Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Domen Vozel | -- | 1729 | 2023-12-27 08:24:40 | | | |
| 2 | Catherine Yang | Meta information modification | 1729 | 2023-12-27 08:37:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Božanić Urbančič, N.; Battelino, S.; Vozel, D. Appropriate Vestibular Stimulation in Children and Adolescents. Encyclopedia. Available online: https://encyclopedia.pub/entry/53163 (accessed on 07 March 2026).
Božanić Urbančič N, Battelino S, Vozel D. Appropriate Vestibular Stimulation in Children and Adolescents. Encyclopedia. Available at: https://encyclopedia.pub/entry/53163. Accessed March 07, 2026.
Božanić Urbančič, Nina, Saba Battelino, Domen Vozel. "Appropriate Vestibular Stimulation in Children and Adolescents" Encyclopedia, https://encyclopedia.pub/entry/53163 (accessed March 07, 2026).
Božanić Urbančič, N., Battelino, S., & Vozel, D. (2023, December 27). Appropriate Vestibular Stimulation in Children and Adolescents. In Encyclopedia. https://encyclopedia.pub/entry/53163
Božanić Urbančič, Nina, et al. "Appropriate Vestibular Stimulation in Children and Adolescents." Encyclopedia. Web. 27 December, 2023.
Copy Citation
The structural development of the vestibular part of the inner ear is completed by birth but its central connections continue to develop until adolescence. Their development is dependent on vestibular stimulation—vestibular experience. Studies have shown that vestibular function, modulated by experience and epigenetic factors, is not solely an instrument for body position regulation, navigation, and stabilization of the head and images but also influences cognition, emotion, the autonomous nervous system and hormones.
dizziness
vertigo
sedentary behavior
screen time
1. Introduction
The vestibular system is one of the most essential senses, proven by the fact that it is the most ancient sensory system in vertebrates. In early vertebrates, the inner ear only provided balance information [1].
Moreover, studies have demonstrated that the auditory portion of the inner ear, which, in Homo sapiens, is of utmost importance for everyday functioning, has evolved out of its vestibular part [2].
The vestibular part of the inner ear consists of semicircular canals and otolith organs that enable the navigation of the head and body in different environments and stabilize a picture on the retina during head movements. This is accomplished by the vestibulo-ocular, vestibulo-cervical and vestibulo-spinal reflex [3]. Structural inner ear development is completed by birth, but the central parts of the vestibular system continue to develop until adolescence.
Vestibular information, in contrast to other sensory information, is spread to different parts of the central nervous system, forming the vestibular system-associated network. There is evidence that vestibular information also influences emotions [4][5][6], cognition [6][7][8][9][10][11], the endocrine system [12], the autonomous system [13][14] and memory and cognition [15].
2. Development of the Vestibular Reflexes
The vestibular reflexes enable gaze and posture stability during everyday activities. Mature neuronal connections, peripheral organs and vestibular nuclei are essential for their function [16]. Neuronal connections between the inner ear and oculomotor nuclei in the brainstem occur between the 12th and 24th week. Myelination of the vestibular nerve begins around the 20th week. It is the first cranial nerve to complete myelination [17]. The vestibular nuclear complex is functional at 21 weeks of gestation. The vestibulo-ocular reflex (VOR) is present at birth. Infants demonstrate inaccurate saccades, frequently requiring more than one to reach the target [18]. Maturation of the saccadic system is expected to be completed by two years of age [18]. Children have a higher gain of the VOR as a response to sinusoidal rotation than adults and, due to immature visual–vestibular interaction, also a poorer suppression of the VOR response. Most normal children have vestibular responses to caloric and rotational stimuli by two months of age. Consequently, an absence of the VOR at ten months of age is considered abnormal [19]. As a consequence of the maturation of visual pathways, the time constant approaches adult values by the age of 2 months. [18].
Compared to the VOR pathway, the vestibulo-spinal pathways are more complex because of the number of muscles and joints that are controlled, the variety of activity patterns and the biomechanical constraints, which vary considerably throughout development [1]. Vestibulo-spinal and vestibulo-colic reflexes mature gradually until adolescence [20]. The central vestibular pathways mature until 11 to 15 years of age [8]. Consequently, differences between children’s and adults’ VEMP (vestibular-evoked myogenic potential) responses can be observed [21][22], suggesting maturational effects from preschool through adolescence. It is not surprising that vestibular stimulation is crucial in that developmental period. Due to suppressed vestibular stimulation (for various possible reasons), various difficulties can appear, such as delayed acquisition of motor skills, problems with cognition, reading (further compromised language skills in hearing loss patients), altered spatial representations, etc. [8][23].
Studies have indicated that the developing nervous system cannot compensate for a vestibular deficit during the early phase of ontogeny [24] (Figure 1). Achievements during the postnatal development of the vestibular system are presented in Figure 1.
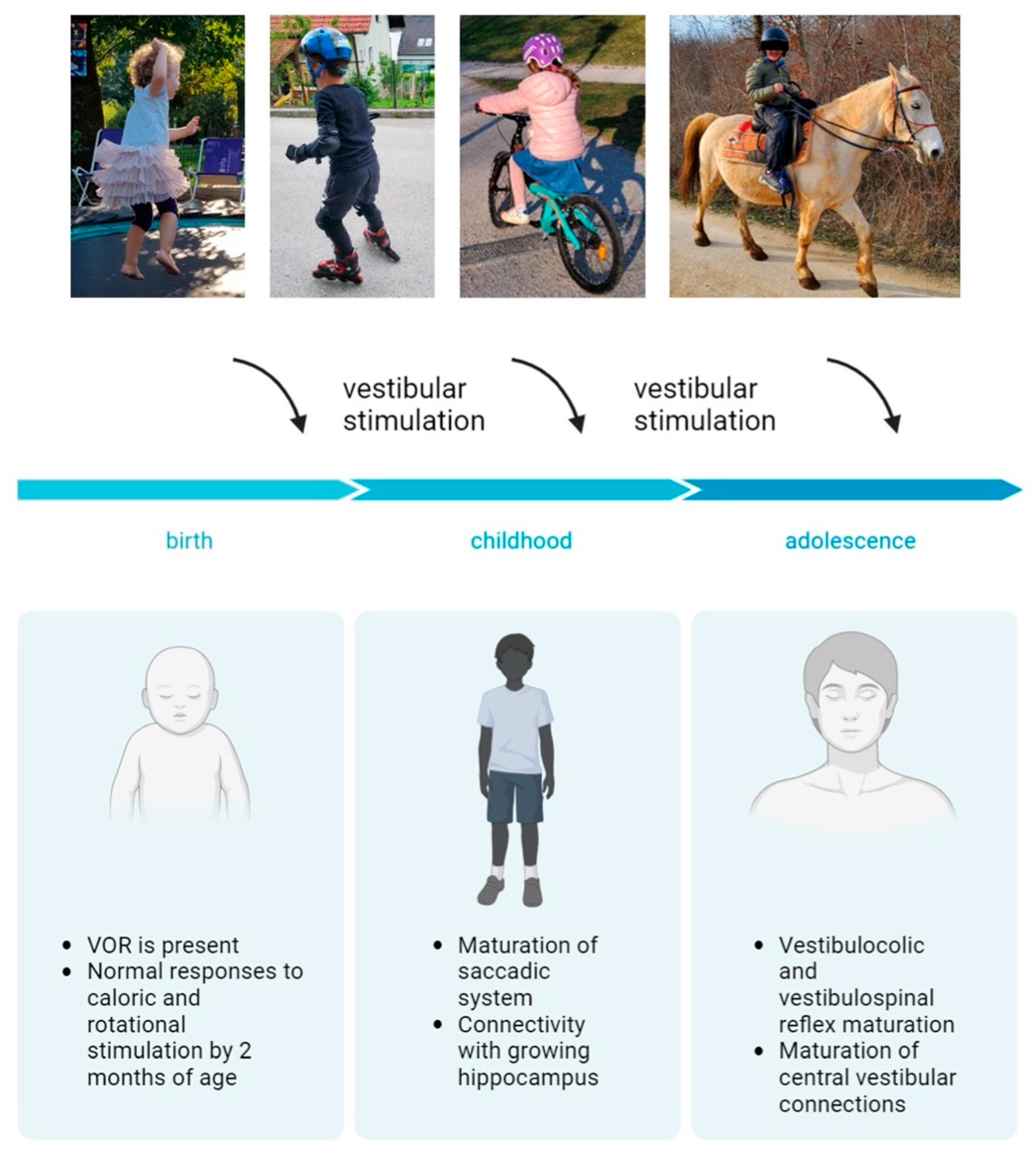
Figure 1. Postnatal development of the vestibular system. Created with BioRender.com.
3. Vestibular Screening—A Necessary Tool for Timely Identification of Children with an Underdeveloped Vestibular System
Universal hearing screening has enabled timely and successful hearing rehabilitation of hard-of-hearing children, contributing significantly to normal child development, socialization and education.
Vestibular screening would enable early detection of vestibular impairment in a child, leading to appropriate vestibular rehabilitation.
The idea of vestibular screening goes back to 1974, when Isabelle Rapin published her observations when performing vestibular tests on hard-of-hearing children and concluded that vestibular assessment should not be limited only to at-risk populations [25].
The screening method should be chosen based on the child’s age at the time of the screening and the expected level of vestibular system development. The method should be applicable for routine use in pediatric outpatient clinics [26]. Some authors use the test of the vestibulo-spinal reflex (Romberg test—the ability to maintain erect posture with the eyes closed, standing on one foot, walking) as a screening method. Since the vestibulo-ocular reflex matures earlier, should consider applying one of the tests for VOR.
The brain builds representations of verticality based on vestibular, somatosensory, proprioceptive and visual information. Knowing how vital the vestibular system and its central connections are for the normal development and function of balance and navigation, cognition, endocrine, vegetative system, etc., we should consider universal vestibular screening in children. Early detection of vestibular dysfunction would enable adequate and timely rehabilitation, normal postural control acquisition, gaze stabilization and prevention of secondary sequelae involving multiple systems.
4. The Vestibular System-Associated Network
The vestibular system is the only sensory system whose information is not directed to a single and specific brain area [27] (Figure 2). Vestibular information is spread from the vestibular nuclei to different parts of the central nervous system, forming a vestibular system-associated network [9][27][28]. Studies have proven that this network significantly impacts the function of various organs and systems, and that there are complex interactions between central vestibular fields and cognitive, oculomotor and emotional circuits [29][30]. The connections between the vestibular nuclei and frontal, prefrontal, parietal, cingulate, occipital, posterior and medial temporal cortex, insula and retroinsular cortex, thalamus, hippocampus, cerebellum, the Sylvian fissure and the brainstem have been described [14][23][28][31] (Figure 2). The vestibular network information and cognitive functions are integrated at the cortical level and within the hippocampal and limbic systems [4][6][10]. Not all of the functions of the above-mentioned cortical areas and their possible interactions and pathways are known [23]. Studies assessing vestibular-associated networks use electrical, caloric, visual or sound stimulation to induce the vestibular response [32][33][34][35][36]. The current testing methods lack the possibility of functional brain imaging during head and body movement.
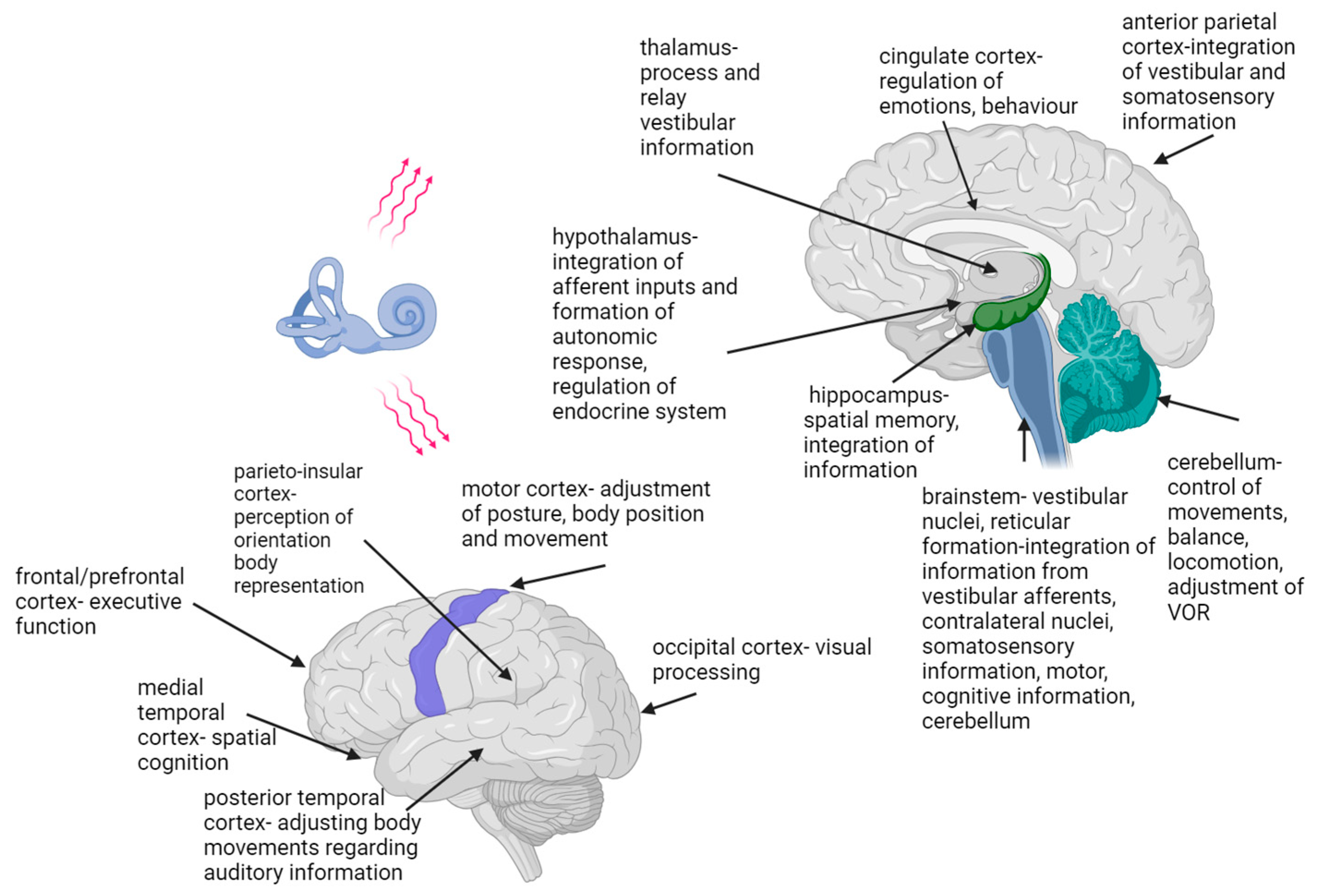
On the basis of an experiment using caloric stimulation, Batchold suggested that vestibular stimulation leads precisely to enhanced functioning of the contralateral cerebral structures, the parahippocampal gyrus and the temporal lobe [35]. As Wilkinson et al. showed, electrical vestibular stimulation positively affects the speed of visual memory recollection [32]. Recent studies have indicated that non-invasive electrical stimulation causes simultaneous activation of semicircular canals and otolith systems and can improve neuronal plasticity in the central pathways [34].
Much has been learned from studies on unilaterally and bilaterally vestibular-impaired subjects. The most straightforward are gaze and fixation problems associated with vestibular dysfunction, which can cause decreased visual acuity and consequent reading problems [40]. Some studies have demonstrated that humans with vestibular disorders show a range of cognitive deficits that are not just the consequence of spatial information deficits, such as spatial navigation, spatial learning and spatial memory, but also of problems with establishing a mental representation of the world, which could be connected to impaired numerical skills in vestibular-impaired subjects [10][23][31]. These cognitive deficits also include non-spatial functions such as object recognition memory, self-motion perception and bodily self-awareness [31][41]. Other cognitive deficits related to vestibular dysfunction include the impairment of short-term memory and concentration and impaired numerical skills, attention and cognitive processing ability [10][11]. In his 2015 review, Lopez argued that vestibular information impacts the perception of the body on different levels: from the perception of touch, pain and body dimensions to the feeling of owning a body, the feeling of being placed within one’s body and the visuospatial perception of the body [42].
The visual interpretation of body posture is used to anticipate emotions and intentions and is also essential in social interactions [43].
The influence of vestibular information on emotions has been known since ancient times. Hanging beds, providing low-frequency vestibular stimulation, were used to induce sleep and relieve pain. Similarly, spinning chairs, with their high-frequency stimulation, have been used to treat mental illnesses, such as manic episodes [3][4]. In relation to the influence on emotions, vestibular dysfunction can not only induce or cause a worsening of anxiety, depression and other affective disorders, but there is evidence that affective conditions may also influence the central vestibular systems [30][44][45][46]. This is not surprising, knowing the effects of vestibular impairment on everyday functioning, daily activities, social interactions and the connections of the vestibular system with the limbic system (Figure 2). There is also robust evidence in the literature of a close functional interplay between vestibular and anxiety systems [47].
Moreover, vestibular–autonomic networks have also been described, which explain the influence of body position on autonomic regulation of blood pressure and respiratory activity [13][14][48]. Interestingly, the receptors of adrenal gland hormones, thyroid hormones, sex hormones and insulin have been identified in inner ear structures, the vestibular nerve and the vestibular nuclei [12]. Electrical activation of the vestibular system alters sympathetic system activity throughout the body, with the renal nerve being the most sensitive to vestibular inputs [13]. The insular cortex has been recognized as a cortical site for vestibular and autonomic integration and modulation [49][50].
Regarding the influence of sex hormones, studies have confirmed the influence of female sex hormones on susceptibility to peripheral and central vestibular disorders [51][52]. A study by Serra et al. found altered glucose and insulin levels in patients with peripheral vestibular disorders [53]. Congenital hypothyroidism, among other organs, also has a negative effect on inner ear development [54]. A connection between the inner ear and thyroid cells has also been found concerning exposure to gravity, which modulated the thyroid cells in a study by Grimm [55]. The positive experience that clinicians dealing with acutely vestibular-impaired patients have had with corticosteroid therapy is one of the signs of vestibule–adrenal interactions. Corticosteroids are found to accelerate compensation after vestibular loss in guinea pigs [56].
There are not as many studies exploring vestibular connections to hormonal balance as there are for other vestibular connections.
References
- Beraneck, M.; Lambert, F.M.; Sadeghi, S.G. Functional Development of the Vestibular System. In Development of Auditory and Vestibular Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 449–487.
- Duncan, J.S.; Fritzsch, B. Evolution of Sound and Balance Perception: Innovations That Aggregate Single Hair Cells into the Ear and Transform a Gravistatic Sensor into the Organ of Corti. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2012, 295, 1760–1774.
- Highstein, S.M.; Fay, R.R.; Popper, A.N. The Vestibular System. In Springer Handbook of Auditory Research; Highstein, S.M., Fay, R.R., Popper, A.N., Eds.; Springer: New York, NY, USA, 2004; Volume 19, ISBN 978-0-387-98314-1.
- Rajagopalan, A.; Jinu, K.; Sailesh, K.; Mishra, S.; Reddy, U.; Mukkadan, J. Understanding the Links between Vestibular and Limbic Systems Regulating Emotions. J. Nat. Sci. Biol. Med. 2017, 8, 11.
- Kumar Goothy, S.S.; McKeown, J. Anxiolytic Effects of Vestibular Stimulation: An Update. J. Basic Clin. Physiol. Pharmacol. 2023, 34, 445–449.
- Bigelow, R.T.; Semenov, Y.R.; Hoffman, H.J.; Agrawal, Y. Association between Vertigo, Cognitive and Psychiatric Conditions in U.S. Children: 2012 National Health Interview Survey. Int. J. Pediatr. Otorhinolaryngol. 2020, 130, 109802.
- Risey, J.; Briner, W. Dyscalculia in Patients with Vertigo. J. Vestib. Res. Equilib. Orientat. 1990, 1, 31–37.
- Wiener-Vacher, S.R.; Hamilton, D.A.; Wiener, S.I. Vestibular Activity and Cognitive Development in Children: Perspectives. Front. Integr. Neurosci. 2013, 7, 92.
- Smith, J.L.; Diekfuss, J.A.; Dudley, J.A.; Ahluwalia, V.; Zuleger, T.M.; Slutsky-Ganesh, A.B.; Yuan, W.; Foss, K.D.B.; Gore, R.K.; Myer, G.D.; et al. Visuo-vestibular and Cognitive Connections of the Vestibular Neuromatrix Are Conserved across Age and Injury Populations. J. Neuroimaging 2023, 33, 1003–1014.
- Hanes, D.A.; McCollum, G. Cognitive-Vestibular Interactions: A Review of Patient Difficulties and Possible Mechanisms. J. Vestib. Res. Equilib. Orientat. 2006, 16, 75–91.
- Ozkul, A.; Konukseven, O. The Development of the Cognitive Vestibular Function Scale in the Elderly Complaints of Imbalance: A Study on Validity and Reliability. Braz. J. Otorhinolaryngol. 2023, 89, 101282.
- El Khiati, R.; Tighilet, B.; Besnard, S.; Chabbert, C. Vestibular Disorders and Hormonal Dysregulations: State of the Art and Clinical Perspectives. Cells 2023, 12, 656.
- Kerman, I.A.; McAllen, R.M.; Yates, B.J. Patterning of Sympathetic Nerve Activity in Response to Vestibular Stimulation. Brain Res. Bull. 2000, 53, 11–16.
- Yates, B.J.; Bronstein, A.M. The Effects of Vestibular System Lesions on Autonomic Regulation: Observations, Mechanisms, and Clinical Implications. J. Vestib. Res. Equilib. Orientat. 2005, 15, 119–129.
- Kumar, S.; Archana, R.; Mukkadan, J. Effect of Vestibular Stimulation on Spatial and Verbal Memory in College Students. Natl. Med. J. India 2017, 30, 337.
- Cullen, K.E.; Taube, J.S. Our Sense of Direction: Progress, Controversies and Challenges. Nat. Neurosci. 2017, 20, 1465–1473.
- Hadders-Algra, M. Early Human Motor Development: From Variation to the Ability to Vary and Adapt. Neurosci. Biobehav. Rev. 2018, 90, 411–427.
- Nandi, R.; Luxon, L.M. Development and Assessment of the Vestibular System. Int. J. Audiol. 2008, 47, 566–577.
- Fife, T.D.; Tusa, R.J.; Furman, J.M.; Zee, D.S.; Frohman, E.; Baloh, R.W.; Hain, T.; Goebel, J.; Demer, J.; Eviatar, L. Assessment: Vestibular Testing Techniques in Adults and Children: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2000, 55, 1431–1441.
- O’Reilly, R.; Grindle, C.; Zwicky, E.F.; Morlet, T. Development of the Vestibular System and Balance Function: Differential Diagnosis in the Pediatric Population. Otolaryngol. Clin. N. Am. 2011, 44, 251–271.
- Kelsch, T.A.; Schaefer, L.A.; Esquivel, C.R. Vestibular Evoked Myogenic Potentials in Young Children: Test Parameters and Normative Data. Laryngoscope 2006, 116, 895–900.
- Young, Y.-H. Assessment of Functional Development of the Otolithic System in Growing Children: A Review. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 435–442.
- Hitier, M.; Besnard, S.; Smith, P.F. Vestibular Pathways Involved in Cognition. Front. Integr. Neurosci. 2014, 8, 59.
- Geisler, H.C.; Gramsbergen, A. Motor Development after Vestibular Deprivation in Rats. Neurosci. Biobehav. Rev. 1998, 22, 565–569.
- Rapin, I. Hypoactive Labyrinths and Motor Development. Clin. Pediatr. 1974, 13, 922–937.
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Déry, V. Revisiting Wilson and Jungner in the Genomic Age: A Review of Screening Criteria over the Past 40 Years. Bull. World Health Organ. 2008, 86, 317–319.
- Raiser, T.M.; Flanagin, V.L.; Duering, M.; van Ombergen, A.; Ruehl, R.M.; zu Eulenburg, P. The Human Corticocortical Vestibular Network. NeuroImage 2020, 223, 117362.
- Brandt, T.; Strupp, M.; Dieterich, M. Towards a Concept of Disorders of Higher Vestibular Function. Front. Integr. Neurosci. 2014, 8, 47.
- Indovina, I.; Bosco, G.; Riccelli, R.; Maffei, V.; Lacquaniti, F.; Passamonti, L.; Toschi, N. Structural Connectome and Connectivity Lateralization of the Multimodal Vestibular Cortical Network. NeuroImage 2020, 222, 117247.
- Nagaratnam, N.; Ip, J.; Bou-Haidar, P. The Vestibular Dysfunction and Anxiety Disorder Interface: A Descriptive Study with Special Reference to the Elderly. Arch. Gerontol. Geriatr. 2005, 40, 253–264.
- Mast, F.W.; Preuss, N.; Hartmann, M.; Grabherr, L. Spatial Cognition, Body Representation and Affective Processes: The Role of Vestibular Information beyond Ocular Reflexes and Control of Posture. Front. Integr. Neurosci. 2014, 8, 44.
- Wilkinson, D.; Nicholls, S.; Pattenden, C.; Kilduff, P.; Milberg, W. Galvanic Vestibular Stimulation Speeds Visual Memory Recall. Exp. Brain Res. 2008, 189, 243–248.
- Frank, S.M.; Greenlee, M.W. The Parieto-Insular Vestibular Cortex in Humans: More than a Single Area? J. Neurophysiol. 2018, 120, 1438–1450.
- Lopez, C.; Cullen, K.E. Electrical Stimulation of the Peripheral and Central Vestibular System. Curr. Opin. Neurol. 2023.
- Bächtold, D.; Baumann, T.; Sándor, P.S.; Kritos, M.; Regard, M.; Brugger, P. Spatial- and Verbal-Memory Improvement by Cold-Water Caloric Stimulation in Healthy Subjects. Exp. Brain Res. 2001, 136, 128–132.
- Carvalho, G.F.; Mehnert, J.; Basedau, H.; Luedtke, K.; May, A. Brain Processing of Visual Self-Motion Stimuli in Patients with Migraine. Neurology 2021, 97, e996–e1006.
- Hernandez, E.; Das, J.M. Neuroanatomy, Nucleus Vestibular. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562261/ (accessed on 16 November 2023).
- Morton, S.M.; Bastian, A.J. Cerebellar Control of Balance and Locomotion. Neuroscientist 2004, 10, 247–259.
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.A.; Hudspeth, A.J. Priciples of Neural Science, 5th ed.; McGraw Hill: New York, NY, USA, 2014; ISBN 978-0-07-139011-8.
- Braswell, J.; Rine, R.M. Evidence That Vestibular Hypofunction Affects Reading Acuity in Children. Int. J. Pediatr. Otorhinolaryngol. 2006, 70, 1957–1965.
- Smith, P.F.; Zheng, Y.; Horii, A.; Darlington, C.L. Does Vestibular Damage Cause Cognitive Dysfunction in Humans? J. Vestib. Res. Equilib. Orientat. 2005, 15, 1–9.
- Lopez, C. Making Sense of the Body: The Role of Vestibular Signals. Multisensory Res. 2015, 28, 525–557.
- Deroualle, D.; Lopez, C. Toward a Vestibular Contribution to Social Cognition. Front. Integr. Neurosci. 2014, 8, 16.
- Bayat, A.; Hoseinabadi, R.; Saki, N.; Sanayi, R. Disability and Anxiety in Vestibular Diseases: A Cross-Sectional Study. Cureus 2020, 12, e11813.
- Jacob, R.G.; Furman, J.M. Psychiatric Consequences of Vestibular Dysfunction. Curr. Opin. Neurol. 2001, 14, 41–46.
- Dieterich, M.; Brandt, T. Central Vestibular Networking for Sensorimotor Control, Cognition, and Emotion. Curr. Opin. Neurol. 2023.
- Brandt, T.; Dieterich, M. ‘Excess Anxiety’ and ‘Less Anxiety’: Both Depend on Vestibular Function. Curr. Opin. Neurol. 2020, 33, 136–141.
- Heyer, G.L.; Young, J.A.; Fischer, A.N. Lightheadedness after Concussion: Not All Dizziness Is Vertigo. Clin. J. Sport Med. 2018, 28, 272–277.
- Bogle, J.M.; Benarroch, E.; Sandroni, P. Vestibular-Autonomic Interactions: Beyond Orthostatic Dizziness. Curr. Opin. Neurol. 2022, 35, 126–134.
- Nagai, M.; Scheper, V.; Lenarz, T.; Förster, C.Y. The Insular Cortex as a Vestibular Area in Relation to Autonomic Function. Clin. Auton. Res. 2021, 31, 179–185.
- Mucci, V.; Hamid, M.; Jacquemyn, Y.; Browne, C.J. Influence of Sex Hormones on Vestibular Disorders. Curr. Opin. Neurol. 2022, 35, 135–141.
- Smith, P.F.; Agrawal, Y.; Darlington, C.L. Sexual Dimorphism in Vestibular Function and Dysfunction. J. Neurophysiol. 2019, 121, 2379–2391.
- Paula Serra, A.; De Carvalho Lopes, K.; Dorigueto, R.S.; Freitas Ganança, F. Blood Glucose and Insulin Levels in Patients with Peripheral Vestibular Disease. Braz. J. Otorhinolaryngol. 2009, 75, 701–705.
- Rastoldo, G.; Tighilet, B. Thyroid Axis and Vestibular Physiopathology: From Animal Model to Pathology. Int. J. Mol. Sci. 2023, 24, 9826.
- Grimm, D.; Kossmehl, P.; Shakibaei, M.; Schulze-Tanzil, G.; Pickenhahn, H.; Bauer, J.; Paul, M.; Cogoli, A. Effects of Simulated Microgravity on Thyroid Carcinoma Cells. J. Gravitational Physiol. J. Int. Soc. Gravitational Physiol. 2002, 9, P253–P256.
- Gliddon, C.M.; Smith, P.F.; Darlington, C.L. Interaction Between the Hypothalamic—Pituitary—Adrenal Axis and Behavioural Compensation Following Unilateral Vestibular Deafferentation. Acta Otolaryngol. 2003, 123, 1013–1021.
More
Information
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
27 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





