 | 2023-12-28 10:49:15 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jacob, S.; Boddu, S.H.S.; Bhandare, R.; Ahmad, S.S.; Nair, A.B. Orodispersible Films. Encyclopedia. Available online: https://encyclopedia.pub/entry/53216 (accessed on 07 February 2026).
Jacob S, Boddu SHS, Bhandare R, Ahmad SS, Nair AB. Orodispersible Films. Encyclopedia. Available at: https://encyclopedia.pub/entry/53216. Accessed February 07, 2026.
Jacob, Shery, Sai H. S. Boddu, Richie Bhandare, Samiullah Shabbir Ahmad, Anroop B. Nair. "Orodispersible Films" Encyclopedia, https://encyclopedia.pub/entry/53216 (accessed February 07, 2026).
Jacob, S., Boddu, S.H.S., Bhandare, R., Ahmad, S.S., & Nair, A.B. (2023, December 28). Orodispersible Films. In Encyclopedia. https://encyclopedia.pub/entry/53216
Jacob, Shery, et al. "Orodispersible Films." Encyclopedia. Web. 28 December, 2023.
Copy Citation
Orodispersible films (ODFs) are thin, mechanically strong, and flexible polymeric films that are designed to dissolve or disintegrate rapidly in the oral cavity for local and/or systemic drug delivery.
orodispersible film
polymers
clinical trials
1. Introduction
Oral medications are the preferred and widely accepted method of drug delivery due to their ease of administration, convenience for repeated and prolonged use, non-invasiveness, adaptability, scalability, and high patient compliance [1]. Nevertheless, certain patient groups, such as the elderly, children, individuals with Parkinson’s disease, and those recovering from anesthesia, often encounter challenges in swallowing or chewing solid dosage forms, particularly tablets and capsules [2]. In the United States, it is estimated that about 15 million people have dysphagia, and this represents about 4.6% of the population. Thus, extensive efforts have been undertaken to create innovative oral drug formulations that dissolve or disperse in the oral cavity, aiming to address the issue of swallowing difficulties [3]. Moreover, highly vascularized oral mucosa may increase permeability to many medications, thereby providing a rapid onset of action and increasing bioavailability, as reported elsewhere [4].
Oral disintegrating tablets (ODTs) are a type of solid oral dosage form designed to disintegrate rapidly within a matter of seconds when placed on the tongue without the necessity of water or chewing [5]. Oral thin films are a drug delivery system consisting of thin, flexible sheets that typically dissolve or disintegrate quickly, often within seconds, when placed in the mouth. They are intended to be placed either on the tongue or cheek and can be used to deliver a variety of medications, including over-the-counter (OTC) and prescription drugs [6][7].
While various names like thin strip, oral film, orally dissolving film, quick dissolve film, melt-away film, and wafer are employed to refer to the oral film dosage form, the European Medicines Agency officially designates it as an orodispersible film (ODF), or, as the United States Food and Drug Administration (U.S. FDA) commonly terms them, soluble films [8]. As per European Pharmacopeia (Ph. Eur.), ODFs are defined as sheets, either single or multilayered, composed of appropriate materials and are intended for rapid dispersion in the mouth. They rapidly disintegrate/dissolve in saliva to form a solution or suspension, thus enabling rapid absorption and delivery of the drug into the bloodstream or a rapid local effect. Moreover, ODFs offer rapid and consistent drug release, which can improve the bioavailability of some medications. The oral cavity is richly vascularized and has low enzymatic activity, which can potentially boost the bioavailability of drugs with low aqueous solubility. This route is advantageous for those drugs classified under the biopharmaceutical classification system (BCS) as Class II and Class IV. Rapid permeation across the mucosal lining of the oral cavity can circumvent acid hydrolysis in the stomach and initial hepatic metabolism. This pathway is particularly well-suited for potent medications, especially those designed for acute conditions, where they have an immediate therapeutic effect, mainly due to oromucosal and pregastric absorption, as well as direct access to the jugular vein [4]. Nevertheless, certain compounds are absorbed exclusively in the gastrointestinal tract after ingestion.
2. Nanoparticle-Embedded ODFs
The advantages of embedding either polymeric or lipid nanoparticles into ODFs are multifaceted, including enhanced drug solubility and permeability, controlled release, targeted drug delivery, taste masking, and improved stability during storage and transportation [9][10][11]. Embedding nanoparticles into ODFs usually requires specialized formulation methods like solvent casting, HME, and spray drying. These techniques offer the necessary control and compatibility for successfully embedding nanoparticles in ODFs while addressing the challenges associated with their integration into the film formulation. A schematic diagram depicting the typical nanoparticle types and methods employed for incorporating nanoparticles into ODFs is presented in Figure 1. These techniques are employed to achieve the even dispersion of nanoparticles while preserving the film’s mechanical characteristics and overall integrity. Despite the promising potential of nanoparticle-loaded ODFs, there are challenges to address, including concerns about nanoparticle toxicity, regulatory compliance, and quality control. It is essential to thoroughly evaluate these aspects to ensure the safety and efficacy of such formulations. Several studies have suggested that formulating APIs in lipid nanoparticles can enhance their oral bioavailability [12][13]. Additionally, lipid formulations have been shown to mitigate the impact of food on API absorption [14]. The main approach to improving the bioavailability of APIs in lipid dispersions revolves primarily around creating surface-active monoglycerides. These monoglycerides, in combination with bile salts, give rise to mixed micelles that can encapsulate drug molecules. These mixed micelles are believed to facilitate the direct absorption of the API in conjunction with the lipids.
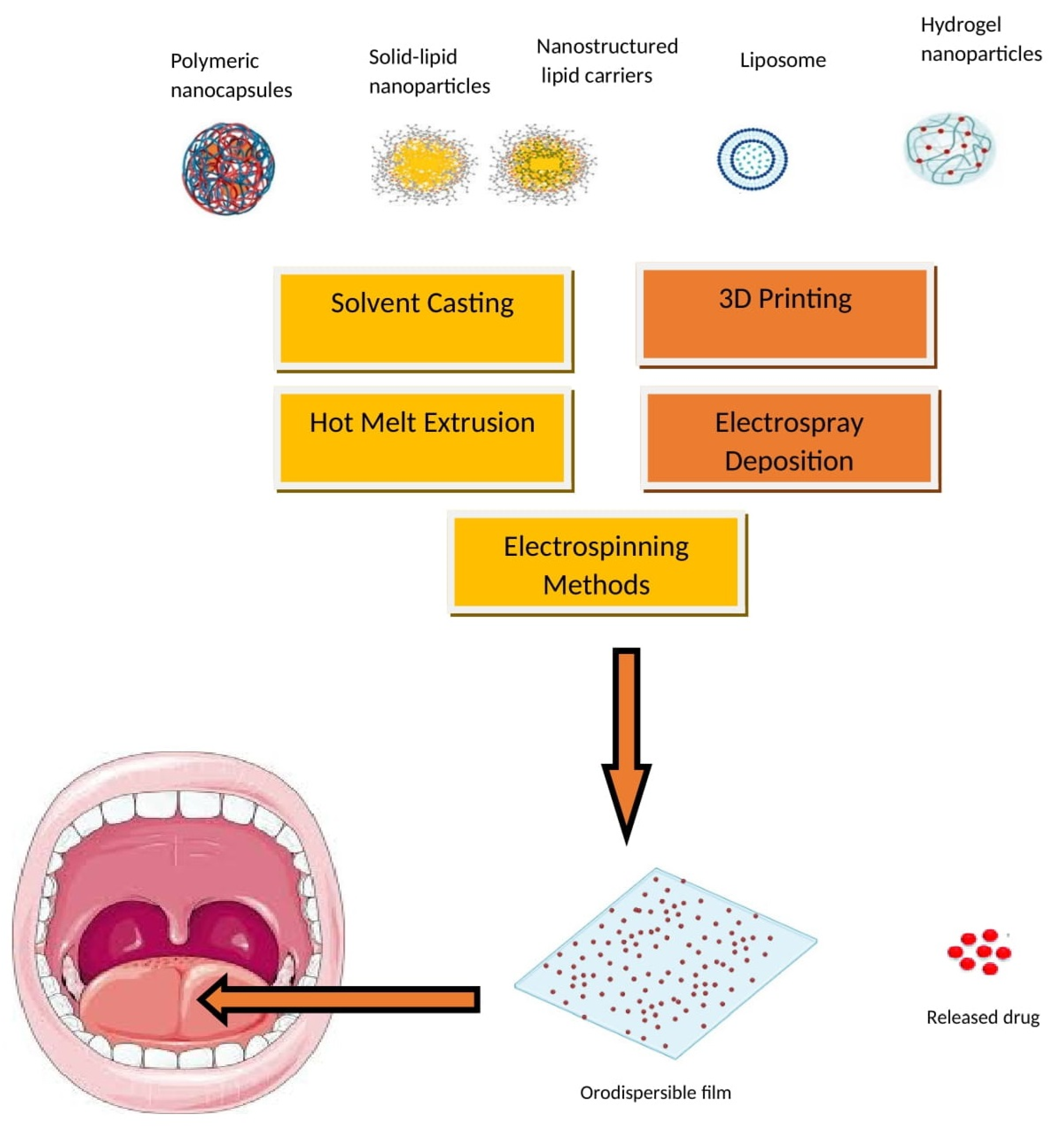
Figure 1. Nanoparticle types and incorporation methods in ODFs.
In addition, the release of bile salts can potentially improve the solubility of orally administered inadequately aqueous soluble APIs, thus facilitating their immediate absorption. While there have been limited studies on integrating solid lipid nanoparticles into mucoadhesive films, there has been even less focus on incorporating lipid microparticles into ODFs [15]. In a recent study [16], researchers explored the potential of utilizing HPMC film as a promising vehicle for solid lipid particles. Their findings demonstrated that the lipid content in the film matrix could reach as high as 54 wt.%, depending on the specific type of triglyceride nanoparticles integrated. To create lipid dispersions for inclusion in ODFs, a high-pressure homogenization process was employed, with formulations composed of 10% lipid, 5% stabilizer, and 0.5% sodium dodecyl sulfate (SDS). Some formulations solely relied on the surfactant SDS for stabilization, with a composition of 10% lipid and 0.5% SDS. During the evaluation of the stabilizers, various particle formulations were tested for three different triglycerides (tristearin, tripalmitin, and trimyristin). The most promising outcomes were observed with combinations of a polymer and the surfactant SDS, as well as formulations containing only SDS. Two specific particle formulations, HPMC/SDS and SDS, stood out as highly promising due to their ability to achieve substantial lipid contents and produce high-quality films. For tristearin, lipid contents of up to 0.50 were achievable in the ODFs with HPMC/SDS stabilization, and they were up to 0.54 for the SDS-based formulation. It is worth noting that the maximum lipid load was lower for the films loaded with tripalmitin (0.40) and trimyristin (0.29) compared to tristearin. A noteworthy aspect was the successful redispersal of lipid nanoparticles from the film matrix without compromising their nanoparticulate properties. Additionally, the films exhibited commendable mechanical properties and disintegration times. Moreover, this study demonstrated the feasibility of substantially stabilizing lipid particles in the metastable α-polymorphic form for a duration of at least 6 months when utilizing HPMC/SDS to stabilize the triasterin suspension [16]. This breakthrough discovery opens up new possibilities for leveraging HPMC films to enhance the delivery of lipid-based drug formulations.
One innovative technique involves integrating a self-micro emulsifying system into the film, as previously explored [17]. However, this method demands a substantial quantity of oil, posing a challenge to the long-term integrity and stability of the film. To address this concern, another strategy was employed, i.e., reducing the drug to the nano-scale before its incorporation into ODFs. Research has shown a remarkable 11-fold increase in the in vitro drug release from polymeric films embedded with nanoparticles in comparison to the pure drug [18]. Other researchers have reported identical improvements in ex vivo permeation, emphasizing the substantial promise of using nanoparticle-loaded ODFs for the delivery of poorly soluble compounds [19][20]. In a recent study, scientists employed the Box–Behnken method to design and fabricate ODFs loaded with ketoprofen nanoparticles [21]. The amorphous state of drug-embedded nanoparticles within the ODFs was verified through the absence of distinct crystalline patterns and the absence of endothermic peaks of ketoprofen in X-ray diffraction and modulated differential scanning calorimetry analyses, respectively. The optimized formulation demonstrated a nearly fourfold increase in permeability compared to pure drugs. Furthermore, the dissolution rates of the drug and the optimized drug-containing ODF in a pH 1.2 solution reached approximately 30% and 95% at the 60 min mark, respectively. Pharmaceutical nanosuspensions are necessary when dealing with problematic drug molecules that cannot form salts, have high molecular weights, require a large dose, possess high log p-values, and have high melting points, as these factors make it difficult to create suitable drug formulations using other methods. A variety of techniques are employed to create nanosuspensions with diverse particle sizes. These methods include top-down approaches like dry and wet milling, co-grinding, and high-pressure homogenization, as well as bottom-up techniques such as antisolvent precipitation, liquid emulsion, and sonoprecipitation methods [22]. Nanosuspension-based oral thin films offer a promising approach to drug delivery, addressing the challenges associated with solubility, bioavailability, and patient compliance for a variety of pharmaceutical compounds. Fast-dissolving oral films of buspirone were created by employing the solvent evaporation method to transform a nanosuspension with the film-forming agents HPMC E5 and PVA. Buspirone oral films exhibited remarkable physical and mechanical characteristics and displayed good stability, and the in vitro assessments revealed an initial rapid drug release followed by a sustained release [23]. The inclusion of nanoparticles in oral films was anticipated to improve the dissolution and permeability properties of various poorly water-soluble drugs. A fast-dissolving oral film containing the poorly aqueous soluble and low bioavailable drug, lercanidipine, was fabricated as nanoparticles through the antisolvent evaporation technique. These formulations demonstrated a substantial enhancement in the in vitro dissolution rate and ex vivo permeation [23].
Pharmacokinetic investigations involving lutein nanocrystals in fast-dissolving oral films in rats demonstrated a significant reduction in the Tmax and a substantial increase in the Cmax compared to the oral solution. Additionally, the AUC0–24 h of nanocrystal fast-dissolving oral films was approximately two times larger than that of the oral solution, confirming a substantial enhancement in both the rate and extent of bioavailability [24].
A research study explores the application of the wet-milling technique to produce the nanosized hydrophobic drug loratadine, resulting in enhanced solubility and quicker dissolution [25]. These nanosized materials (<400 nm) were then employed in the formulation of ODFs that disintegrate rapidly, typically in less than 60 s. Although the drug’s crystalline structure remains unaltered, there is a significant improvement in its bioavailability. When administered to rats, the nanocrystal ODF leads to a 5.69-fold increase in the AUC0–24 h compared to the original drug, with a faster onset of action, typically within 30 min. The simplification of the formulation process and the enhancements in drug solubility and bioavailability have positioned the ODF as a promising drug delivery method for loratadine. In another interesting investigation, poorly aqueous soluble drugs such as fenofibrate and naproxen were formulated as nanoparticles using various strategies and were incorporated into ODFs [26]. The research findings revealed that the dose of API that can be administered in a single ODF significantly relies on the chosen formulation approach and the physicochemical characteristics of the API. The amorphous solid dispersion-ODFs and films incorporating API-loaded lipid nanoemulsions emerged as the most favorable film formulations. These formulations resulted in a decrease in the API dissolution time when compared to ODFs containing non-formulated API microparticles. Despite slightly compromised mechanical film properties compared to API-free film formulations, these ODFs achieved rapid disintegration times. For naproxen in the solid dispersion-ODFs, an API loading of up to 8 wt.% was attained without encountering recrystallization. In contrast, during the formulation of FENO in the films, the API content was limited to 2 wt.%, indicating unique intermolecular interactions between the APIs and the film-forming matrix.
References
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411.
- He, M.; Zhu, L.; Yang, N.; Li, H.; Yang, Q. Recent advances of oral film as platform for drug delivery. Int. J. Pharm. 2021, 604, 120759.
- Lee, Y.; Kim, K.; Kim, M.; Choi, D.H.; Jeong, S.H. Orally disintegrating films focusing on formulation, manufacturing process, and characterization. J. Pharm. Investig. 2017, 47, 183–201.
- Jacob, S.; Nair, A.B.; Boddu, S.H.S.; Gorain, B.; Sreeharsha, N.; Shah, J. An Updated Overview of the Emerging Role of Patch and Film-Based Buccal Delivery Systems. Pharmaceutics 2021, 13, 1206.
- Food and Drug Administration. Guidance for Industry: Orally Disintegrating Tablets; Food and Drug Administration: Silver Spring, MD, USA, 2008.
- Agency, E.M. Guideline on Pharmaceutical Development of Medicines for Paediatric Use; European Medicines Agency: London, UK, 2013.
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574.
- Borges, A.F.; Silva, C.; Coelho, J.F.; Simões, S. Oral films: Current status and future perspectives: I—Galenical development and quality attributes. J. Control. Release 2015, 206, 1–19.
- Jacob, S.; Nair, A.B.; Shah, J.; Gupta, S.; Boddu, S.H.S.; Sreeharsha, N.; Joseph, A.; Shinu, P.; Morsy, M.A. Lipid Nanoparticles as a Promising Drug Delivery Carrier for Topical Ocular Therapy-An Overview on Recent Advances. Pharmaceutics 2022, 14, 533.
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Patel, S.S.; Morsy, M.A.; Patel, V.; Chavda, V.; Jacob, S.; Sreeharsha, N.; Shinu, P. Development of asialoglycoprotein receptor-targeted nanoparticles for selective delivery of gemcitabine to hepatocellular carcinoma. Molecules 2019, 24, 4566.
- Kotta, S.; Aldawsari, H.M.; Badr-Eldin, S.M.; Nair, A.B.; Yt, K. Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics 2022, 14, 1636.
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997.
- Kamboj, S.; Bala, S.; Nair, A.B. Solid lipid nanoparticles: An effective lipid based technology for poorly water soluble drugs. Int. J. Pharm. Sci. Rev. Res. 2010, 5, 78–90.
- Vinarov, Z.; Abrahamsson, B.; Artursson, P.; Batchelor, H.; Berben, P.; Bernkop-Schnürch, A.; Butler, J.; Ceulemans, J.; Davies, N.; Dupont, D.; et al. Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network. Adv. Drug Deliv. Rev. 2021, 171, 289–331.
- Tzanova, M.M.; Hagesaether, E.; Tho, I. Solid lipid nanoparticle-loaded mucoadhesive buccal films—Critical quality attributes and in vitro safety & efficacy. Int. J. Pharm. 2021, 592, 120100.
- Steiner, D.; Emmendörffer, J.F.; Bunjes, H. Orodispersible Films: A Delivery Platform for Solid Lipid Nanoparticles? Pharmaceutics 2021, 13, 2162.
- Talekar, S.D.; Haware, R.V.; Dave, R.H. Evaluation of self-nanoemulsifying drug delivery systems using multivariate methods to optimize permeability of captopril oral films. Eur. J. Pharm. Sci. 2019, 130, 215–224.
- Shankar Raman, S.; Narayanan, V.H.B.; Durai, R. Lamotrigine Nanoparticle Laden Polymer Composite Oral Dissolving Films for Improving Therapeutic Potential of the Hydrophobic Antiepileptic Molecule. Assay Drug Dev. Technol. 2021, 19, 2–16.
- Steiner, D.; Finke, J.H.; Kwade, A. Model-based description of disintegration time and dissolution rate of nanoparticle-loaded orodispersible films. Eur. J. Pharm. Sci. 2019, 132, 18–26.
- Sinha, S.; Sonali; Garg, V.; Thapa, S.; Singh, S.; Chauhan, M.; Dutt, R.; Singh, R.P. Empagliflozin containing chitosan-alginate nanoparticles in orodispersible film: Preparation, characterization, pharmacokinetic evaluation and its in-vitro anticancer activity. Drug Dev. Ind. Pharm. 2022, 48, 279–291.
- Shah, H.G.; Rathod, V.; Basim, P.; Gajera, B.; Dave, R.H. Understanding the Impact of Multi-factorial Composition on Efficient Loading of the Stable Ketoprofen Nanoparticles on Orodispersible Films Using Box-Behnken Design. J. Pharm. Sci. 2022, 111, 1451–1462.
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3.
- Bharti, K.; Mittal, P.; Mishra, B. Formulation and characterization of fast dissolving oral films containing buspirone hydrochloride nanoparticles using design of experiment. J. Drug Deliv. Sci. Technol. 2019, 49, 420–432.
- Liu, C.; Chang, D.; Zhang, X.; Sui, H.; Kong, Y.; Zhu, R.; Wang, W. Oral fast-dissolving films containing lutein nanocrystals for improved bioavailability: Formulation development, in vitro and in vivo evaluation. AAPS PharmSciTech 2017, 18, 2957–2964.
- Van Nguyen, K.; Nguyen, H.T.; Nghiem, L.H.T.; Van Can, M.; Tran, T.H. Nanosized-Loratadine Embedded Orodispersible Films for Enhanced Bioavailability: Scalable Preparations and Characterizations. AAPS PharmSciTech 2022, 23, 78.
- Steiner, D.; Tidau, M.; Finke, J.H. Embedding of Poorly Water-Soluble Drugs in Orodispersible Films-Comparison of Five Formulation Strategies. Pharmaceutics 2022, 15, 17.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
28 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




