You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Linda Abenaim | -- | 5052 | 2023-12-28 00:24:23 | | | |
| 2 | Linda Abenaim | + 3545 word(s) | 8597 | 2023-12-28 00:26:53 | | | | |
| 3 | Camila Xu | -53 word(s) | 8544 | 2024-01-18 03:51:49 | | | | |
| 4 | Camila Xu | -3545 word(s) | 4997 | 2024-01-18 04:00:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Abenaim, L.; Conti, B. Chitosan as a Control Tool for Insect Pest. Encyclopedia. Available online: https://encyclopedia.pub/entry/53199 (accessed on 16 December 2025).
Abenaim L, Conti B. Chitosan as a Control Tool for Insect Pest. Encyclopedia. Available at: https://encyclopedia.pub/entry/53199. Accessed December 16, 2025.
Abenaim, Linda, Barbara Conti. "Chitosan as a Control Tool for Insect Pest" Encyclopedia, https://encyclopedia.pub/entry/53199 (accessed December 16, 2025).
Abenaim, L., & Conti, B. (2023, December 28). Chitosan as a Control Tool for Insect Pest. In Encyclopedia. https://encyclopedia.pub/entry/53199
Abenaim, Linda and Barbara Conti. "Chitosan as a Control Tool for Insect Pest." Encyclopedia. Web. 28 December, 2023.
Copy Citation
Chitosan is a biopolymer derived from chitin that gained much attention due to its biological activities. Chitosan can be produced by the exoskeleton of arthropods (crustaceans and insects) and structural membranes and spores of fungi. Its application has spread to many sectors, including pharmaceutical, medical, veterinary, food and agricultural. In the latter one, chitosan is applied to improve the interaction between plants and microorganisms and metabolisms of plants, fruits and germination. In addition, chitosan is demonstrated to enhance the availability and stabilization of insecticides and essential oils. Several chitosan formulations were studied as tools for insect pest control.
biopolymer
chitin
insect control
chitosan formulations
chitosan nanoparticles
1. Introduction
In recent decades, research has increasingly sought alternative, biodegradable, and ecological materials for various scientific applications. Biopolymers such as cellulose, starch, gelatines, and chitosan have gained much attention due to their valuable characteristics, including biodegradability, biocompatibility, and non-toxicity for humans [1][2]. Chitosan is the most important derivative of chitin. It was discovered in mushrooms by Henri Braconnot back in 1811, who named it “fungine”. Later, in 1821, Auguste Odier isolated the same compound from beetle cuticles and named it “chitin” [3][4].
Chitin was, thus, the first polysaccharide identified by man, preceding cellulose by about 30 years, and the most abundant polymer after the latter. Indeed, chitin is the most abundant component in the exoskeleton of arthropods and in the structural membranes and spores of fungi [2][5]. Shrimps and crabs, characterized by a high chitin content (around 20–30%), are the most common commercial sources of this polysaccharide, although their use as a source of chitin has been much debated recently [6][7].
As the global demand for chitosan is increasing, with an annual growth rate of 15.4%, there is a need for alternative sources to meet the market demand. In recent years, research has also focused on the study of insects, characterized by 10–15% of chitin, as an alternative source of the latter [8]. The extraction of chitin from insects is also advantageous in terms of the rearing method, substance consumption, and time of production [9]. The most investigated insect species for chitosan production are Hermetia illucens (Linnaeus, 1758) (Diptera Stratiomyidae), Tenebrio molitor (Linnaeus, 1758) (Coleoptera Tenebrionidae), and Alphitobius diaperinus (Panzer, 1797) (Coleoptera Tenebrionidae). Chitosan production from chitin, regardless of the source, is mainly characterized by three steps of extraction, as follows: demineralization, deproteinization, and deacetylation [2][9]. However, different conditions during the extraction process, such as concentration of reagents, time and temperature, pH, presence, or absence of metal cations, pKa, molecular weight, and degree of deacetylation, may affect the properties of the biopolymer [10].
Chitosan has well-known antimicrobial, antioxidant, and antitumoral properties [10][11][12], and its application has spread to many sectors, including the pharmaceutical, medical, veterinary, food, and agricultural sectors. In the pharmaceutical industry, chitosan is used in drug formulas to prevent stomach irritation thanks to its antacid and antiulcer properties [2]. In the cosmetic industry, it is used for hair and skin treatments; in medicine, it is used for orthopaedic and periodontal implants, and, in the textile field, it is employed for its anti-static properties and in water purification [1][2][13]. In animal nutrition, chitosan is used as an antimicrobial to protect feed from Escherichia coli and Salmonella spp. contamination, while in the food industry it is used for food packaging [14]. In agriculture, chitosan is applied to soil, promoting symbiotic interaction between plants and microorganisms and improving the metabolisms of fruits and plants and germination [2][13].
2. Use of Chitosan against Insects
Chitosan can enhance the availability and stabilization of some insecticides or botanicals, while some chitosan derivatives have demonstrated insecticide activity towards various agricultural pests [12][15]. For this reason, chitosan is currently authorised in the EU as an active and non-toxic basic substance for plant protection as an environmentally friendly and safe alternative to synthetic pesticides (Regulation EC No 1107/2009) [16][17]. Various formulations of chitosan, supplemented with natural or synthetic insecticides, were studied as insect pest control tools in agriculture and public health but also as instruments for protecting foodstuffs [18][19][20][21]. In particular, the polymer has been used in a variety of formulations, including a plain chitosan formulation sprayed on leaves, wood, and paper, chitosan derivatives, chitosan–metal complexes, chitosan nanoparticles loaded with essential oils (EOs) and insecticides, chitosan coating, and chitosan formulations with nematodes.
The use of chitosan in different formulations has been the subject of many studies. Thus, the purpose of this research is to provide the reader with updated information concerning the use and potential applications of chitosan formulations, summarized in Table 1, as pest control tools.
Table 1. Chitosan formulations as insect pest control strategies in the literature from the last 20 years (from 2003 to 2023).
| Chitosan Formulation | Insect Order | Insect Species/Family | Target Instars |
References |
|---|---|---|---|---|
| Plain chitosan incorporated into a diet |
Diptera | Musca domestica (Muscidae) | Adults | [22] |
| Tabanus nigrovittatus (Tabanidae) | Adults | [22] | ||
| Phormia regina (Calliphoridae) | Adults | [22] | ||
| Plain chitosan added to paper and wood | Blattodea | Reticulitermes virginicus (Rhinotermitidae) | Adults | [23] |
| Reticulitermes flavipes (Rhinotermitidae) | Adults | [24] | ||
| Plain chitosan coating | Diptera | Anastrepha ludens (Tephritidae) | Larvae | [25] |
| Anastrepha obliqua (Tephritidae) | Adults | [26] | ||
| Chitosan coating with EOs | Lepidoptera | Plutella xylostella (Plutellidae) | Adults | [27] |
| Hemiptera | Myzus persicae (Aphididae) | Adults | [27] | |
| Diptera | Bactrocera carambolae (Tephritidae) |
Adults | [28] | |
| Calliphora vomitoria (Calliphoridae) |
Adults | [29] | ||
| Coleoptera | Acanthoscelides obtectus (Bruchidae) | Adults | [30] | |
| Sitophilus oryzae (Curculionidae) | Adults | [31] | ||
| Chitosan coating in active packaging |
Coleoptera | Sitophilus zeamais (Curculionidae) |
Adults | [32] |
| Chitosan with nematodes |
Coleoptera | Ryncgophorus ferrugineus (Curculionidae) |
Larvae | [33][34] |
| Chitosan oligosaccharides |
Lepidoptera | Helicoverpa armigera (Noctuidae) | Larvae | [35] |
| Plutella xylostella (Plutellidae) | Larvae | [35] | ||
| Hemiptera | Sitobion avenae (Aphididae) | Adults | [33] | |
| Metopolophium dirhodum (Aphididae) | Adults | [35] | ||
| Myzus persicae (Aphididae) | Adults | [35] | ||
| Hyalopterus pruni (Aphididae) | Adults | [35] | ||
| Aphis gossypii (Aphididae) | Adults | [35] | ||
| Rhopalosiphum padi (Aphididae) | Adults | [35] | ||
| Chitosan derivatives | Lepidoptera | Spodoptera littoralis (Noctuidae) | Larvae | [36][37][38] |
| Chitosan–metal complexes |
Lepidoptera | Spodoptera littoralis (Noctuidae) | Larvae | [39] |
| Hemiptera | Aphis nerii (Aphididae) | Adults | [39] | |
| Chitosan nanoparticles loaded with EOs | Coleoptera | Tribolium castaneum (Tenebrionidae) | Adults | [20][21][40][41][42] |
| Sitophilus oryzae (Curculionidae) | Adults | [21] | ||
| Oryzaephilus surinamensis (Silvanidae) |
Adults | [43] | ||
| Carpophilus hemipterus (Nitidulidae) |
Adults | [44] | ||
| Diptera | Anopheles stephensi (Culicidae) | Larvae | [45] | |
| Culex pipiens (Culicidae) | Larvae | [46] | ||
| Aedes aegypti (Culicidae) | Larvae | [19][47] | ||
| Musca domestica (Muscidae) | Adults | [48] | ||
| Hemiptera | Bemisia tabaci (Aleyrodidae) | Adults | [49] | |
| Chitosan nanoparticles loaded with agrochemicals |
Lepidoptera | Helicoverpa armigera (Noctuidae) | Larvae | [18] |
| Spodoptera litura (Noctuidae) | Larvae | [50] | ||
| Diptera | Drosophila melanogaster (Drosophilidae) |
Adults/ larvae |
[51] | |
| Chitosan-g-poly acrylic acid nanoparticles | Hemiptera | Aphis gossypii (Aphididae) | Adults | [52] |
| Coleoptera | Cassida vittata (Chrysomelidae) | Larvae | [53] | |
| Myristic acid chitosan nanoparticles | Coleoptera | Sitophilus granarius (Curculionidae) |
Adults | [54][55] |
| Tribolium confusum (Tenebrionidae) |
Adults | [54][55] | ||
| Chitosan nanoparticles RNAi | Diptera | Anopheles gambie (Culicidae) | Larvae | [56][57] |
| Aedes aegypti (Culicidae) | Larvae | [57][58] |
2.1. Plain Chitosan
Recently, some papers have pointed to chitosan as a tool for controlling insect pests in agriculture, foodstuffs, and public health. However, the insecticidal activity seems related to the antimicrobial effect of the chitosan that impairs the function of the insects’ gut microbiota.
In fact, Stoffolano and co-authors reported that a 2% chitosan solution incorporated in an artificial diet induced a statistically significant reduction in the survival period of Musca domestica (Linnaeus, 1758) (Diptera Muscidae) from 13 to 4 days in the control diet. This trend is also confirmed for Tabanus nigrovittatus (Macquart, 1847) (Diptera Tabanidae) (from 16 to 4.5 days) and for Phormia regina (Meigen, 1826) (Diptera Calliphoridae) (from 24 to 6 days). The insect mortality shown in this study appears to be due to physiological and structural changes in the midgut tract, even if the mode of action has not yet been clarified [20].
Even Muryeti and co-authors [59] reported that milled paper supplemented with chitosan and administered to termites showed an increase in their mortality compared to the control (80 and 45% mortality, respectively). This seems to be due to the fact that chitosan paper interferes with termite feeding, disturbing the ability of symbiotic protists in the termites’ digestive tract. This prompted the research to discover the effect of chitosan on the diversity and number of protists in termites’ guts. Chitosan was also reported as being potentially effective in preserving wood against termites [60]. In particular, in the work of Raji et al. [24], chitosan-treated wood showed a statistically significant mortality against subterranean termites of more than 94% for Reticulitermes flavipes (Kollar, 1837) (Blattodea, Rhinotermitidae) when exposed for 28 days to the chitosan (2% of concentration). On the contrary, for the other species tested, such as R. virginicus, more than 90% mortality was obtained at the lowest concentrations of chitosan (0.5%). Moreover, in Telmadarrehei et al. [23], the effect of chitosan wood treatment was investigated against the protists of Reticulitermes virginicus (Banks, 1907) (Blattodea, Rhinotermitidae). From the results, termites of the control group showed ten protist species in the hindgut, while in the specimens treated with chitosan the presence of protists was reduced to only two species.
2.2. Chitosan Coating
Because of its film-forming and biochemical capabilities, chitosan is a good preservative for fresh fruits and vegetables, according to numerous studies [61][62]. Furthermore, besides its antimicrobial activity, unlike other coating materials, plain chitosan displays antifungal activity against a variety of fungi, including Aspergillus niger, Botrytis cinetica, and Rhizopus stolonifer [63][64].
Plain chitosan coating is also safe for humans [65] and semi-permeable and has the potential to change the internal atmosphere of food, e.g., by minimizing transpiration losses, delaying spoilage, and, thus, extending the shelf-life of various foods [66][67].
In agriculture, plain chitosan coatings have also been tested for seed and fruit treatment for plant growth stimulation and disease control [61][68]. In particular, some studies have reported that chitosan coating repels insects and inhibits the settling of certain developmental instars. Indeed, in Salvador-Figueroa et al. [25] and Limon et al. [26], a chitosan coating on mango fruit inhibited the development of eggs and larvae of Mexican fruit flies, Anastrepha ludens (Loew, 1873) and Anastrepha obliqua (Macquart, 1835) (Diptera Tephritidae). In both cases, the chitosan coating significantly slowed the ripening rate and reduced the weight of the fruit. Regarding the mode of action, the authors [25][26] indicated an increase in the concentration of phenolic compounds and in the gas exchange of the fruit, both responsible for the inhibition of the development of eggs and larvae. In addition, due to the different colours and penetrability of the treated fruits, the chitosan coating behaved as a barrier, reducing the fruits’ attractiveness to pests and inhibiting the insects’ oviposition [69].
2.3. Chitosan Coating with Essential Oils
In the relevant literature from the last 30 years, essential oils (EOs) have been proposed as eco-friendly insecticides and/or repellents for insects [70][71][72][73][74][75] even in different formulations [76]. However, they present some use limitations due to their pronounced volatility. Chitosan nanoparticles loaded with EOs can improve their bioactivity and stability by decreasing their volatility. Indeed, EOs, known for their insecticidal and insect-repellent activity, added to chitosan’s results in increases in their bioactivity. In fact, by applying on the fruit of Psidium guajava (common guava) the film produced by adding 2% of citronella EOs and, as scaffolding excipients, polyethylene glycol (PEG) and carboxymethylcellulose (CMC), the oviposition of Bactrocera carambolae (Drew & Hancock, 1994) (Diptera Tephritidae) was significantly inhibited by 85% when compared to the control [28]. In a similar work [27], cabbage seeds coated with chitosan, enriched with jasmonic acid, induced a 57% preimaginal mortality of Plutella xylostella (Linnaeus, 1758) (Lepidoptera Plutellidae) and Myzus persicae (Sulzer, 1776) (Hemiptera, Aphididae) before reaching adulthood, preventing adult emergence.
The coating of chitosan loaded with different EOs has demonstrated antifeedant and anti-toxic properties, enhancing EOs’ effectiveness, durability, and availability. In fact, in Ascrizzi et al. [30], the chitosan coating film, obtained with the addition of Ferulago campestis EO, tested on bean seeds showed a statistically significant dose-dependent repellent effect (93.3% repellence at the highest concentration 57.7 µL/L of air) against the seed pest Acanthoscelides obtectus (Say, 131) (Coleoptera Bruchidae), without leading to adverse effects on bean plant germination and growth but resulting, at the same time, effective in suppressing weed seed germination in vitro.
For the protection of fresh foods, chitosan loaded with EOs has been tested as a protective coating to preserve meat against the oviposition of Calliphora vomitoria (Linnaeus, 1758) (Diptera Calliphoridae) [29]. From the results, chitosan at a 1% loaded with 0.1% of L. nobilis and P. nigrum EOs (chosen following the sensory profile analysis) showed a protective effect on meat with statistically significant oviposition reduction in C. vomitoria of 84.9 and 93.3%, respectively, for the two EOs compared to the control. Moreover, the treatment was effective in preserving the quality and shelf-life of the meat, delaying meat desiccation and lipid peroxidation by 7 days.
Hossain et al. [31] developed a chitosan coating containing eight different EOs also in combination with ionizing radiation (specifically gamma radiation at 100 and 300 Gy). The eucalyptus and tea tree EOs loaded in a chitosan coating in rice showed the most effectiveness towards Sitophilus oryzae (Linnaeus, 1758) (Coleoptera Curculionidae), causing 100% mortality at the lowest concentrations of 0.2 µL/mL for an incubation period between 24 and 48 h. With the combination of gamma radiation, the mortality of insects was 100% even after 14 days of treatment, providing complete pest control for the food’s packaging.
2.4. Chitosan Coating in Active Packaging
Chitosan can also be used as a secondary compound in active packaging systems to control foodstuff insect pests [77]. Active packaging refers to the incorporation of some active compounds into the packaging material, in this case chitosan-based, to maintain the quality and extend the shelf-life of food products. Recently, De Fàtima Silva et al. [32] developed a sustainable active packaging material based on the coating of a cardboard surface with chitosan and lemongrass EO, and they evaluated the insecticidal activity towards Sitophilus zeamais (Motschulsky, 1885) (Coleoptera, Curculionidae). In particular, in this formulation, chitosan, thanks to its amino groups, formed hydrogen bonds with the hydroxyl groups of the cardboard cellulose, providing good strength for the paper and improving the barrier properties of the paper matrix whilst conserving biodegradability, while the EO contributed to an anti-insect effect. In fact, the active packaging material was fully effective against the maize weevil attack, with 100% toxicity observed even after 360 h from the treatment. Moreover, the air permeability and water absorption capacity of the active packaging material were improved compared to the uncoated packaging.
2.5. Chitosan and Nematodes
Entomopathogenic nematodes (EPNs) are used in traditional, conservation, and supplemental biological control programs [78][79]. Most of the existing applied research concerns their potential as biological control agents [80][81][82]. According to Abbas et al. [83], Steinernema carpocapsae is the most available, versatile, and effective EPN, mutualistically associated with Xenorhabdus nematophila, against Rhynchophorus ferrugineus (Oliver, 1790) (Coleoptera Curculionidae). Recent laboratory and semi-field investigations have demonstrated the efficacy of combining S. carpocapsae with chitosan against R. ferrugineus on a Phoenix canariensis palm [33]. According to these results, in both Llácer et al. [33] and Dembilio et al. [34], chitosan activated the defence mechanisms in the palm, increasing lignification and root development. The chitosan formulation with nematodes, already patented in Spain [84], showed interesting results through curative assays (81.3% larval mortality after 28 days of application) and preventive assays (98.2% immature stage infestation after 15 days of application) [33]. Consistent with these results, Dembilio et al. [34] found 99.7% mortality of the immature stages of R. ferrugineus in a palm stipe treated with S. carpocapsae formulated with chitosan.
2.6. Chitosan Chemical Modification
Since chitosan itself has no toxic properties, its use against harmful insects is usually as an additive to other toxic substances. Nevertheless, its use in water is often limited due to its intermolecular and intramolecular hydrogen bonds, which are highly crystalline, hindering the material’s ability to interact with water molecules [85]. For this reason, research has turned to the study of new derivatives and chemical modifications of chitosan that can compensate for this lack of solubility and toxicity and improve its physicochemical properties in order to broaden its applications. An example is the chitosan oligosaccharide (COS) (Figure 1), which is produced using various methods, including enzymatic and acid hydrolysis, which convert chitosan to low molecular weight units and provide it with the ability to be soluble in aqueous solutions. These properties of chitosan oligosaccharides also improve the biological activities of chitosan, as reported in numerous studies. In particular, the properties of chitosan derivatives have been studied against certain insects harmful to agriculture [86][87][88]. Zhang et al. [35] reported the insecticidal activity of a chitosan oligosaccharide solution sprayed on the leaves of plants attacked by some of the most common pests in agriculture, including the Lepidoptera Helicoverpa armigera (Hübner, 1808) (Noctuidae) and P. xylostella and the Hemiptera Aphididae Rhopalosiphum padi (Linnaeus, 1758)), Sitobion avenae (Fabricius, 1775), Metopolophium dirhodum (Walker, 1849), M. persicae, Hyalopterus pruni (Geoffroy, 1762), and Aphis gossypii (Glover,1877). The results obtained using the chitosan solution showed an insecticidal activity of 40% for H. armigera and 72% for P. xylostella after 72 h. The treatment was proved to also be effective against aphids, especially against H. pruni, (93% mortality).
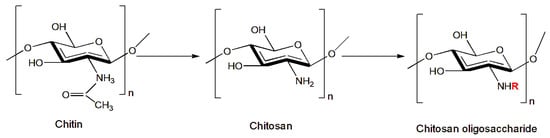
Figure 1. Formation of chitosan oligosaccharide (COS). R is replaced with H or an acetyl group depending on the degree of deacetylation (DD).
Other authors have demonstrated that chitosan derivatives, with the introduction of functional groups at the N- and O- positions (Figure 2), show improved biodegradability and chemical solubility in water or organic solvents as well as enhance chitosan’s antimicrobial properties [89].
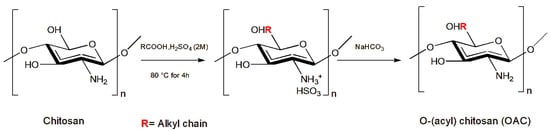
Figure 2. Synthesis of OAC. Redesigned from [38].
The new chitosan derivatives N-alkyl chitosan (NAC), N-benzyl chitosan (NBC), and O-(acyl) chitosan (OAC) were administrated in an artificial diet to the larvae of the Lepidoptera Noctuidae Spodoptera littoralis (Boisduval, 1833). Among the chitosan derivatives tested, N-(2-chloro-6-fluorbenzyl) chitosan was the most effective against S. littoralis larvae (LC50 of 0.32 g/kg and LC100 of 0.625 g/kg of diet), even if not statistically significant [36]. Moreover, N-(propyl) chitosan, N-(undecanyl) chitosan, N-(3-phenyl propyl) chitosan [37], and O-(decanoyl) chitosan [38] showed larval growth inhibition with larval weight reductions of 76, 66, 65, and 64%, respectively, after 4 days of administration, even if the results were not statistically significant.
Moreover, chitosan complexes with metals modify both the activity and the physiochemical characteristics of plain chitosan [90]. As a chelating agent, chitosan may easily form complexes with transition or heavy metals (Figure 3). Metal ions such as Ag+, Cu2+, Zn2+, and others are known to be antimicrobial agents [91]. In this regard, some studies have focused on the antibacterial properties of chitosan–metal complexes. In fact, in Wang et al. [92], chitosan–metal complexes exhibited pronounced antimicrobial activities indicating that the inhibitory effect of the chitosan–metal complexes depended on the property of the metal ions and their molecular weight. Knowledge of the mode of action of these complexes against insects has also been considered of great interest. Metal complexes like AgNO3, CuSO4-5H2O, NiCl2-6H2O, and HgCl2 were added to a solution of different molecular weights of chitosan to obtain some chitosan–metal complexes that were then tested against S. littoralis and Aphis nerii (Boyer de Fonscolombe, 1841) (Hemiptera Aphididae) [39]. After 7 days of administration to S. littoralis larvae, the chitosan at a low molecular weight (2.27 × 105 g/mol) incorporated in the artificial diet exhibited 50% larval mortality, while the Chitosan–Ni and Chitosan–Hg complexes exhibited significantly higher larval mortality (93 and 83%, respectively) when compared to the control but also to the other chitosan–metal complexes. Instead, for A. nerii, the three solutions of chitosan at different molecular weights (2.27 × 105, 3.60 × 105, and 5.97 × 105 g/mol) all had significant high efficacy (96, 87, and 100%, respectively) when compared to the chitosan–metal complexes. However, against A. nerii, the chitosan–Cu complex was the statistically significant most effective chitosan–metal complex, resulting in a 94% mortality at 48 h.
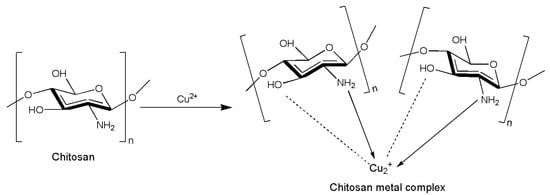
Figure 3. Possible formation of a chitosan–metal complex. Redesigned from Wang et al. [92].
2.7. Chitosan Nanoparticles
Polymeric nanoparticles have recently gained popularity in a wide variety of contexts, mainly in the agricultural sector [15][93][94]. In particular, chitosan nanoparticles are being investigated as carriers of active substances, thanks to their controlled delivery systems, and for the stabilization of biological components such as proteins, peptides, or genetic material [95].
Ionotropic gelation, polyelectrolyte complex, microemulsion, emulsion solvent diffusion, and inverse micellar techniques are the systems employed to synthesize chitosan nanoparticles. However, the most common techniques are the first two mentioned above [96].
According to their morphology, nanoparticles are divided into two major groups: nanospheres, solid structures with a homogeneous matrix in which the active ingredient uniformly coats them externally, and nanocapsules, which are hollow structures with a polymer membrane and an inner core containing the active ingredient [97]
With diameters ranging from 1 to 100 nm, the size, shape, surface area, and area/volume ratio of nanoparticles are crucial characteristics for their successful use in pest control. The smaller size of nanoparticles (Figure 4) ensures better coverage, permeability, and bioavailability of the active ingredient, and it also enhances water solubility for otherwise-insoluble active ingredients, formulation stability, slow-release capability, resistance to degradation, mobility, and increased insecticidal activity. Consequently, nanometre-sized particles are the most commonly utilized in chitosan formulations [93].
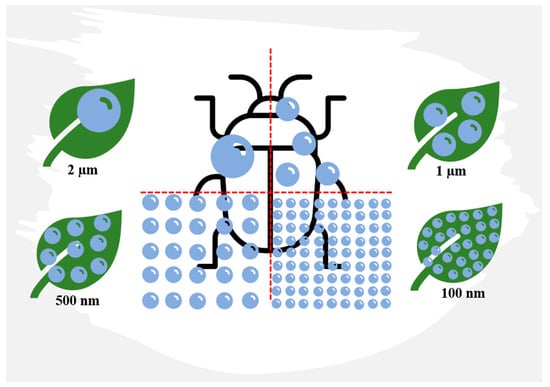
Figure 4. The ratio of particle size to the surface area to be treated. The smaller the particle size, the better the coverage, permeability, and bioavailability of the active ingredient as a tool for pest control. Redesigned from An et al. [94].
One of the most common uses of this formulation is loading chitosan nanoparticles with EOs to increase the bioactivity and stability of the oils. In Rajkumar et al. [20], chitosan nanoparticles loaded with Piper nigrum EO were evaluated against Tribolium castaneum (Herbst, 1797) (Coleoptera Tenebrionidae) and S. oryzae. In fumigation toxicity tests, carried out using an impregnated paper assay [40], chitosan nanoparticles loaded with P. nigrum EO showed statistically significant prominent mortality after 24 h with LC50 of 25.03 and 29.02 μL/L air for S. oryzae and T. castaneum, respectively, compared to the pure EO (LC50 of 48.97 and 55.77 μL/L air, respectively). Moreover, in antifeedant bioassays, the nanoparticles showed greater wheat grain protection from S. oryzae and T. castaneum attack (0% of damages observed for both species) when compared to the control (74% damages observed for S. oryzae and 86% for T. castaneum). The toxicity of chitosan nanoparticles loaded with EOs was confirmed in a later work by the same authors [21], in which chitosan nanoparticles loaded with peppermint EO (administered using the same methodology) showed a similar toxic effect with LC50 of 28.61 and 34.79 μL/L air against S. oryzae and T. castaneum, respectively, also resulting more effective when compared to the control (LC50 of 56.48 and 62.94 μL/L air, respectively). Similarly, chitosan nanoparticles loaded with the EO of Melissa officinalis showed higher statistically significant toxicity against T. castaneum by fumigation (LC50 of 0.048 μL/mL air) than the control (LC50 of 0.071 μL/mL air). The antifeeding potential of chitosan nanoparticles loaded with M. officinalis EO was tested in dedicated bioassays, which revealed that T. castaneum was deterred from feeding on the treated flour discs (maximum deterrence of 80%) [41].
Moreover, chitosan nanoparticles contribute to a slow release of EOs and prolong their effect over time, compared to the neat product. In fact, in Soltani and co-authors [42], fumigation tests with nanocapsules of chitosan loaded with Rosmarinus officinalis EO showed an extension of mortality over time of T. castaneum (20% mortality after 60 days compared to the pure EO after the same time, at 2.1%). These results were confirmed by Soltani and co-authors [43][44], who reported that the rosemary oil nanoencapsulated by chitosan exhibited 82% and 50.7% mortality after 60 days against Oryzaephilus surinamensis (Linnaeus, 1758) (Coleoptera, Silvanidae) and Carpophilus hemipterus (Linnaeus, 1758) (Coleoptera, Nitidulidae) compared to the neat EO (62% and 19% mortality, respectively). Following these results, the EO nanoencapsulation in chitosan was more effective than the neat EO even over time.
Chitosan nanoparticles loaded with EOs were also tested against mosquitoes, with good results. In fact, Elettaria cardamomum and Cinnamomum zeylanicum EOs loaded in chitosan nanoparticles showed significant larvicidal activity, after 24 h, against Anopheles stephensi (Liston, 1901) (Diptera Culicidae), with LC50 values of 7.58 and 2.98 μg/mL, respectively [45]. In other studies, chitosan nanoparticles loaded with the EOs of Geranium maculatum and Citrus bergamia showed statistically significant larvicidal activity against Culex pipiens (Linnaeus, 1758) (Diptera Culicidae) with LC50 of 22.63 and 38.52 ppm, respectively, and also mortality over time. In detail, mortality was more than 50% even 5 days after treatment [46].
Similar results were obtained with chitosan nanoparticles loaded with Lippia sidoides EO, which showed significant high larvicidal activity after 3 days (specifically 85% after 24 h and 92% after 48 and 72 h) against Ae. aegypti [47]. Likewise, in another study, chitosan nanoparticles loaded with Siparuna guianensis EO, at various concentrations, induced significant larval mortality of Ae. aegypti in a time- and dose-dependent way [19]. In this case, first, instar mosquito larvae were changed daily for 2 weeks to observe the effect of the nanoparticles over time. The nanoparticles prepared with a 1:2 ratio of chitosan/EO showed 100% mortality after 2 days at the lowest concentration (0.83 mg/mL), while the nanoparticles with the ratio 1:1 of chitosan/EO at the highest concentration (6.67 mg/mL) showed more than 80% mortality even 2 weeks after treatment.
In some cases, chitosan nanoparticles loaded with EOs or their compounds can be attractive to Bemisia tabaci (Gennadius, 1889) (Hemiptera, Aleyrodidae), with a prolonged effect on pests, as reported by De Olivera et al. [49]. In the latter’s work, chitosan nanoparticles loaded with geraniol (5 mg/mL), one of the main components of some EOs, produced a stable attractive effect even after 60 days. On the contrary, Ferreira et al. [19], obtained a high toxicity at 6.67 mg/mL of the OE of S. guianensis loaded in chitosan nanoparticles. In fact, it has been shown that the bioactivity of EOs is vectorial, i.e., it changes sign depending on the EO and on the dose used. The nanoencapsulation of limited amounts of EOs, which also results in a controlled release of their volatile components, can, therefore, turn repellence into attractiveness [98][99][100]. These results suggest that nanoencapsulation of a small amount of EOs in chitosan could be used in potential traps.
Vegetable plant extracts possess natural protective properties against pests [101]. Moreover, when combined with chitosan nanoparticles, the insecticidal effect of plant extracts appears to be enhanced. In fact, the addition of chitosan nanoparticles loaded with Nerium oleander leaf extract to an artificial diet resulted in a statistically significant larvicidal effect against M. domestica 48 h after exposure. The LC50 was 0.64 ppm, and there was a reduction in the pupation rate and adult emergence of 27% and 60%, respectively [48].
Chitosan nanoparticles have been also applied as nanocarriers for agrochemicals [102]. In this regard, the nanoencapsulation of Poneem (a botanical pesticide containing neem oil, Karanja oil, azadirachtin, and Karanjin) in chitosan was tested against H. amigera larvae [18]. The chitosan nanoparticles were obtained by tripolyphosphate (TPP) or glutaraldehyde (GLA), commonly used as crosslinking agents in chitosan nanoparticle preparation, increasing their stability. The chitosan–TPP–Poneem and chitosan–GLA–Poneem obtained showed significant antifeedant activity of larvae up to 88.5 and 72.3%, respectively, and larvicidal activity up to 90.2% for chitosan–TPP–Poneem and 87.5% for chitosan–GLA–Poneem. Like some of the botanicals contained in the agrochemicals, these nanoparticles induced growth and developmental abnormalities in the larvae and pupae.
Moreover, the chitosan nanoparticles loaded with insecticidal metabolites extracted from the fungal biocontrol agent Nomuraea rileyi and sprayed on leaves showed up to 99% larvicidal activity for the fourth larval stage of Spodoptera litura (Fabricius, 1775) (Lepidoptera Noctuidae) after 48 h from the treatment [40].
Chitosan nanoparticles have been also loaded with commercial insecticides (Spinosad and Permethrin), showing a significant dose-dependent ovicide effect against Drosophila melanogaster (Meigen, 1830) (Diptera Drosophilidae) [51].
Even chitosan-g-poly acrylic acid nanoparticles, obtained by grafting an acrylic acid monomer onto chitosan and administrated on castor leaves, negatively influence the percentage of A. gossypii growth performance and adult emergence (77.8% and 75% decrease in growth and adult emergence compared to the control) [52].
Instead, the nanochitosan-g-poly acrylic acid tested against Cassida vittata (Villers, 1789) (Coleoptera Chrysomelidae) showed a statistically significant decrease in oviposition compared to plain chitosan (3 ± 8.9 and 266 ± 8.7 eggs, respectively). The new product also induced 100% egg mortality and 91% larval mortality [53].
Self-assembled chitosan nanoparticles with fatty myristic acid (MA–chitosan nanoparticles) loaded with Cuminum cyminum EO were tested against foodstuff insect pests. The self-assembled compound showed, after 48 h, significant mortality of 100% of the Sitophilus granarius (Linnaeus, 1758) (Coleoptera Curculionidae) and Tribolium confusum (Jaquelin du Val, 1863) (Coleoptera Tenebrionidae) adults, with efficacy persistence over time (50% mortality after 12 days from exposure for S. granarius and 50% after 24 days for T. confusum) [54]. These results have been confirmed in a similar study by the same authors, where MA–chitosan nanogel loaded with Carum copticum EO was tested against the same pest species [55]. Also, in this last case, the mortality was maintained over time, with 89% and 80% mortality after 48 h for S. granaries and T. confusum, respectively, and 20% mortality after 12 days for S. granaries and 40% after 24 days for T. confusum.
Recently, chitosan nanoparticles have gained popularity as a component of small interfering RNA (siRNA) and double-strand RNA (dsRNA) formulations [15]. The cationic nature of chitosan enables the application of chitosan nanoparticles for RNAi, a non-invasive mechanism by which specific RNA fragments (dsRNA and siRNA) are able to silence gene expression in mosquito larvae by feeding [56][57][58]. In particular, RNAi technology using chitosan nanoparticles has improved its efficacy as a pest control tool, particularly for Anopheles gambie (Giles, 1902) (Diptera Culicidae) and Ae. aegypti, by interfering with the locomotion and metabolic processes responsible for growth and development.
3. Conclusions and Future Perspectives
Besides its various properties and applications, chitosan, in its different formulations, is also known as a supportive tool (generally as a matrix for controlled release compounds) for the control of many insect species harmful to agriculture, food, veterinary and public health.
Among the different formulations of chitosan (such as plain chitosan, chitosan coating and film, chitosan and nematodes, chitosan chemical modification, chitosan nanoparticles loaded with EOs, insecticides, and different metabolites) the chitosan added with EOs used as a coating film proved to be a promising technology to protect fruit, vegetables, and foodstuffs against insect pests and in most cases preserved the organoleptic characteristics and increased the shelf-life of fresh food products. This technique can be used to develop protective food coatings to discourage attack and oviposition by insect pests, but also to create an active packaging material that could prevent fungi infestation and extend the shelf-life of products.
As reported in many articles, the nanoencapsulation of EOs in a chitosan matrix is also a promising formulation against insect pests. In fact, nanoparticles exhibit greater mobility than plain materials such as EOs or insecticides, resulting in better penetration into insect tissues and increasing insecticidal activity [103]. Moreover, chitosan-based nanoparticles showed an increase in the release time of EOs or insecticides compared to starting products, allowing protection over time even at lower concentrations. In particular, the chitosan nanoparticle formulations loaded with EOs could be considered the most promising EOs application thanks to their eco-friendliness, effectiveness, protection over time, and delayed-release effect.
Unfortunately, the application of chitosan is limited by its poor solubility, which can be, however, improved by chemical modification by, for instance, introducing hydrophilic groups into the macromolecular chain of chitosan. Indeed, the chitosan derivatives NAC, NBC, and OAC with alkyl, benzyl, and acyl groups are more soluble than simple chitosan and exhibit, above all, insecticidal activity than plain chitosan. Among the different types of modified chitosan investigated, such as oligosaccharides, and chitosan-metal complexes, some could be considered suitable in terms of applicability, effectiveness, and eco-friendliness.
In conclusion, despite the positive results reported here, and available in the recent literature, there are still a limited number of studies on the effect of various chitosan formulations on insect pests.
References
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27.
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083.
- Odier, A. Mémoire sur la composition chimique des parties cornées des insectes. Mem. Soc. Hist. Paris 1823, 1, 29–42.
- Muzzarelli, R.A.A. Chitin, 1937. In EPST, 2nd ed.; Mark, H.F., Bikales, N.M., Overberger, C.G., Menges, G., Eds.; Wiley: New York, NY, USA, 1985; Volume 3, pp. 430–449.
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94.
- Gillett, R. Global study of shrimp fisheries. FAO Fish Tech. Pap. 2008, 475, 25–29.
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795.
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600.
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. 2020, 105, 17–42.
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63.
- Vinsova, J.; Vavrikova, E. Chitosan Derivatives with Antimicrobial, Antitumour and Antioxidant Activities—A Review. Curr. Pharm. Des. 2011, 17, 3596–3607.
- Sharif, R.; Mujtaba, M.; Ur Rahman, M.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The Multifunctional Role of Chitosan in Horticultural Crops; A Review. Molecules 2018, 23, 872.
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981.
- Ma, J.; Faqir, Y.; Tan, C.; Khaliq, G. Terrestrial insects as a promising source of chitosan and recent developments in its application for various industries. Food Chem. 2022, 373, 131407.
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51.
- Žabka, M.; Pavela, R. The dominance of chitosan hydrochloride over modern natural agents or basic substances in efficacy against Phytophthora infestans, and its safety for the non-target model species Eisenia fetida. Horticulturae 2021, 7, 366.
- Orzali, L.; Allagui, M.B.; Chaves-Lopez, C.; Molina-Hernandez, J.B.; Moumni, M.; Mezzalama, M.; Romanazzi, G. Basic Substances and Potential Basic Substances: Key Compounds for a Sustainable Management of Seedborne Pathogens. Horticulturae 2023, 9, 1220.
- Paulraj, G.M.; Ignacimuthu, S.; Gandhi, M.R.; Shajahan, A.; Ganesan, P.; Packiam, S.M.; Al-Dhabi, N.A. Comparative studies of tripolyphosphate and glutaraldehyde cross-linked chitosan-botanical pesticide nanoparticles and their agricultural applications. Int. J. Biol. Macromol. 2017, 104, 1813–1819.
- Ferreira, T.P.; Haddi, K.H.; Corrêa, F.T.; Zapata, V.L.B.; Piau, T.B.; Souza, L.F.N.; Santos, S.G.; Oliveira, E.E.; Jumbo, L.O.; Ribeiro, B.M.; et al. Prolonged mosquitocidal activity of Siparuna guianensis essential oil encapsulated in chitosan nanoparticles. PLoS Negl. Trop. Dis. 2019, 13, e0007624.
- Rajkumar, V.; Gunasekaran, C.; Dharmaraj, J.; Chinnaraj, P.; Paul, C.A.; Kanithachristy, I. Structural characterization of chitosan nanoparticle loaded with Piper nigrum essential oil for biological efficacy against the stored grain pest control. Pestic. Biochem. Phys. 2020, 166, 104566.
- Rajkumar, V.; Gunasekaran, C.; Paul, C.A.; Dharmaraj, J. Development of encapsulated peppermint essential oil in chitosan nanoparticles: Characterization and biological efficacy against stored-grain pest control. Pestic. Biochem. Phys. 2020, 170, 104679.
- Stoffolano, J.; Wong, R.; Lo, T.; Ford, B.; Geden, C.J. Effect of chitosan on adult longevity when fed, in no-choice experiments, to Musca domestica L., Tabanus nigrovittatus Macquart, and Phormia regina (Meigen) adults and its consumption in adult Musca domestica L. Pest Manag. Sci. 2020, 76, 4293–4300.
- Telmadarrehei, T.; Tang, D.J.; Raji, O.; Rezazadeh, A.; Jeremic, D. Effect of chitosan on diversity and number of protists in subterranean termites. In Proceedings of the 114th Annual Meeting of the American Wood Protection, Seattle, WA, USA, 22–24 April 2018; pp. 22–24.
- Raji, O.; Tang, J.D.; Telmadarrehei, T.; Jeremic, D. Termiticidal activity of chitosan against the subterranean termites Reticulitermes flavipes and Reticulitermes virginicus. Pest Manag. Sci. 2018, 74, 1704–1710.
- Salvador-Figueroa, M.; Hernández-Ortiz, E.; Ventura-González, C.; Ovando-Medina, I.; Adriano-Anaya, L. Effect of chitosan coatings on the development of Anastrepha ludens (Loew) in mango fruits (Mangifera indica L.) cv. Ataulfo. Rev. Iberoam. De Tecnol. Postcosecha 2013, 14, 14–20.
- Limon, T.; Birke, A.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A.; Altúzar-Molina, A.; Carrión, G.; Goycoolea, F.M.; Moerschbacher, B.M.; Aluja, M. Chitosan coatings reduce fruit fly (Anastrepha obliqua) infestation and development of the fungus Colletotrichum gloeosporioides in Manila mangoes. J. Sci. Food Agric. 2021, 101, 2756–2766.
- Haas, J.; Lozano, E.R.; Haida, K.S.; Mazaro, S.M.; de Souza Vismara, E.; Poppy, G.M. Getting ready for battle: Do cabbage seeds treated with jasmonic acid and chitosan affect chewing and sap-feeding insects? Entomol. Exp. Appl. 2018, 166, 412–419.
- Perwita, M.G.C.S.; Wahyuningsih, T.D.; Astuti, E.; Pranowo, D. Synthesis Edible Film Chitosan/Polyethylene Glycol/Carboxymethylcellulose with Lemongrass Oils as Insect Ovipositing Repellent. Key Eng. Mater. 2020, 840, 142–148.
- Farina, P.; Ascrizzi, R.; Bedini, S.; Castagna, A.; Flamini, G.; Macaluso, M.; Mannucci, A.; Pieracci, Y.; Ranieri, A.; Sciampagna, M.C.; et al. Chitosan and Essential Oils Combined for Beef Meat Protection against the oviposition of Calliphora vomitoria, Water Loss, Lipid Peroxidation, and Colour Changes. Foods 2022, 11, 3994.
- Ascrizzi, R.; Flamini, G.; Bedini, S.; Tani, C.; Giannotti, P.; Lombardi, T.; Conti, B.; Fraternale, D. Ferulago campestris Essential Oil as Active Ingredient in Chitosan Seed-Coating: Chemical Analyses, Allelopathic Effects, and Protective Activity against the Common Bean Pest Acanthoscelides obtectus. Agronomy 2021, 11, 1578.
- Hossain, F.; Follett, P.; Salmieri, S.; Vu, K.D.; Harich, M.; Lacroix, M. Synergistic effects of nanocomposite films containing essential oil nanoemulsions in combination with ionizing radiation for control of rice weevil Sitophilus oryzae in stored grains. J. Food Sci. 2019, 84, 1439–1446.
- De Fátima Silva, M.; Maciel, V.B.V.; Noletto, A.P.R.; Venturini, A.C.; de Carvalho, R.A.; Yoshida, C.M.P. Chitosan active coating on paperboard surface forming an anti-insect grain-based food packaging. Packag. Technol. Sci. 2022, 35, 361–372.
- Llácer, E.; Martínez de Altube, M.M.; Jacas, J.A. Evaluation of the efficacy of Steinernema carpocapsae in a chitosan formulation against the red palm weevil, Rhynchophorus ferrugineus, in Phoenix canariensis. BioControl 2009, 54, 559–565.
- Dembilio, O.; Llacer, E.; Martínez de Altube, M.D.M.; Jacas, J.A. Field efficacy of imidacloprid and Steinernema carpocapsae in a chitosan formulation against the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in Phoenix canariensis. Pest Manag. Sci. 2010, 66, 365–370.
- Zhang, M.I.; Tan, T.; Yuan, H.; Rui, C. Insecticidal and fungicidal activities of chitosan and oligo-chitosan. J. Bioact. Compat. Polym. 2003, 18, 391–400.
- Rabea, E.I.; Badawy, M.E.; Rogge, T.M.; Stevens, C.V.; Höfte, M.; Steurbaut, W.; Smagghe, G. Insecticidal and fungicidal activity of new synthesized chitosan derivatives. Pest Manag. Sci. 2005, 61, 951–960.
- Rabea, E.I.; Badawy, M.E.; Rogge, T.M.; Stevens, C.V.; Steurbaut, W.; Höfte, M.; Smagghe, G. Enhancement of fungicidal and insecticidal activity by reductive alkylation of chitosan. Pest Manag. Sci. 2006, 62, 890–897.
- Badawy, M.E.I.; Rabea, E.I.; Rogge, T.M.; Stevens, C.V.; Steurbaut, W.; Höfte, M.; Smagghe, G. Fungicidal and Insecticidal Activity of O-Acyl Chitosan Derivatives. Polym. Bull. 2005, 54, 279–289.
- Badawy, M.E.I.; El-Aswad, A.F. Insecticidal activity of chitosans of different molecular weights and chitosan-metal complexes against cotton leafworm Spodoptera littoralis and oleander aphid Aphis nerii. Plant Prot. Sci. 2012, 48, 131–141.
- Rajkumar, V.; Gunasekaran, C.; Christy, I.K.; Dharmaraj, J.; Chinnaraj, P.; Paul, C.A. Toxicity, antifeedant and biochemical efficacy of Mentha piperita L. essential oil and their major constituents against stored grain pest. Pestic. Biochem. Phys. 2019, 156, 138–144.
- Upadhyay, N.; Singh, V.K.; Dwivedy, A.K.; Das, S.; Chaudhari, A.K.; Dubey, N.K. Assessment of Melissa officinalis L. essential oil as an eco-friendly approach against biodeterioration of wheat flour caused by Tribolium castaneum Herbst. Environ. Sci. Pollut. Res. 2019, 26, 14036–14049.
- Soltani, A.; Labidi, A.; Ben Jemaa, J. Development of formulation based on essential oils of rosemary to manage pests of stored cereal foodstuffs. In Proceedings of the 1st International Electronic Conference on Entomology, 1–15 July 2021; p. 10407.
- Soltani, A.; Ncibi, S.; Djebbi, T.; Sadraoui, I.A.; Abada, M.B.; Chargui, H.; Hassine, K.; Laabidi, A.; Mahmoudi, H.; Majdoub, H.; et al. Microencapsulation of rosemary Rosmarinus officinalis essential oil by Chitosan—Gum Arabic and its application for the control of two secondary pests of stored cereals. Res. Sq. 2022. Preprint.
- Soltani, A.; Haouel-Hamdi, S.; Sadraoui Ajmi, I.; Djebbi, T.; Ben Abada, M.; Yangui, I.; Chouachi, N.; Hassine, K.; Majdoub, H.; Messaoud, C.; et al. Insights for the control of dried-fruit beetle Carpophilus hemipterus (Nitidulidae) using rosemary essential oil loaded in chitosan nanoparticles. Int. J. Environ. Health Res. 2022, 33, 1243–1253.
- Sanei-Dehkordi, A.; Moemenbellah-Fard, M.D.; Sereshti, H.; Shahriari-Namadi, M.; Zarenezhad, E.; Osanloo, M. Chitosan nanoparticles containing Elettaria cardamomum and Cinnamomum zeylanicum essential oils; repellent and larvicidal effects against a malaria mosquito vector, and cytotoxic effects on a human skin normal cell line. Chem. Pap. 2021, 75, 6545–6556.
- Werdin González, J.O.; Jesser, E.N.; Yeguerman, C.A.; Ferrero, A.A.; Fernández Band, B. Polymer nanoparticles containing essential oils: New options for mosquito control. Environ. Sci. Pollut. Res. 2017, 24, 17006–17015.
- Paula, H.C.; Sombra, F.M.; Abreu, F.O.; Paul, R. Lippia sidoides essential oil encapsulation by angico gum/chitosan nanoparticles. J. Braz. Chem. Soc. 2010, 21, 2359–2366.
- El-Monairy, O.M.; Abdel-Meguid, A.D.; Emara, M.M. Efficacy of Methanol Leaf Extract, Biosynthesized Silver and Chitosan Nanoparticles Using Nerium oleander against Musca domestica. Egypt. Acad. J. Biol. Sci. F. Toxicol. Pest Control 2020, 12, 35–45.
- De Oliveira, J.L.; Campos, E.V.R.; Pereira, A.E.; Nunes, L.E.; Da Silva, C.C.; Pasquoto, T.; Lima, R.; Smaniotto, G.; Polanczyk, R.A.; Fraceto, L.F. Geraniol encapsulated in chitosan/gum arabic nanoparticles: A promising system for pest management in sustainable agriculture. J. Agric. Food Chem. 2018, 66, 5325–5334.
- Namasivayam, K.R.S.; Arvind Bharani, R.S.; Karunamoorthy, K. Insecticidal fungal metabolites fabricated chitosan nanocomposite (IM-CNC) preparation for the enhanced larvicidal activity—An effective strategy for green pesticide against economic important insect pests. Int. J. Biol. Macromol. 2018, 120, 921–944.
- Sharma, A.; Sood, K.; Kaur, J.; Khatri, M. Agrochemical loaded biocompatible chitosan nanoparticles for insect pest management. Biocatal. Agric. Biotechnol. 2019, 18, 101079.
- Sahab, A.F.; Waly, A.I.; Sabbour, M.M.; Nawar, L.S. Synthesis, antifungal and insecticidal potential of Chitosan (CS)-g-poly (acrylic acid) (PAA) nanoparticles against some seed borne fungi and insects of soybean. Int. J. ChemTech Res. 2015, 8, 589–598.
- Sabbour, M.M.; Abdel-Hakim, E.A. Control of Cassida vittata (Vill) (Coleoptera: Chrysomelidae) using chitosan and nano chitosan. Sciences 2018, 8, 141–144.
- Ziaee, M.; Moharramipour, S.; Mohsenifar, A. Toxicity of Carum copticum essential oil-loaded nanogel against Sitophilus granarius and Tribolium confusum. J. Appl. Entomol. 2014, 138, 763–771.
- Ziaee, M.; Moharramipour, S.; Mohsenifar, A. MA-chitosan nanogel loaded with Cuminum cyminum essential oil for efficient management of two stored product beetle pests. J. Pest Sci. 2014, 87, 691–699.
- Zhang, X.; Zhang, J.; Zhu, K.Y. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693.
- Zhang, X.; Mysore, K.; Flannery, E.; Michel, K.; Severson, D.W.; Zhu, K.Y.; Duman-Scheel, M. Chitosan/interfering RNA nanoparticle mediated gene silencing in disease vector mosquito larvae. J. Vis. Exp. 2015, 97, e52523.
- Kumar, D.; Saravana Kumar, P.; Gandhi, M.R.; Al-Dhabi, N.A.; Paulraj, M.G.; Ignacimuthu, S. Delivery of chitosan/dsRNA nanoparticles for silencing of wing development vestigial (vg) gene in Aedes aegypti mosquitoes. Int. J. Biol. Macromol. 2016, 86, 89–95.
- Muryeti, M.; Pratiwi, F.E.; Yuniastuti, R.T.; Mulyani, E.B. Termiticidal activity of chitosan on paper. Prog. Chem. Appl. Chitin Deriv. 2020, 25, 164–173.
- Liibert, L.; Treu, A.; Meier, P. A two-step wood protection process using alternative wood protection agents in combination with an oil treatment. In Proceedings of the 7th meeting of the Nordic-Baltic Network in Wood Material Science & Engineering (WSE), Oslo, Norway, 27–28 October 2011.
- Jianglian, D.; Shaoying, Z. Application of Chitosan Based Coating in Fruit and Vegetable Preservation: A Review. J. Food Process. 2013, 4, 227.
- Shiekh, R.A.; Malik, M.A.; Al-Thabaiti, S.A.; Shiekh, M.A. Chitosan as a novel edible coating for fresh fruits. Food Sci. Technol. Res. 2013, 19, 139–155.
- Reddy, M.B.; Belkacemi, K.; Corcuff, R.; Castaigne, F.; Arul, J. Effect of pre-harvest chitosan sprays on post-harvest infection by Botrytis cinerea and quality of strawberry fruit. Postharvest Biol. Technol. 2000, 20, 39–51.
- Salgado-Cruz, M.D.L.P.; Salgado-Cruz, J.; García-Hernández, A.B.; Calderón-Domínguez, G.; Gómez-Viquez, H.; Oliver-Espinoza, R.; Fernández-Marínez, C.M.; Yáñez-Fernández, J. Chitosan as a coating for biocontrol in postharvest products: A bibliometric review. Membranes 2021, 11, 421.
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299.
- Shiri, M.A.; Bakhshi, D.; Ghasemnezhad, M.; Dadi, M.; Papachatzis, A.; Kalorizou, H. Coating improves the shelf life and postharvest quality of table grape (Vitis vinifera) cultivar Shahroudi. Turk. J. Agric. For. 2013, 37, 148–156.
- Sikder, M.B.H.; Islam, M.M. Effect of shrimp chitosan coating on physico-chemical properties and shelf-life extension of banana. Int. J. Eng. Technol. Sci. 2019, 6, 41–54.
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and chitosan fragments responsible for plant elicitor and growth stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211.
- Aluja, M.; Mangan, R.L. Fruit Fly (Diptera: Tephritidae) Host Status Determination: Critical Conceptual, Methodological, and Regulatory Considerations. Annu. Rev. Entomol. 2008, 53, 473–502.
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Artemisia spp. Essential oils against the disease-carrying blowfly Calliphora vomitoria. Parasites Vectors 2017, 10, 80.
- Bedini, S.; Cosci, F.; Girardi, J.; Bocchino, R.; Conti, C. Aromatic plant essential oils for the control of blowflies in the production of dry-cured meat. In Proceedings of the IOBC-WPRS Bulletin Bulletin OILB-SROP Proceeding of the meeting at Ljubljana (Slovenia), Ljubljana, Slovenia, 3–5 July 2017; Volume 130, pp. 75–80.
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Echeverria, M.C.; Gomez, E.V.; Guidi, L.; Landi, M.; Lucchi, A.; Conti, B. Toxicity and oviposition deterrence of essential oils of Clinopodium nubigenum and Lavandula angustifolia against the myiasis inducing blowfly Lucilia sericata. PLoS ONE 2019, 14, e0212576.
- Bedini, S.; Guarino, S.; Echeverria, M.C.; Flamini, G.; Ascrizzi, R.; Loni, A.; Conti, B. Allium sativum, Rosmarinus officinalis, and Salvia officinalis essential oils: A spiced shield against blowflies. Insects 2020, 11, 143.
- Bedini, S.; Farina, P.; Napoli, E.; Flamini, G.; Ascrizzi, R.; Verzera, A.; Conti, B.; Zappalà, L. Bioactivity of different chemotypes of oregano essential oil against the blowfly Calliphora vomitoria vector of foodborne pathogens. Insects 2021, 12, 52.
- Farina, P.; Venturi, F.; Ascrizzi, R.; Flamini, G.; Chiriboga Ortega, R.D.; Echeverría, M.C.; Ortega, S.; Zinnai, A.; Bedini, S.; Conti, B. Andean plants essential oils: A scented alternative to synthetic insecticides for the control of blowflies. Insects 2021, 12, 894.
- Pierattini, E.C.; Bedini, S.; Venturi, F.; Ascrizzi, R.; Flamini, G.; Bocchino, R.; Girardi, J.; Giannotti, P.; Ferroni, G.; Conti, B. Sensory Quality of Essential Oils and Their Synergistic Effect with Diatomaceous Earth, for the Control of Stored Grain Insects. Insects 2019, 10, 114.
- Kumar, N.; Kaur, P.; Bhatia, S. Advances in bio-nanocomposite materials for food packaging: A review. Nutr. Food Sci. 2017, 47, 591–606.
- Hadwiger, L.A.; Loschke, D.C. Molecular Communication in Host-Parasite Interactions: Hexosamine Polymers (Chitosan) as Regulator Compounds in Race-Specific and Other Interactions. Phytopathology 1981, 71, 756–762.
- Ait Barka, E.; Eullaffroy, P.; Clément, C.; Vernet, G. Chitosan improves development and protects Vitis vinifera L. against Botrytis cinerea. Plant Cell Rep. 2004, 22, 608–614.
- Smart, G.C., Jr. Entomopathogenic nematodes for the biological control of insects. J. Nematol. 1995, 27, 529.
- Lacey, L.A.; Georgis, R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 2012, 44, 218.
- Shapiro-Ilan, D.; Dolinski, C. Entomopathogenic nematode application technology. In Nematode Pathogenesis of Insects and Other Pests: Ecology and Applied Technologies for Sustainable Plant and Crop Protection; Springer: Berlin/Heidelberg, Germany, 2015; pp. 231–254.
- Abbas, M.S.T.; Saleh, M.M.E.; Akil, A.M. Laboratory and field evaluation of the pathogenicity of entomopathogenic nematodes to the red palm weevil, Rhynchophorus ferrugineus (Oliv.) (Col.: Curculionidae). Anz. Schädlingskunde 2001, 74, 167–168.
- Peña, A.M. Biological Pesticide Based on Chitosan and Entomopathogenic Nematodes. WO Patent 037966, 16 May 2002.
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487.
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72.
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181.
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179.
- Sahariah, P.; Másson, M. Antimicrobial chitosan and chitosan derivatives: A review of the structure–activity relationship. Biomacromolecules 2017, 18, 3846–3868.
- Wang, X.; Du, Y.; Liu, H. Preparation, characterization and antimicrobial activity of chitosan–Zn complex. Carbohydr. Polym. 2004, 56, 21–26.
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93.
- Wang, X.; Du, Y.; Fan, L.; Liu, H.; Hu, Y. Chitosan-metal complexes as antimicrobial agent: Synthesis, characterization and Structure-activity study. Polym. Bull. 2005, 55, 105–113.
- Athanassiou, C.G.; Kavallieratos, N.G.; Benelli, G.; Losic, D.; Usha Rani, P.; Desneux, N. Nanoparticles for pest control: Current status and future perspectives. J. Pest Sci. 2018, 91, 1–15.
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C.; et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: Strategies towards sustainable agriculture. J. Nanobiotechnology 2022, 20, 11.
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011, 29, 792–803.
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112.
- Shoueir, K.R.; El-Desouky, N.; Rashad, M.M.; Ahmed, M.K.; Janowska, I.; El-Kemary, M. Chitosan based-nanoparticles and nanocapsules: Overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2021, 167, 1176–1197.
- Maganga, M.E.; Gries, G.; Cries, R. Repellency of various oils and pine oil constituents to house flies (Diptera: Muscidae). Environ. Entomol. 1996, 25, 1182–1187.
- Katerinopoulos, H.E.; Pagona, G.; Afratis, A.; Stratigakis, N.; Roditakis, N. Composition and insect attracting activity of the essential oil of Rosmarinus officinalis. J. Chem. Ecol. 2005, 31, 111–122.
- Lacotte, V.; Rey, M.; Peignier, S.; Mercier, P.E.; Rahioui, I.; Sivignon, C.; Razy, L.; Benhamou, S.; Livi, S.; da Silva, P. Bioactivity and chemical composition of forty plant essential oils against the pea aphid Acyrthosiphon pisum revealed peppermint oil as a promising biorepellent. Ind. Crops Prod. 2023, 197, 116610.
- Lahlali, R.; El Hamss, H.; Mediouni-Ben Jemâa, J.; Barka, E.A. The Use of Plant Extracts and Essential Oils as Biopesticides. Front. Agron. 2022, 4, 921965.
- Maluin, F.N.; Hussein, M.Z. Chitosan-based agronanochemicals as a sustainable alternative in crop protection. Molecules 2020, 25, 1611.
- Werdin González, J.O.; Gutiérrez, M.M.; Ferrero, A.A.; Fernández Band, B. Essential oils nanoformulations for stored-product pest control—Characterization and biological properties. Chemosphere 2014, 100, 130–138.
More
Information
Subjects:
Entomology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
570
Revisions:
4 times
(View History)
Update Date:
18 Jan 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




