Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Caesar Ferrari | -- | 4906 | 2023-12-27 15:23:58 | | | |
| 2 | Camila Xu | Meta information modification | 4906 | 2023-12-28 02:01:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ferrari, C.; Tadros, M. The Quality of Upper Gastrointestinal Endoscopy. Encyclopedia. Available online: https://encyclopedia.pub/entry/53183 (accessed on 08 February 2026).
Ferrari C, Tadros M. The Quality of Upper Gastrointestinal Endoscopy. Encyclopedia. Available at: https://encyclopedia.pub/entry/53183. Accessed February 08, 2026.
Ferrari, Caesar, Micheal Tadros. "The Quality of Upper Gastrointestinal Endoscopy" Encyclopedia, https://encyclopedia.pub/entry/53183 (accessed February 08, 2026).
Ferrari, C., & Tadros, M. (2023, December 27). The Quality of Upper Gastrointestinal Endoscopy. In Encyclopedia. https://encyclopedia.pub/entry/53183
Ferrari, Caesar and Micheal Tadros. "The Quality of Upper Gastrointestinal Endoscopy." Encyclopedia. Web. 27 December, 2023.
Copy Citation
Upper gastrointestinal endoscopy, or EGD, is essential for diagnosing and managing ailments of the upper gastrointestinal tract. The quality of EGD is crucial and carries significant consequences for patient outcomes, the employment of healthcare resources, and the future course of gastroenterology as a medical specialty.
upper gastrointestinal endoscopy
esophagogastroduodenoscopy
quality indicators (QIs)
gastrointestinal diseases
1. Introduction
Upper gastrointestinal endoscopy, or EGD, is essential for diagnosing and managing ailments of the upper gastrointestinal tract [1][2]. Despite its importance, pronounced variability exists in EGD examinations due to operator proficiency, impacting patient outcomes and healthcare efficiency [3]. Even with guidelines for standardized endoscopic protocols, inconsistent adherence remains a concern [4]. Notably, there is no globally accepted systematic examination protocol for EGD, leading to false-negative rates between 10% and 20% [1][4][5]. Especially significant for high-risk gastric cancer patients, EGD’s practice standardization becomes paramount [6][7].
Unlike colonoscopy, EGD lacks distinct Quality Indicators (QIs), making detecting subtle changes in gastric mucosa challenging [8][9][10]. Subpar EGD practices result in misdiagnoses, increased healthcare expenses, and potential complications [11].
2. EGD Procedure: A Landmark-Driven Examination
The Technique: An EGD employs an endoscope, a flexible tube with an attached camera and light, allowing visualization of the internal surfaces of the upper gastrointestinal (GI) tract.
Preparation and Sedation: Fasting is mandated for several hours before the procedure. Mild sedatives are often administered for patient comfort and to suppress natural gag reflexes [12][13][14].
Landmark Exploration (Figure 1) [15]:
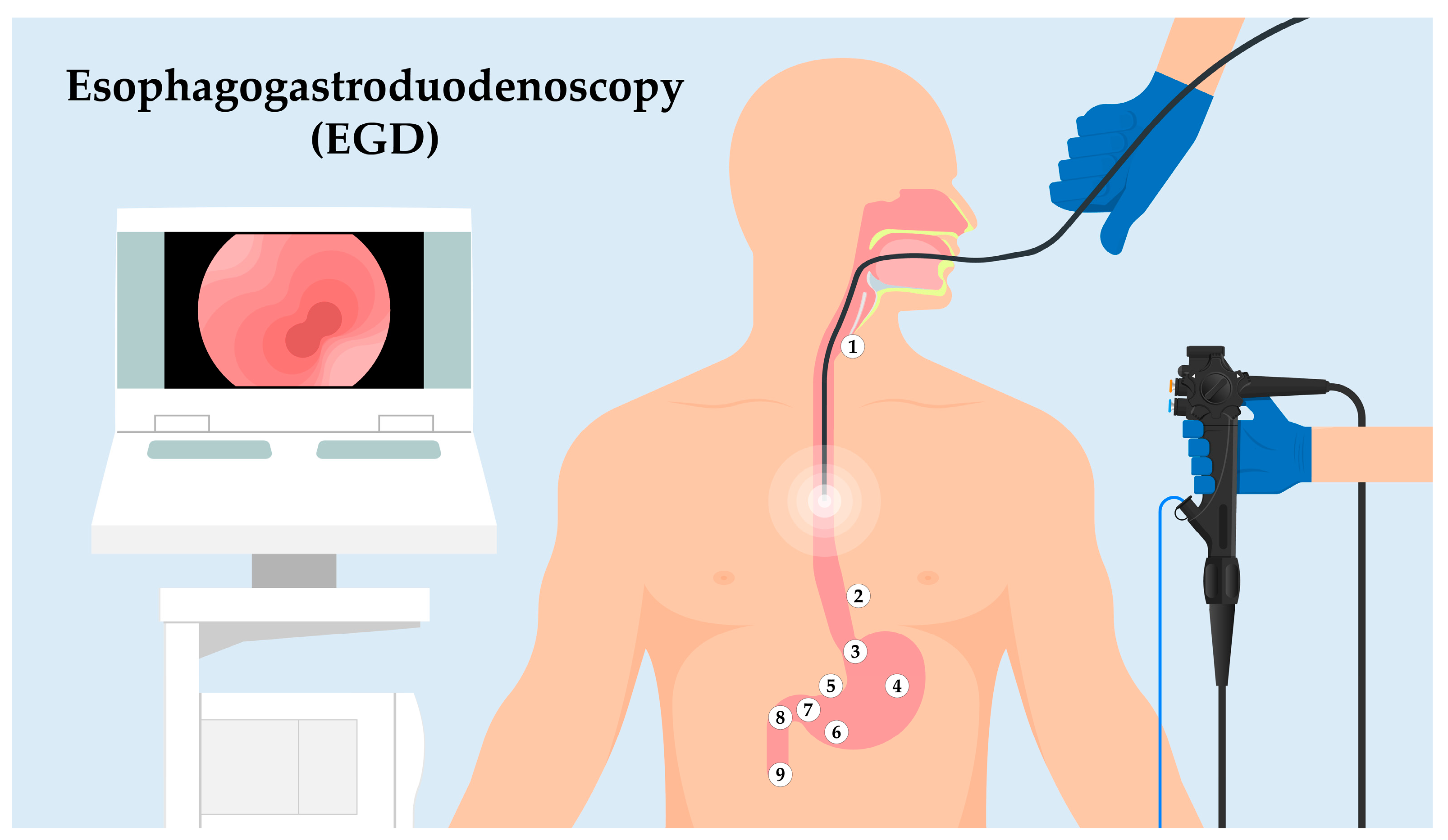
Figure 1. Sequential landmarks during EGD. Utilizing an endoscope to traverse the upper GI tract, this figure illustrates the key anatomic landmarks. Starting at the UES (1), the journey highlights the Z-line (2), indicative of the esophagus–stomach transition. This path proceeds through the Cardia (3), then the Body of the Stomach (4). The Angularis Incisure (5) serves as a notable bend before reaching the Antrum (6), adjacent to the Pyloric Canal and Ring (7). The exploration continues to the Duodenal Bulb (8) and slightly extends into the Descending Duodenum (9), showcasing the ampulla of Vater. This overview facilitates understanding a structured EGD exploration and its intrinsic significance.
- ⮚
-
Upper Esophageal Sphincter (UES): As the endoscope enters the esophagus, the first landmark encountered is the UES. This muscular ring divides the pharynx from the esophagus and acts as a valve, ensuring a unidirectional flow of ingested contents.
- ⮚
-
Z-line: Moving distally, the endoscope will visualize the “Z-line” or “squamocolumnar junction.” This zone demarcates the junction between the esophagus’s squamous epithelium and the stomach’s columnar epithelium. The appearance and location of the Z-line can offer insights into conditions like Barrett’s esophagus.
- ⮚
-
Cardia: As the endoscope progresses into the stomach, the cardia is encountered, a small area surrounding the esophagogastric junction.
- ⮚
-
Body of the Stomach: The main, central region of the stomach is examined next, noting the appearance of the gastric folds and assessing for abnormalities like ulcers or masses.
- ⮚
-
Angularis Incisure: A notable bend in the stomach’s structure, this landmark can be a reference for the division between the body of the stomach and the antrum.
- ⮚
-
Antrum: This portion of the stomach is closer to the pyloric canal and is essential to assess as it is a common site for peptic ulcers.
- ⮚
-
Pyloric Canal and Pyloric Ring: The distal stomach section leading into the duodenum. It acts as a valve to regulate the release of gastric contents into the duodenum.
- ⮚
-
Duodenal Bulb: The first part of the duodenum, immediately after the pylorus. It is a common site to inspect for ulcers, especially in patients with Helicobacter pylori infection.
- ⮚
-
Descending (Second) Part of the Duodenum: The endoscope can typically be advanced a short distance beyond the bulb to visualize this segment. The presence of the ampulla of Vater, the joint opening for the bile and pancreatic ducts, can be identified in this region.
Post-Procedural Care: Once the procedure concludes, patients are monitored until the sedative effects dissipate. Mild symptoms such as a sore throat or bloating are commonplace but usually transient. Activities requiring keen attention are generally discouraged for 24 h post-EGD due to potential sedation after-effects.
Potential Risks: Though largely safe, the procedure can sometimes lead to minor discomfort in the throat or bloating. On rare occasions, complications like GI tract perforation or adverse reactions to sedation can occur, necessitating swift medical attention [16].
3. Best Practice Guidelines for High-Quality EGD: Guidelines from Leading Medical Societies
Numerous esteemed medical societies, including the American Society for Gastrointestinal Endoscopy/American College of Gastroenterology (ASGE/ACG), the Asian Consensus, the British Society of Gastroenterology (BSG), and the European Society of Gastrointestinal Endoscopy (ESGE), have issued extensive guidelines outlining the finest methodologies for conducting a high-caliber EGD [17][18][19][20]. It is incumbent upon practicing endoscopists to remain current with these guidelines and incorporate the recommended practices into their routine clinical work. The convergence of insights from these reputable organizations is a cornerstone for establishing best practices in EGD procedures. However, beyond mere adherence to guidelines, endoscopists are tasked with the vital responsibility of translating these recommendations into tangible actions within their everyday clinical practice. To facilitate a holistic understanding of the intricate nuances involved in achieving the highest standards of quality, Table 1 offers an in-depth comparison of the pre-procedural, intra-procedural, and post-procedural QIs as outlined by these key organizations. This comprehensive analysis not only underscores the convergence of these societies on critical aspects but also highlights potential variations and distinctive perspectives that can enrich the decision-making process for endoscopists, promoting optimal patient outcomes and contributing to the continuous advancement of upper endoscopic practices.
Table 1. Comparing EGD guidelines between the ASGE/ACG, Asian Consensus, BSG/AUGIS, and ESGE.
| PRE-, INTRA-, AND POST- PROCEDURAL | CRITERIA/CATEGORY | ASGE/ACG [21] | ASIAN CONSENSUS [22] | BSG/AUGIS [23] | ESGE [1] |
|---|---|---|---|---|---|
| PRE-PROCEDURAL | EGD Indications | Accepted Indications | Risk stratification for UGI cancers; High-risk factors | Adequate preparation, indications, fitness assessment, and consent | - |
| Informed Consent | Risk discussion; proper documentation | Identification of high-risk patients for UGI cancers | Consent; endoscopist competency: JAG/JET accreditation with a minimum of 100 procedures/year | Proper instructions and informed consent | |
| Prophylactic Measures | Antibiotics for cirrhosis, PEG tube; PPI for suspected ulcer bleeding; vasoactive drugs for suspected variceal bleeding | - | Fasting protocol: 2 h for liquids, 6 h for solids; continuing professional development emphasizing lesion recognition | Water allowed until 2 h before procedure; safe fasting duration ≥6 h for solids | |
| INTRA-PROCEDURAL | Organ Examination | Complete organ examination, including stomach retroflexion | Sedation enhances detection of superficial neoplasms; systematic endoscopic mapping for detection of UGI superficial neoplasms; longer OGD times | Midazolam use; optimal procedure time: 7–8 min; high-definition systems for improved images and biopsies | Inclusion of esophagus, stomach, and duodenum in inspection; inspection duration should be ≥7 min |
| Biopsy Protocol | Gastric ulcer biopsy for malignancy; biopsy for suspected BE; adequate sample collection | Systematic photo-mapping; enhanced lesion recognition in high-risk and surveillance populations | Prague classification for Barrett’s lesions; Paris classification for lesion description | Minimum of 10 pictures for normal exam; use validated classifications for reporting | |
| Clinical Documentation and Visualization | Primary hemostasis; second treatment modality for bleeding ulcers | Iodine chromoendoscopy; NBI; indigo carmine chromoendoscopy | Photo mapping to enhance mucosal inspection; standardized terminology for reporting findings | Visualize major duodenal papilla; high-quality reporting with photo documentation | |
| POST-PROCEDURAL | Adverse Event Monitoring | Contact patients to document adverse events after EGD | Contact patients to document adverse events after EGD | Audit complications, readmissions, and mortality; review histology results from procedures | Implement software for reporting enhancement; monitor dysplasia incidence in Barrett’s surveillance |
| Patient Communication | - | - | Provide written and verbal post-EGD instructions; escalate malignant lesions promptly to multidisciplinary team meetings | Contact patients to document adverse events after EGD |
4. QIs for Upper Gastrointestinal Endoscopy
In the realm of EGD, QIs play an indispensable role as measurable benchmarks pivotal to evaluating healthcare delivery processes and outcomes. Serving as a yardstick for assessing excellence, QIs pinpoint opportunities for quality enhancement, monitor the impacts of changes, and guarantee the delivery of superlative patient care.
4.1. Fundamental Elements of High-Quality EGD: Patient Selection, Patient Preparation, Procedure, Follow-Up
Achieving a high-quality EGD begins with appropriate patient selection and extends beyond the procedure to include adequate follow-up [24].
-
Patient Selection: A judicious evaluation of the patient’s symptoms, medical history, physical examination, and, where appropriate, non-invasive tests are critical in deciding when EGD is indicated.
-
Patient Preparation: Effective communication with the patient about the purpose, process, and potential risks of the procedure, in addition to providing clear instructions for pre-procedure fasting and medication management, is essential to minimize the risk and maximize the diagnostic or therapeutic yield.
-
Procedure: The endoscopist should adhere to established procedural guidelines, which include a systematic examination of the upper gastrointestinal tract, adequate documentation of findings, taking biopsies when indicated, and performing therapeutic interventions safely and effectively.
-
Follow-Up: Post-procedure care includes monitoring for complications, communicating findings to the patient and their primary care provider, arranging for pathological evaluation of biopsies, and scheduling appropriate follow-up based on the results of the EGD.
4.2. Pre-Procedural QIs
Before executing the EGD procedure, myriad indicators are crucial in determining and ensuring procedural quality. Foremost among these is the justification for the EGD. It is vital that the procedure adheres strictly to accepted indications and established clinical guidelines, as emphasized by organizations such as the ASGE/ACG [21]. Equally significant is the role of informed consent. Comprehensive informed consent involves a thorough discussion of the procedure’s risks, benefits, and alternatives. The ASGE/ACG, BSG/AUGIS, and ESGE highlight this fundamental aspect [1][22][23][25]. This aligns seamlessly with the age-old practice of accurately documenting a patient’s medical history and emphasizes the importance of effective communication with the patient.
Fasting conventions have evolved. Historically, patients fasted for between four and six hours before endoscopy. However, recent BSG/AUGIS and ESGE guidelines highlight that while solids require a minimum fasting duration of six hours, clear liquids can be consumed up to two hours before the procedure. This modern approach alleviates patient discomfort and anxiety without increasing the risk of regurgitating gastric contents. Furthermore, the ESGE underlines the importance of reporting on stomach contents and water jet usage to better gauge gastric preparation [1].
The use of premedication has been spotlighted in the recent literature, demonstrating its role in enhancing visualization. Agents like simethicone, N-acetylcysteine, and pronase significantly improve visibility during the procedure, with pronase, in particular, having added benefits during biopsy due to reducing mucus thickness and biopsy depth, thereby refining diagnostic evaluations [26][27][28].
Prophylactic measures, as the ASGE/ACG recommends, play an instrumental role in preventing potential complications. These include prescribing prophylactic antibiotics in particular situations, such as cirrhosis and PEG tube placements, and utilizing proton pump inhibitors (PPIs) for suspected ulcer bleeding and vasoactive drugs for anticipated variceal bleeding [29][30][31].
Furthermore, the Asian Consensus guidelines accentuate risk stratification. It is essential to identify high-risk patients for UGI cancers and to maintain a lower biopsy threshold for suspicious lesions, especially in the context of high-risk factors such as esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma [22].
Another essential facet of procedural quality is the competency of the endoscopist. The BSG/AUGIS stresses the importance of continuous professional development, adequate experience, especially in high-risk populations, meeting benchmarks like the JAG/JET accreditation, and performing a specified number of procedures annually [23].
Lastly, the choice of sedation is pivotal. While the debate on the optimal sedation regimen remains, evidence suggests that patient satisfaction is markedly improved among those who receive sedation [13]. Specifically, propofol has been shown to lead to a more comprehensive inspection during esophagogastroduodenoscopy (OGD), yielding a superior-quality examination [14].
4.3. Intra-Procedural QIs
During the technical execution of the EGD procedure, intra-procedural indicators take precedence in ascertaining optimal outcomes. A cornerstone of these indicators is the thoroughness of the examination. To thoroughly inspect the UGI tract, specific reference points need to be systematically assessed. The evaluation should initiate at the upper esophageal sphincter and extend to the second segment of the duodenum, covering areas such as the upper esophagus, gastro–esophageal junction, fundus, stomach’s main body, incisura, antrum, duodenal bulb, and the end of the duodenum. Using a J-maneuver, the fundus should be observed in every individual. Additionally, the diaphragmatic constriction should be examined in cases with a hiatus hernia during retroflexion [23][32]. The ASGE/ACG and ESGE have accentuated the necessity of a comprehensive view from the esophagus to the duodenum [1][21].
The duration of the inspection has been spotlighted as a critical determinant of quality. While studies like those by Barclay et al. highlighted a mean withdrawal time of six minutes for colonoscopies, the recommendations across the Asian Consensus, BSG/AUGIS, and ESGE guidelines recommend an optimal EGD duration of 7–8 min. Extended durations might be required in certain instances, such as Barrett’s surveillance. Extended EGD durations have correlated with enhanced detection rates of premalignant and neoplastic lesions [33][34][35].
Technological advancements have further elevated the standard of intra-procedural indicators. The Asian Consensus guidelines enumerate the value of imaging enhancement equipment (IEE) types, encompassing narrow band imaging (NBI), flexible spectral imaging color enhancement (FICE), blue laser/light imaging (BLI), i-SCAN, and optical enhancement [22][36]. Concurrently, the BSG/AUGIS accentuates the significance of leveraging high-definition video endoscopy systems for excellent imaging and biopsies. Enhanced detection techniques such as iodine chromoendoscopy, NBI, and magnifying NBI were spotlighted for their potency in improving the detection accuracy for conditions ranging from esophageal SCC to EGC [37][38][39].
An understanding of the biopsy protocols is critical to refine the quality of EGD further. As such, researchers propose the introduction of Table 2, which outlines a comprehensive overview of the biopsy protocols for various gastrointestinal conditions. This table, informed by current guidelines and the literature, provides a succinct yet detailed guide for performing biopsies within the EGD procedure, ensuring adherence to best practices and enhancing diagnostic accuracy.
Table 2. Biopsy protocols for EGD.
| Disease | Biopsy Site | Number of Biopsies | Method and Considerations | Reference |
|---|---|---|---|---|
| Barrett’s Esophagus | Esophagus above the GEJ | Every 1–2 cm in quadrants | Seattle protocol for quadrants; targeted biopsies of visible lesions; consider advanced imaging like NBI for identification of dysplastic areas; surveillance based on degree of dysplasia | [40][41] |
| Celiac Disease |
Duodenal bulb and descending duodenum | 4–6 | Biopsies from the duodenal bulb and at least one other site in the duodenum; ensure adequate sampling of the intestinal mucosa for assessment of villous atrophy; orientation of biopsies for histological evaluation is important; four to six biopsies recommended, including one from the bulb | [40][42] |
| Eosinophilic Esophagitis | Esophagus | At least 6 | Biopsies from different locations focusing on areas with endoscopic mucosal abnormalities; eosinophil count ≥15 per high power field for diagnosis; Hematoxylin-eosin staining for assessment; two to four biopsies each from the proximal and distal esophagus recommended | [40][43] |
| Gastric Polyps | Polyp | Depends on polyp size | Small polyps (<5 mm): biopsy; larger polyps: removal and histological examination; multiple biopsies from large sessile polyps to rule out malignancy; polypectomy recommended for solitary polyps, with representative biopsies from smaller polyps in cases of multiple polyps | [40] |
| Helicobacter pylori | Antrum and corpus | Multiple from both sites | Sidney protocol: One biopsy each from lesser and greater curvatures of the antrum and body, and one from the incisura angularis; alternatively, three biopsies protocol: one from the incisura angularis, one from the greater curvature of the body, one from the greater curvature of the antrum | [40] |
| Infectious Esophagitis | Ulcers or lesions | As indicated | Biopsies from base of ulcers for CMV, edges for HSV; multiple biopsies may be needed for fungal esophagitis; consider PCR testing for definitive pathogen identification | [40] |
| Peptic Ulcer Disease | Ulcer and surrounding mucosa | ≥8 around the base | Biopsies of the ulcer margin and adjacent mucosa; consider testing for H. pylori; in cases of gastric ulcers, biopsy the ulcer base as well to rule out malignancy; recommended to perform multiple biopsies (≥8) in the base | [40] |
| Upper GI Neoplasia | Lesion site | 4–8 (optimal: 3–4) | Targeted biopsies of suspected malignant lesions; additional biopsies from the margins may be required for larger or irregular lesions; enhanced imaging techniques like chromoendoscopy may be used to identify subtle lesions; three or four biopsies considered optimal; exact targeting of appropriate site and viable tissue acquisition crucial for diagnosis; image enhanced endoscopy-assisted biopsy can aid in targeting and reduce the number of biopsies needed | [6] |
The biopsy technique and the number of samples retrieved are critical for an accurate pathological diagnosis in assessing gastric ulcers. A study emphasized the importance of multiple biopsies, recommending at least four to six pieces from various ulcer sites, as single biopsies often result in “pseudo-negative” outcomes, potentially delaying diagnosis and treatment [44]. Consensus guidelines from gastroenterological societies, like the ASGE, corroborate the necessity of multiple biopsies to differentiate between benign and malignant ulcers, especially considering the potential for carcinoma in the background of a benign-appearing gastric ulcer. Moreover, literature reviews suggest a minimum of seven to eight biopsies to significantly decrease the false-negative rate for detecting gastric malignancies. These findings align with expert opinions and meta-analyses that indicate a higher biopsy count leads to an improved diagnostic yield for gastric malignancies. Considering the current evidence and expert recommendations, researchers propose a standardized protocol for biopsy in gastric ulcers, advocating for a minimum of six biopsies optimally distributed between the antrum and body of the stomach, encompassing the ulcer margins and base. This protocol is designed to increase the likelihood of detecting malignancy and ensure a more reliable diagnostic pathway.
The fidelity of characterization and adherence to biopsy protocols are indispensable. The ASGE/ACG and Asian Consensus guidelines underscore the essence of practices like gastric ulcer biopsies to ascertain malignancy, measuring Barrett’s esophagus (BE) length, and conducting a biopsy for suspected BE cases [45][46]. The ASGE/ACG further insists on documenting primary hemostasis achievements and deploying a secondary treatment modality for bleeding ulcers following epinephrine injections [47][48][49].
Maintaining the clarity of visualization remains pivotal. The BSG/AUGIS advocates employing tools like water jets, mucolytics, and defoaming agents for enhanced mucosal cleansing [50][51][52]. Such clear views are foundational for excluding early UGI lesions, which aligns with the ESGE’s emphasis on superior-quality reporting and photo-documentation, necessitating at least 10 pictures even for standard exams. These images should cover the following areas: the beginning of the esophagus, its end, the Z-line paired with the diaphragm’s impression, the cardia and fundus when inverted, the body of the stomach with a focus on the lesser curvature from a straightforward perspective, a retroflexed view of the stomach body highlighting the greater curvature, a semi-inverted view of the angulus, the antrum, the initial section of the duodenum, and its subsequent segment [1].
Recent developments in ulcer bleeding and treatment necessitate comprehensive treatment approaches integrating pharmacological and endoscopic interventions. This includes using proton pump inhibitors and endoscopic techniques such as clipping and hemospray, which have effectively managed gastrointestinal bleeding, particularly from ulcers. Alongside this, the Forrest classification system is essential for classifying peptic ulcers based on the risk of rebleeding, guiding clinicians in therapeutic decisions. By categorizing ulcers from low to high risk, this system plays a pivotal role in determining the urgency and nature of the interventions required. These advanced strategies, combined with the enhanced understanding and application of biopsy protocols, provide a more robust framework for managing gastric ulcers and associated complications [53][54][55].
Different hemostatic protocols are tailored based on the variceal type—esophageal or gastric—and the severity of the bleeding in managing variceal hemorrhage. For esophageal varices, Endoscopic Variceal Ligation (EVL) is preferred for its efficacy in achieving hemostasis, lower complication rates, and reduced early rebleeding risk compared to sclerotherapy. Esophageal varices, the most common type of gastrointestinal varices, present a notable risk of bleeding, particularly in more severe cases (Child–Pugh class B and C). The EVL technique involves placing bands on the varices during endoscopy, effectively reducing the risk of hemorrhage. This method is favored over nonselective beta-blockers or the previously more commonly used Transjugular Intrahepatic Portosystemic Shunt (TIPS) for primary prophylaxis. Screening for esophageal varices is recommended for patients newly diagnosed with cirrhosis, with subsequent monitoring depending on the size of the varices and the presence of liver injury or other cofactor diseases. EVL is typically performed in a series of treatments until the varices are eradicated, with follow-up endoscopy scheduled every 6–12 months [56]. In gastric varices, endoscopic cyanoacrylate glue injection is the first-line treatment, with TIPS reserved for cases of acute bleeding not controlled by endoscopic means [57][58].
In the context of variceal hemorrhage, promptness in terms of the intervention is paramount. Practice society guidelines advocate that endoscopic intervention for variceal bleeding should be executed as swiftly as possible, ideally within 12 h from patient presentation. This timely approach is crucial in managing the acute phase of upper gastrointestinal bleeding and optimizing patient outcomes. The rapid initiation of endoscopic treatment improves hemostasis and significantly reduces the risk of rebleeding and associated complications [57].
Prokinetic agents, particularly metoclopramide, have gained significance regarding pharmacological management during EGD. A meta-analysis investigating the impact of prokinetics in gastroscopy for patients with acute upper gastrointestinal bleeding highlighted the efficacy of metoclopramide. When administered before endoscopy, this agent effectively decreased the need for repeat endoscopy in selected patients, particularly those with active bleeding likely to exhibit blood in the stomach. While metoclopramide did not significantly improve other clinical outcomes, such as endoscopic visualization, blood transfusions, hospitalization duration, or surgery, its role in enhancing gastric emptying and managing gastroesophageal reflux is noteworthy. This underscores its utility as an adjunctive pharmacological option in EGD procedures, improving procedural efficiency and potentially improving patient outcomes [59].
The benefits of sedation are universally acknowledged, including enhanced detection rates and increased patient satisfaction and cooperation. However, the Asian Consensus, BSG/AUGIS, and ESGE emphasize that its administration should be judiciously determined, considering age, comorbidities, and aspiration risks, while potentially incorporating a blend of IV sedation and local anesthetic throat sprays [1][12][22][23].
4.4. Post-Procedural QIs
Post-procedural QIs are indispensable in ensuring comprehensive patient management following EGD. At the core of these indicators lies the necessity for thorough and precise documentation of findings and recommendations. The BSG/AUGIS and ESGE underscore the need to encapsulate these insights into comprehensive reports, ensuring accessibility for both patients and their healthcare providers [1][23].
Seamless patient communication emerges as a pivotal theme in post-procedural care. This resonates across the guidelines by the ASGE/ACG, Asian Consensus, and ESGE, each accentuating the imperative of reaching out to patients post-EGD to document any untoward incidents meticulously. The ASGE/ACG emphasizes the evolution of robust systems capable of capturing both immediate and delayed adverse events, advocating for all-encompassing post-procedural surveillance [21].
In conjunction with patient communication, the BSG/AUGIS elaborates on the merit of routinely auditing post-procedural outcomes. Tracking metrics like complications, subsequent hospital readmissions, and even mortality rates can shed light on the effectiveness of the procedure and potential avenues for refinement. Moreover, to bolster patient adherence to post-EGD care and empower them with knowledge, it is crucial to provide written and verbal instructions after the procedure [23].
As researchers navigate the intricacies of biopsies and histological evaluations, timely and efficient communication emerges as a linchpin. Beyond the elemental importance of biopsy handling and swift review, as previously highlighted, the BSG/AUGIS delves deeper by emphasizing the need to review histology results garnered during EGDs promptly. Further accentuating this, they underscore the urgency of formulating adept pathways that facilitate the rapid escalation of detected malignant lesions, ensuring their swift incorporation into multidisciplinary discussions and consequent decisions.
Lastly, the ESGE introduces a technological dimension to post-procedural care, endorsing the deployment of software tools geared toward enhancing report generation. Such tools champion precision and clarity in documentation and fortify standardization processes [1]. Furthermore, in the context of surveillance, especially for conditions like Barrett’s esophagus, vigilant monitoring of dysplasia occurrences is paramount. This proactive approach paves the way for prompt detection and intervention, fine-tuning patient outcomes [60].
For a comprehensive yet succinct overview of the pre-, intra-, and post-procedural QIs, please refer to the QIs checklist chart provided (Table 3) and its visual representation in Figure 2 for a concise visualization.
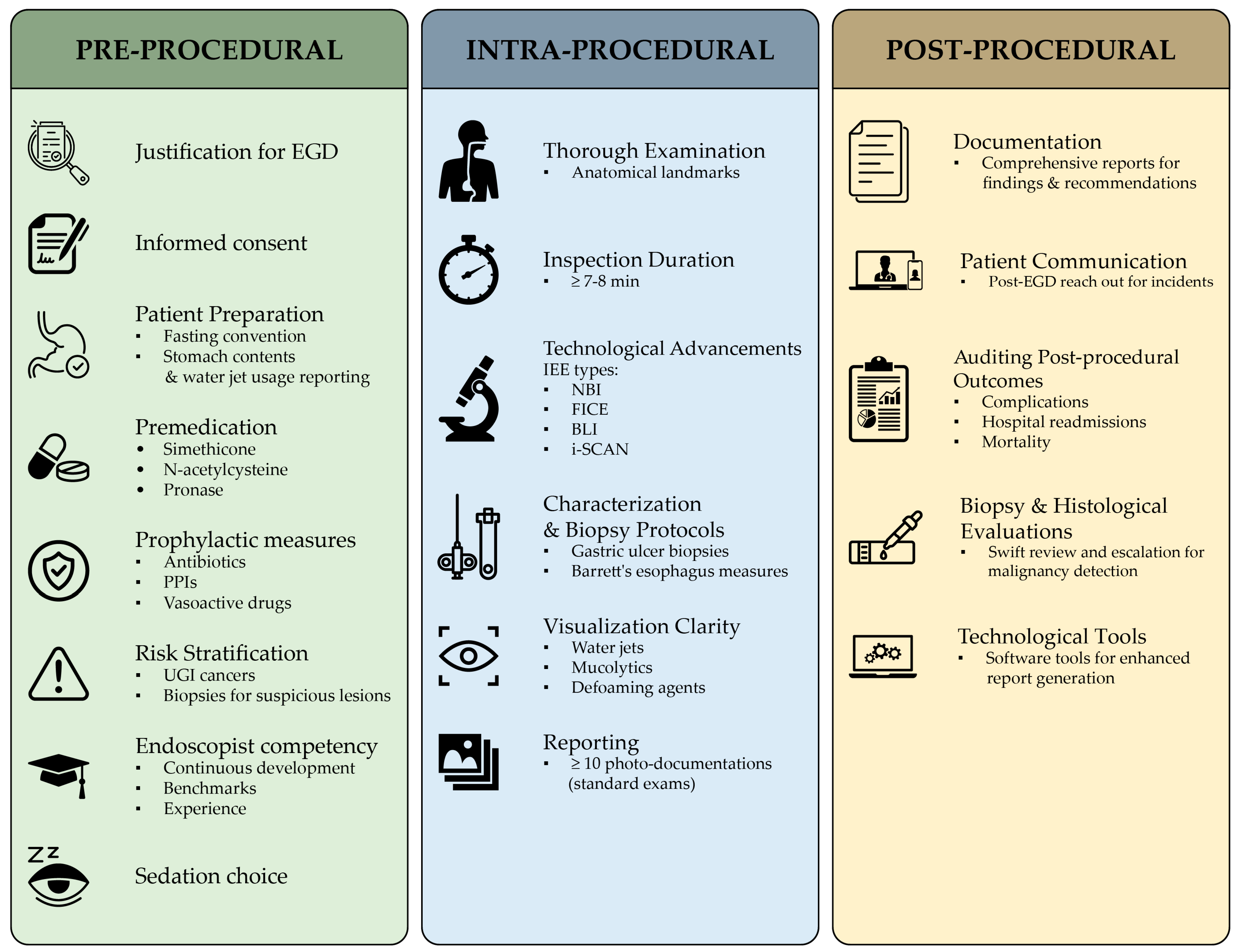
Figure 2. Concise representation of the pre-procedural, intra-procedural, and post-procedural QIs for an EGD procedure. This visualization provides a quick reference for the measurable benchmarks crucial for assessing each EGD healthcare delivery process phase. For a detailed checklist of these indicators, refer to Table 3.
Table 3. Pre-, intra-, and post-procedural QIs for EGD checklist.
| Quality Indicator | Definition | Criteria Met? | |||
|---|---|---|---|---|---|
| PRE-PROCEDURAL | Justification for EGD | The EGD is performed for a valid indication. | Yes | No | N/A |
| Informed consent | The patient is informed of risks and benefits and provides consent. | Yes | No | N/A | |
| Patient preparation | The patient is properly prepared for the EGD, including fasting and taking prescribed medications. | Yes | No | N/A | |
| Premedication | The patient is given appropriate premedication to prevent complications. | Yes | No | N/A | |
| Prophylactic measures | Prophylactic antibiotics are administered to patients at risk for infection. | Yes | No | N/A | |
| Risk stratification | The patient’s risk of complications is assessed and mitigated. | Yes | No | N/A | |
| Endoscopist competency | The endoscopist is qualified and experienced to perform EGDs. | Yes | No | N/A | |
| Sedation | The patient is sedated safely and comfortably for the EGD procedure. | Yes | No | N/A | |
| INTRA-PROCEDURAL | Thoroughness of the examination | The entire UGI tract is examined thoroughly. | Yes | No | N/A |
| Duration of the examination | The examination is performed in a timely manner. | Yes | No | N/A | |
| Imaging enhancement equipment | Appropriate imaging enhancement is used to improve visualization. | Yes | No | N/A | |
| Adherence to biopsy protocols | Biopsies are taken from suspicious lesions and characterized appropriately. | Yes | No | N/A | |
| Clarity of visualization | The endoscopist can visualize the entire UGI tract clearly. | Yes | No | N/A | |
| Photo-documentation | The EGD is documented photographically. | Yes | No | N/A | |
| POST-PROCEDURAL | Reporting | The EGD findings are reported accurately and in a timely manner. | Yes | No | N/A |
| Documentation of findings | The EGD findings and recommendations are documented | Yes | No | N/A | |
| Patient communication | The patient is informed of the EGD findings and recommendations. | Yes | No | N/A | |
| Auditing post-procedural outcomes | The post-procedural outcomes are audited to ensure quality. | Yes | No | N/A | |
| Biopsy handling and review | Biopsies are handled and reviewed appropriately. | Yes | No | N/A | |
| Escalation of malignant lesions | Detected malignant lesions are escalated to the appropriate level of care. | Yes | No | N/A | |
| Report generation | The EGD report is generated accurately and in a timely manner. | Yes | No | N/A | |
| Surveillance for dysplasia | Patients with Barrett’s esophagus are monitored for dysplasia. | Yes | No | N/A | |
4.5. Role of Advanced Endoscopic Techniques and Equipment
Emerging technology and innovative techniques have significantly contributed to enhancing EGD quality. High-definition endoscopes, image enhancement technologies like narrow-band imaging, and digital chromoendoscopy can improve the visualization of the mucosa, thereby increasing the detection of subtle lesions [61]. Endoscopic ultrasound (EUS) allows for detailed examination of the deeper layers of the gastrointestinal tract and adjacent structures, which is invaluable in staging cancers and diagnosing submucosal lesions [62]. Recent advancements such as confocal laser endomicroscopy (CLE) and optical coherence tomography (OCT) further augment mucosal imaging, enabling early detection of malignancies. These technologies have benefited Barrett’s esophagus, where targeted biopsies guided by CLE have improved diagnostic accuracy [63].
Additionally, therapeutic techniques like endoscopic submucosal dissection (ESD) have evolved, allowing for the precise and minimally invasive removal of early-stage tumors, a significant step forward from traditional surgical approaches [64]. While potentially offering improved outcomes, these advanced tools and techniques necessitate ongoing endoscopist education and training. It is crucial to stay up-to-date with annual recommendations from societies like the ASGE and ESGE, which incorporate these new techniques and their optimal timing into clinical practice [65].
AI has begun to revolutionize EGD, particularly in enhancing lesion detection and diagnosis. AI algorithms, including computer-assisted detection (CADe) for lesion detection and computer-assisted diagnosis (CADx) for optical biopsy and lesion characterization, offer unprecedented accuracy and efficiency in endoscopic procedures [66]. AI has demonstrated considerable efficiency in managing early gastric cancer (EGC) at different levels, from diagnosis to staging and automated lesion delineation. AI systems are also being employed to diagnose H. Pylori, showing high sensitivity and specificity, potentially reducing the number of unnecessary biopsies and providing real-time diagnoses [67].
The evolving landscape of EGD procedures, marked by integrating AI and advanced imaging techniques, underscores the importance of comprehensive training and continuous learning. A restructured approach to endoscopist training is essential for endoscopists to evolve with technological advancements and new educational methodologies. Modern training programs must incorporate modules on AI and other emerging technologies, focusing on their technical aspects and practical application in clinical settings. This includes understanding the strengths and limitations of AI in endoscopy, ethical considerations, and the interpretation of AI-assisted findings in the context of patient management [68].
Simulation-based training (SBT) has emerged as a critical component of teaching these advanced techniques. SBT allows for hands-on experience in a risk-free environment, enabling trainees to develop proficiency in using AI-assisted tools and interpreting complex imaging modalities. High-fidelity simulations, including virtual reality (VR) and augmented reality (AR) platforms, can replicate various endoscopic scenarios, from routine procedures to complex interventions [69]. Adaptive learning, another crucial element, personalizes the training experience, allowing trainees to focus on areas where they need the most improvement. This approach, often supported by AI algorithms, tailors the educational content and difficulty level based on individual performance, ensuring efficient skill development [70]. Furthermore, continuous education programs and workshops should be implemented, offering established endoscopists opportunities to update their skills and knowledge about the latest advancements. These programs could include case studies, interactive sessions, and peer discussions, promoting a culture of lifelong learning and adaptation to technological advancements [71].
Viewing the endoscopic procedure as a seamless part of the patient’s journey, commencing from the initial consultation and extending through follow-up, becomes paramount. Attaining high-quality EGD outcomes hinges not solely on the endoscopist’s technical expertise but also on embracing a holistic patient care approach that seamlessly integrates evolving best practices. A greater emphasis on patient-centered metrics is imperative, in line with evolving best practices. These metrics should encompass patient satisfaction, comfort during the procedure, and understanding of the process and outcomes. Tailoring EGD approaches to individual patient needs and preferences can significantly enhance patient experience and compliance.
References
- Bisschops, R.; Areia, M.; Coron, E.; Dobru, D.; Kaskas, B.; Kuvaev, R.; Pech, O.; Ragunath, K.; Weusten, B.; Familiari, P.; et al. Performance measures for upper gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy 2016, 48, 843–864.
- Matsubara, M.; Manabe, N.; Ayaki, M.; Nakamura, J.; Murao, T.; Fujita, M.; Kuinose, M.; Yamatsuji, T.; Naomoto, Y.; Haruma, K. Clinical significance of esophagogastroduodenoscopy in patients with esophageal motility disorders. Dig. Endosc. 2021, 33, 753–760.
- Uedo, N.; Gotoda, T.; Yoshinaga, S.; Tanuma, T.; Morita, Y.; Doyama, H.; Aso, A.; Hirasawa, T.; Yano, T.; Uchita, N.; et al. Differences in routine esophagogastroduodenoscopy between Japanese and international facilities: A questionnaire survey. Dig. Endosc. 2016, 28, 16–24.
- Yalamarthi, S.; Witherspoon, P.; McCole, D.; Auld, C.D. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy 2004, 36, 874–879.
- Sasako, M.; Inoue, M.; Lin, J.-T.; Khor, C.; Yang, H.-K.; Ohtsu, A. Gastric Cancer Working Group Report. Ultrasound Med. Biol. 2010, 40 (Suppl. S1), i28–i37.
- Kim, S.Y.; Park, J.M. Quality indicators in esophagogastroduodenoscopy. Clin. Endosc. 2022, 55, 319–331.
- Hamashima, C.; Ogoshi, K.; Narisawa, R.; Kishi, T.; Kato, T.; Fujita, K.; Sano, M.; Tsukioka, S. Impact of endoscopic screening on mortality reduction from gastric cancer Observational Study. World J. Gastroenterol. 2015, 21, 2460–2466.
- Park, J.H.; Kim, S.J.; Hyun, J.H.; Han, K.S.; Kim, B.C.; Hong, C.W.; Lee, S.-J.; Sohn, D.K. Correlation Between Bowel Preparation and the Adenoma Detection Rate in Screening Colonoscopy. Ann. Coloproctology 2017, 33, 93–98.
- Brunner, K.T.; Calderwood, A.H. Quality in Colonoscopy. Curr. Gastroenterol. Rep. 2015, 17, 38.
- Jain, R.; Chetty, R. Gastric Hyperplastic Polyps: A Review. Dig. Dis. Sci. 2009, 54, 1839–1846.
- Kavic, S.M.; Basson, M.D. Complications of endoscopy. Am. J. Surg. 2001, 181, 319–332.
- Triantafillidis, J.K. Sedation in gastrointestinal endoscopy: Current issues. World J. Gastroenterol. 2013, 19, 463–481.
- Quinn, L.; Kelly, M.E.; Khan, A.; Irwin, R.; Khan, W.; Barry, K.; Waldron, R.; Khan, I.Z. Sedation for gastroscopy: Is it an adequately understood and informed choice? Ir. J. Med. Sci. 2016, 185, 785–789.
- Meining, A.; Semmler, V.; Kassem, A.; Sander, R.; Frankenberger, U.; Burzin, M.; Reichenberger, J.; Bajbouj, M.; Prinz, C.; Schmid, R. The effect of sedation on the quality of upper gastrointestinal endoscopy: An investigator-blinded, randomized study comparing propofol with midazolam. Endoscopy 2007, 39, 345–349.
- Park, K.S. Introduction to Starting Upper Gastrointestinal Endoscopy: Proper Insertion, Complete Observation, and Appropriate Photographing. Clin. Endosc. 2015, 48, 279–284.
- Waddingham, W.; Kamran, U.; Kumar, B.; Trudgill, N.J.; Tsiamoulos, Z.P.; Banks, M. Complications of diagnostic upper Gastrointestinal endoscopy: Common and rare—Recognition, assessment and management. BMJ Open Gastroenterol. 2022, 9, e000688.
- Quality Indicators for Gastrointestinal Endoscopic Procedure…: Official Journal of the American College of Gastroenterology|ACG. Available online: https://journals.lww.com/ajg/Citation/2006/04000/Quality_Indicators_for_Gastrointestinal_Endoscopic.31.aspx (accessed on 20 July 2023).
- Cohen, J.; Safdi, M.A.; Deal, S.E.; Baron, T.H.; Chak, A.; Hoffman, B.; Jacobson, B.C.; Mergener, K.; Petersen, B.T.; Petrini, J.L.; et al. Quality indicators for esophagogastroduodenoscopy. Gastrointest. Endosc. 2006, 63, S10–S15.
- Waschke, K.A.; Anderson, J.; Valori, R.M.; MacIntosh, D.G.; Kolars, J.C.; DiSario, J.A.; Faigel, D.O.; Petersen, B.T.; Cohen, J. ASGE principles of endoscopic training. Gastrointest. Endosc. 2019, 90, 27–34.
- Januszewicz, W.; Kaminski, M.F. Quality indicators in diagnostic upper gastrointestinal endoscopy. Ther. Adv. Gastroenterol. 2020, 13, 1756284820916693.
- Park, W.G.; Shaheen, N.J.; Cohen, J.; Pike, I.M.; Adler, D.G.; Inadomi, J.M.; Laine, L.A.; Lieb, J.G.; Rizk, M.K.; Sawhney, M.S.; et al. Quality indicators for EGD. Gastrointest. Endosc. 2015, 81, 17–30.
- Chiu, P.W.Y.; Uedo, N.; Singh, R.; Gotoda, T.; Ng, E.K.W.; Yao, K.; Ang, T.L.; Ho, S.H.; Kikuchi, D.; Yao, F.; et al. An Asian consensus on standards of diagnostic upper endoscopy for neoplasia. Gut 2019, 68, 186–197.
- Beg, S.; Ragunath, K.; Wyman, A.; Banks, M.; Trudgill, N.; Pritchard, M.D.; Riley, S.; Anderson, J.; Griffiths, H.; Bhandari, P.; et al. Quality standards in upper gastrointestinal endoscopy: A position statement of the British Society of Gastroenterology (BSG) and Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS). Gut 2017, 66, 1886–1899.
- Lee, S.-H.; Park, Y.-K.; Cho, S.-M.; Kang, J.-K.; Lee, D.-J. Technical skills and training of upper gastrointestinal endoscopy for new beginners. World J. Gastroenterol. 2015, 21, 759–785.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Asplund, J.; Kauppila, J.H.; Mattsson, F.; Lagergren, J. Survival Trends in Gastric Adenocarcinoma: A Population-Based Study in Sweden. Ann. Surg. Oncol. 2018, 25, 2693–2702.
- Elvas, L.; Areia, M.; Brito, D.; Alves, S.; Saraiva, S.; Cadime, A.T. Premedication with simethicone and N-acetylcysteine in improving visibility during upper endoscopy: A double-blind randomized trial. Endoscopy 2017, 49, 139–145.
- Kim, G.H.; Cho, Y.K.; Cha, J.M.; Lee, S.-Y.; Chung, I.-K. Effect of pronase as mucolytic agent on imaging quality of magnifying endoscopy. World J. Gastroenterol. 2015, 21, 2483–2489.
- Lipp, A.; Lusardi, G. Systemic antimicrobial prophylaxis for percutaneous endoscopic gastrostomy. Cochrane Database Syst. Rev. 2013, 11, CD005571.pub3.
- Jain, N.K.; Larson, D.E.; Schroeder, K.W.; Burton, D.D.; Cannon, K.P.; Thompson, R.L.; DiMAGNO, E.P. Antibiotic Prophylaxis for Percutaneous Endoscopic Gastrostomy. Am. J. Gastroenterol. 1996, 91, 2301–2304.
- Thomas, S.; Cantrill, S.; Waghorn, D.J.; Mcintyre, A. The role of screening and antibiotic prophylaxis in the prevention of percutaneous gastrostomy site infection caused by methicillin-resistant Staphylococcus aureus. Aliment. Pharmacol. Ther. 2007, 25, 593–597.
- Leung, W.K.; Wu, M.-S.; Kakugawa, Y.; Kim, J.J.; Yeoh, K.-G.; Goh, K.L.; Wu, K.-C.; Wu, D.-C.; Sollano, J.; Kachintorn, U.; et al. Screening for gastric cancer in Asia: Current evidence and practice. Lancet Oncol. 2008, 9, 279–287.
- Barclay, R.L.; Vicari, J.J.; Doughty, A.S.; Johanson, J.F.; Greenlaw, R.L. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. New Engl. J. Med. 2006, 355, 2533–2541.
- Teh, J.L.; Tan, J.R.; Lau, L.J.F.; Saxena, N.; Salim, A.; Tay, A.; Shabbir, A.; Chung, S.; Hartman, M.; So, J.B.-Y. Longer examination time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy. Clin. Gastroenterol. Hepatol. 2015, 13, 480–487.e2.
- Gupta, N.; Gaddam, S.; Wani, S.B.; Bansal, A.; Rastogi, A.; Sharma, P. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest. Endosc. 2012, 76, 531–538.
- Kaltenbach, T.; Sano, Y.; Friedland, S.; Soetikno, R. American Gastroenterological Association (AGA) Institute Technology Assessment on Image-Enhanced Endoscopy. Gastroenterology 2008, 134, 327–340.
- Katagiri, A.; Kaneko, K.; Konishi, K.; Ito, H.; Kushima, M.; Mitamura, K. Lugol staining pattern in background epithelium of patients with esophageal squamous cell carcinoma. Hepatogastroenterology 2004, 51, 713–717.
- Ishihara, R.; Yamada, T.; Iishi, H.; Kato, M.; Yamamoto, S.; Yamamoto, S.; Masuda, E.; Tatsumi, K.; Takeuchi, Y.; Higashino, K.; et al. Quantitative analysis of the color change after iodine staining for diagnosing esophageal high-grade intraepithelial neoplasia and invasive cancer. Gastrointest. Endosc. 2009, 69, 213–218.
- Shimizu, Y.; Omori, T.; Yokoyama, A.; Yoshida, T.; Hirota, J.; Ono, Y.; Yamamoto, J.; Kato, M.; Asaka, M. Endoscopic diagnosis of early squamous neoplasia of the esophagus with iodine staining: High-grade intra-epithelial neoplasia turns pink within a few minutes. J. Gastroenterol. Hepatol. 2008, 23, 546–550.
- Peixoto, A.; Silva, M.; Pereira, P.; Macedo, G. Biopsies in Gastrointestinal Endoscopy: When and How. GE Port. J. Gastroenterol. 2016, 23, 19–27.
- Sanghi, V.; Thota, P.N. Barrett’s esophagus: Novel strategies for screening and surveillance. Ther. Adv. Chronic Dis. 2019, 10, 204062231983785.
- Husnoo, N.; Ahmed, W.; Shiwani, M.H. Duodenal biopsies for the diagnosis of coeliac disease: Are we adhering to current guidance? BMJ Open Gastroenterol. 2017, 4, e000140.
- Lucendo, A.J.; Molina-Infante, J.; Arias, Á.; Von Arnim, U.; Bredenoord, A.J.; Bussmann, C.; Dias, J.A.; Bove, M.; González-Cervera, J.; Larsson, H.; et al. Guidelines on eosinophilic esophagitis: Evidence-based statements and recommendations for diagnosis and management in children and adults. United Eur. Gastroenterol. J. 2017, 5, 335–358.
- Aruin, L.I. Importance of biopsy in stomach ulcer. Arkhiv Patol. 1989, 51, 70–76.
- Rugge, M.; Zaninotto, G.; Parente, P.; Zanatta, L.; Cavallin, F.; Germanà, B.; Macrì, E.; Galliani, E.; Iuzzolino, P.; Ferrara, F.; et al. Barrett’s Esophagus and Adenocarcinoma Risk: The experience of the North-Eastern Italian Registry (EBRA). Ann. Surg. 2012, 256, 788–795.
- Sikkema, M.; Looman, C.W.N.; Steyerberg, E.W.; Kerkhof, M.; Kastelein, F.; van Dekken, H.; van Vuuren, A.J.; Bode, W.A.; van der Valk, H.; Ouwendijk, R.J.T.; et al. Predictors for Neoplastic Progression in patients with barrett’s esophagus: A prospective cohort study. Am. J. Gastroenterol. 2011, 106, 1231–1238.
- García-Iglesias, P.; Villoria, A.; Suarez, D.; Brullet, E.; Gallach, M.; Feu, F.; Gisbert, J.P.; Barkun, A.; Calvet, X. Meta-analysis: Predictors of rebleeding after endoscopic treatment for bleeding peptic ulcer. Aliment. Pharmacol. Ther. 2011, 34, 888–900.
- Marmo, R.; Koch, M.; Cipolletta, L.; Capurso, L.; Grossi, E.; Cestari, R.; Bianco, M.A.; Pandolfo, N.; Dezi, A.; Casetti, T.; et al. Predicting mortality in non-variceal upper gastrointestinal bleeders: Validation of the italian pned score and prospective comparison with the rockall score. Am. J. Gastroenterol. 2010, 105, 1284–1291.
- Chiu, P.W.; Ng, E.K.; Cheung, F.K.; Chan, F.K.; Leung, W.; Wu, J.C.; Wong, V.W.; Yung, M.; Tsoi, K.; Lau, J.Y.; et al. Predicting mortality in patients with bleeding peptic ulcers after therapeutic endoscopy. Clin. Gastroenterol. Hepatol. 2009, 7, 311–316.
- Kuo, C.H.; Sheu, B.S.; Kao, A.W.; Wu, C.H.; Chuang, C.H. A Defoaming agent should be used with pronase premedication to improve visibility in upper gastrointestinal endoscopy. Endoscopy 2002, 34, 531–534.
- Neale, J.R.; James, S.; Callaghan, J.; Patel, P. Premedication with N-acetylcysteine and simethicone improves mucosal visualization during gastroscopy: A randomized, controlled, endoscopist-blinded study. Eur. J. Gastroenterol. Hepatol. 2013, 25, 778–783.
- Chang, C.-C. Premedication with pronase or N-acetylcysteine improves visibility during gastroendoscopy: An endoscopist-blinded, prospective, randomized study. World J. Gastroenterol. 2007, 13, 444–447.
- Laine, L.; Barkun, A.N.; Saltzman, J.R.; Martel, M.; Leontiadis, G.I. ACG clinical guideline: Upper gastrointestinal and ulcer bleeding. Am. J. Gastroenterol. 2021, 116, 899–917.
- Yen, H.-H.; Wu, P.-Y.; Wu, T.-L.; Huang, S.-P.; Chen, Y.-Y.; Chen, M.-F.; Lin, W.-C.; Tsai, C.-L.; Lin, K.-P. Forrest classification for bleeding peptic ulcer: A new look at the old endoscopic classification. Diagnostics 2022, 12, 1066.
- Jacques, J.; Legros, R.; Chaussade, S.; Sautereau, D. Endoscopic haemostasis: An overview of procedures and clinical scenarios. Dig. Liver Dis. 2014, 46, 766–776.
- Li, Y. Management of Variceal Hemorrhage. Gastroenterol. Res. 2009.
- Boregowda, U.; Umapathy, C.; Halim, N.; Desai, M.; Nanjappa, A.; Arekapudi, S.; Theethira, T.; Wong, H.; Roytman, M.; Saligram, S. Update on the management of gastrointestinal varices. World J. Gastrointest. Pharmacol. Ther. 2019, 10, 1–21.
- Barkun, A.N.; Almadi, M.; Kuipers, E.J.; Laine, L.; Sung, J.; Tse, F.; Leontiadis, G.I.; Abraham, N.S.; Calvet, X.; Chan, F.K.; et al. Management of nonvariceal upper gastrointestinal bleeding: Guideline recommendations from the international consensus group. Ann. Intern. Med. 2019, 171, 805.
- Barkun, A.N.; Bardou, M.; Martel, M.; Gralnek, I.M.; Sung, J.J. Prokinetics in acute upper GI bleeding: A meta-analysis. Gastrointest. Endosc. 2010, 72, 1138–1145.
- Dan, Y.Y.; So, J.; Yeoh, K.G. Endoscopic Screening for Gastric Cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 709–716.
- Muto, M.; Horimatsu, T.; Ezoe, Y.; Morita, S.; Miyamoto, S. Improving visualization techniques by narrow band imaging and magnification endoscopy. J. Gastroenterol. Hepatol. 2009, 24, 1333–1346.
- Brand, B.; Oesterhelweg, L.; Binmoeller, K.; Sriram, P.; Bohnacker, S.; Seewald, S.; De Weerth, A.; Soehendra, N. Impact of endoscopic ultrasound for evaluation of submucosal lesions in gastrointestinal tract. Dig. Liver Dis. 2002, 34, 290–297.
- Fugazza, A.; Gaiani, F.; Carra, M.C.; Brunetti, F.; Lévy, M.; Sobhani, I.; Azoulay, D.; Catena, F.; De’angelis, G.L.; De’angelis, N. Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases: A systematic review and meta-analysis. BioMed Res. Int. 2016, 2016, 4638683.
- Gotoda, T.; Jung, H.-Y. Endoscopic resection (endoscopic mucosal resection/ endoscopic submucosal dissection) for early gastric cancer. Dig. Endosc. 2013, 25, 55–63.
- Parasher, G.; Wong, M.; Rawat, M. Evolving role of artificial intelligence in gastrointestinal endoscopy. World J. Gastroenterol. 2020, 26, 7287–7298.
- Gora, M.J.; Suter, M.J.; Tearney, G.J.; Li, X. Endoscopic optical coherence tomography: Technologies and clinical applications . Biomed. Opt. Express 2017, 8, 2405–2444.
- El Hajjar, A.; Rey, J.-F. Artificial intelligence in gastrointestinal endoscopy: General overview. Chin. Med. J. 2020, 133, 326–334.
- Grover, S.C.; Walsh, C.M. Integrating artificial intelligence into endoscopy training: Opportunities, challenges, and strategies. Lancet Gastroenterol. Hepatol. 2023, 9, 11–13.
- Mahmood, T.; Scaffidi, M.A.; Khan, R.; Grover, S.C. Virtual reality simulation in endoscopy training: Current evidence and future directions. World J. Gastroenterol. 2018, 24, 5439–5445.
- Maulahela, H.; Annisa, N.G.; Konstantin, T.; Syam, A.F.; Soetikno, R. Simulation-based mastery learning in gastrointestinal endoscopy training. World J. Gastrointest. Endosc. 2022, 14, 512–523.
- Khan, R.; Scaffidi, M.A.; Grover, S.C.; Gimpaya, N.; Walsh, C.M. Simulation in endoscopy: Practical educational strategies to improve learning. World J. Gastrointest. Endosc. 2019, 11, 209–218.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
625
Revisions:
2 times
(View History)
Update Date:
28 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




