Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Daryoush Shahbazi-Gahrouei | -- | 5312 | 2023-12-26 09:11:39 | | | |
| 2 | Mona Zou | Meta information modification | 5312 | 2023-12-26 09:51:16 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Aminolroayaei, F.; Shahbazi-Gahrouei, S.; Khorasani, A.; Shahbazi-Gahrouei, D. Nanoparticles for Breast Cancer Diagnosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/53129 (accessed on 07 February 2026).
Aminolroayaei F, Shahbazi-Gahrouei S, Khorasani A, Shahbazi-Gahrouei D. Nanoparticles for Breast Cancer Diagnosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/53129. Accessed February 07, 2026.
Aminolroayaei, Fahimeh, Saghar Shahbazi-Gahrouei, Amir Khorasani, Daryoush Shahbazi-Gahrouei. "Nanoparticles for Breast Cancer Diagnosis" Encyclopedia, https://encyclopedia.pub/entry/53129 (accessed February 07, 2026).
Aminolroayaei, F., Shahbazi-Gahrouei, S., Khorasani, A., & Shahbazi-Gahrouei, D. (2023, December 26). Nanoparticles for Breast Cancer Diagnosis. In Encyclopedia. https://encyclopedia.pub/entry/53129
Aminolroayaei, Fahimeh, et al. "Nanoparticles for Breast Cancer Diagnosis." Encyclopedia. Web. 26 December, 2023.
Copy Citation
Breast cancer (BC) is the foremost common cause of death in women, and its early diagnosis will help treat and increase patients’ survival. BC is about 100 times more common in women than in men. The risk factors for BC are alcohol intake, body mass index, hormone replacement therapy with estrogen and progesterone, radiation exposure, early and late menarche, and late age in first childbirth. Also, current age (increasing age increases the risk of developing BC), history of BC, breast biopsy, cytology, family history, inherited mutation in the BRCA1 or BRCA2 gene, and the risk of BC and interval cancers is four to six times higher in women with very dense breast (DB) tissue than in those with fatty breasts.
breast cancer
diagnostic
mammography
ultrasound
magnetic resonance imaging
1. Mammography (MG)
Mammography includes imaging the breast using low-energy X-rays, which detect BC, benign tumors, and cysts before detection by touch. It has a sensitivity or true positive of about 75%, but in middle-aged people with higher breast tissue density, the sensitivity decreases to about 50% [1]. MG has some advantages. First, it is the gold standard for diagnosing BC patients. Second, MG is suitable as a screening method for disease prevention, as it helps in the finding and removal of premalignant precursors of cancer and in the early detection of cancer for BC [2]. It is demonstrated that the risk of death from BC in women who are invited for screening is reduced by 22% compared to women who are not invited [3].
It should be noted that screening methods such as DM, DBT, and CEM should be considered to have small or negligible risks of radiation-induced cancer or death. Still, screening methods with radionuclide injection have significantly higher cancer risks unless efficient detection systems and prescribed dose reductions are used [4]. Third, MG can find mammary gland calcification. It is known that approximately 25 to 43% of non-palpable cancers are detected in MG due to microcalcifications [5]. Of course, MG is not suitable for people under 40 years of age, it cannot be undertaken more than twice a year [6], and it is limited in imaging DB tissue [7].
1.1. Full-Field Digital Mammography (FFDM)
Mammography can be performed using screen film (SFM) or DM. The advantages of SFM include its high contrast and the high spatial resolution of about 15–20-line pairs/mm. Limitations include the limited dynamic range, and film display characteristics such as brightness and contrast are fixed after the film is developed in a chemical processor. Compared to SFM, DM has advantages such as a more comprehensive dynamic range and better contrast resolution, especially for DB tissue. It is also possible to use post-processing on the digital image to increase the quality of the image [8]. It has been found that DM has similar accuracy, specificity, and sensitivity as SFM in diagnosing BC [9]. A comparison of SFM and DM showed that digital MG has a statistically higher cancer detection rate, an increase in recall and false positive screens, and no effect on interval cancer rates [10]. Digital mammography is also more accurate in women below 50 years of age, those with DB, and premenopausal or postmenopausal women [11]. DM has a lower average radiation dose than SFM without compromising diagnostic accuracy [11].
Posso et al. [12] led an investigation on reducing the masking effect of DM breast density compared to SFM and improving cancer detection, especially in women with high DB [13]. The study showed that high breast density harms DM, and its sensitivity and positive predictive value decrease. Of course, the authors noted that although sensitivity decreases, cancer detection increases.
Although FFDM is the gold standard for effective screening and diagnosis of BC in the early stages because of its low cost, fast speed, and non-invasive technique, it leads to false positives and negatives due to overlapping breast tissue. Therefore, DBT improves cancer detection rates [14], because DBT can reduce the overlap of normal breast parenchyma and reveal clinically obscure lesions [15]. One of the differences between FFDM and DBT is the kVp values used. In MG, the kVp is chosen to provide high contrast, but contrast is not required in tomosynthesis, because the DBT information is reconstructed in the images. For this reason, more penetrating X-ray photons are used to achieve a high signal-to-noise ratio and increase patients’ radiation doses [16].
1.2. Digital Breast Tomosynthesis (DBT)
Digital breast tomosynthesis is a 3D mammogram with multiple projections rotating through an arc of 15 and 60 degrees in a plane parallel to the chest wall. It should be noted that by sampling a wide angular range, tomosynthesis has advantages such as the obtainment of more information, superior depth resolution, and better contrast than narrow-angle sampling. However, patient movement may occur due to the longer imaging duration [17].
Compared to MG, DBT shows an increase in cancer detection and a decrease in the recall rate, but it is associated with an increase in the radiation dose [18]. Østerås et al. [19] showed that compared to DM, DBT can identify more cancers in all density and age groups, and false positive findings due to asymmetric density are less frequent. Another comparison between DBT and FFDM showed that DBT identified more cancers of all sizes, grades, and hormone receptor statuses, with or without node involvement. Similarly, more cancers were detected with DBT than FFDM regardless of age group, density classification, or the presence or absence of prior examination [20].
Heindel et al. and You et al. [21][22] compared DM and DBT with synthetic mammography (SM gives a virtual 2D MG image obtained from DBT, which looks similar to that from FFDM). According to their analysis, it was found that the detection rate of invasive BC is significantly higher with DBT with SM. In another study, Choi et al. [23] showed that SM might be slightly more sensitive than DM for detecting and characterizing microcalcifications. Also, SM plus DBT can replace DM plus DBT for detecting microcalcifications. It was also shown that DBT with SM is a better method than FFDM for detecting mass, calcification, and asymmetry [24], which is shown in Figure 1.
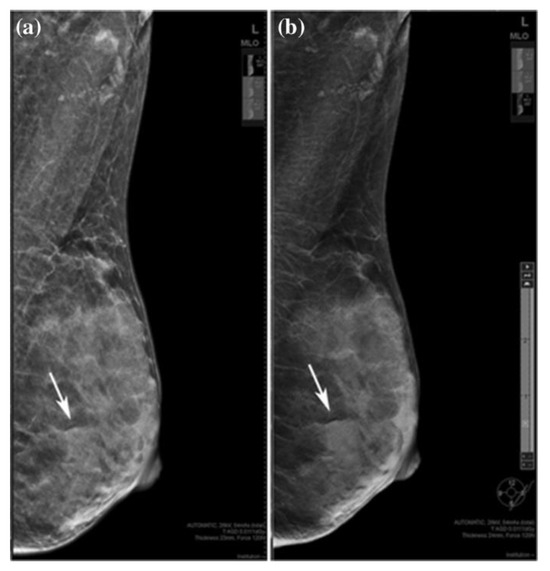
Figure 1. A 36-year-old woman with a fibroadenoma in the left breast. (a) FFDM and (b) SM mediolateral oblique images. Rows indicate benign lesion, which is asymmetric on SM and an obscured mass on DBT. “Reprinted with permission from Ref. [24]. 2019, Springer”. More details on “Copyright and Licensing” are available via the following link: https://link.springer.com/article/10.1007/s12282-019-00992-1 (accessed on 1 December 2023).
Furthermore, DBT increases cancer detection rates, reduces recall rates compared to FFDM, and improves sensitivity and specificity. DBT with SM (instead of FFDM) has a radiation dose similar to that of FFDM alone [25]. A comparison between the image quality of SM and DM demonstrated that the spatial resolution and contrast detail curve in SM is lower than that in DM. Still, SM has some advantages, like decreasing the radiation dose, reducing imaging time [26], and improving diagnostic efficacy for detecting malignant breast lesions [22]. Theoretically, DBT may show only a few calcifications of a clinically significant micro-calcification cluster, but FFDM has a higher sensitivity in detecting and characterizing calcifications. In the study of Murakami et al. [27], it was found that SM can compensate for the disadvantages of DBT in underestimating calcification.
Adding DBT to FFDM has advantages such as increased sensitivity, specificity, and positive predictive value, reducing the false positive rate in diagnostic and screening cases and increasing the cancer detection rate [28]. Skaane et al.’s study has determined that adding DBT to DM significantly increases sensitivity and specificity. Though using SM instead of DM in combination with DBT causes a slight change in sensitivity or specificity, it can be a suitable alternative to DM when using DBT [29]. The results of a study by Yi et al. [30] showed that the tumor visibility and diagnostic performance of DBT added to FFDM in the evaluation of women with T1 non-calcified invasive BC depend on the composition of the breast and the probability of failed diagnosis in both DBT and FFDM images in small isodense non-calcified cancer that is in the tissue, where dense fibro glandular glands are hidden. Therefore, complementary imaging other than DBT should be considered for screening women with very DB. A study by Alabousi et al. showed that combined DBT and DM or combined DBT and SM resulted in higher cancer detection rates, higher invasive cancer detection rates, and higher positive predictive value than DM alone. The combination of DBT and SM reduced the recall rate for additional imaging and biopsy. However, DBT alone has no advantage compared to DM alone [31]. Another study showed that the diagnostic accuracy and sensitivity of DBT plus SM are higher than that of FFDM alone, and its recall rate for DB is lower than that for FFDM [32].
Although DBT combined with DM can increase diagnostic accuracy and reduce the recall rate, it leads to more extended time necessary for interpretation and a higher radiation dose [33][34] due to two separate acquisitions. Hence, synthesized mammography (SM) reconstructed from DBT images can be a potential alternative to DM, which leads to a significant reduction in the total radiation dose [16] without compromising diagnostic accuracy [35].
1.3. Contrast-Enhanced Mammography (CEM)
Contrast-enhanced mammography is an imaging procedure that combines digital MG with copper filtration and additional software to perform dual-energy imaging at about 26–33 kVp and 44–50 kVp and administer intravenous nonionic low-osmolar iodinated contrast media [36]. The reason for using contrast media in CEM is the low difference in the absorption of X-rays between the tissues and, thus, the similarity of the image contrast of the tumor tissue compared to the glandular tissue in DB [37]. Rasouli et al. (47) recently demonstrated the advantages of iodine nanoparticles compared to gold nanoparticles. In this study, they reported that iodine works better than gold nanoparticles, and the cytotoxicity of gold nanoparticles is higher than that of iodine. Therefore, it was stated that in general, iodine has a better performance than gold nanoparticles.
In CEM, two images of each view are obtained at two energy levels. The first image is a low-energy image that shows breast morphology and is equivalent to a standard 2D mammogram and another image provides low- and high-energy images showing areas of contrast absorption [38]. Imaging breast tissue with one direction of X-ray exposure is ineffective for BC detection. A dual-energy imaging technique can overcome this problem, resulting in a high dose. Li et al. [39] investigated the use of CEM with photon counting detectors (PCDs) using cadmium zinc telluride (CZT) and total variation (TV) denoising algorithms. The study showed that CEM with PCD can be used to solve the problem of high doses in dual-energy imaging systems, and TV can improve the image quality for BC diagnosis.
Contrast-enhanced mammography in BC detection has advantages such as a performance similar to breast MRI, a cost identical to conventional MG, and time imaging similar to an abbreviated MRI protocol and inferior to MRI. Less time is needed by the radiologist to perform the procedure and interpret the findings [40][41]. As mentioned, the sensitivity and specificity of standard MG are affected by breast tissue density. The US depends on the operator’s experience. Although MRI is the most sensitive breast imaging method, it has a high rate of false positive results. Therefore, Bozzini et al. [42] evaluated CEM in DB patients with histologically proven malignant breast lesions and compared its diagnostic performance with US, FFDM, and MRI. According to the study, CEM had a detection rate similar to US and MRI, and it was significantly higher than that of FFDM (Figure 2). Invasive tumor size obtained by CEM matched pathological data in 64.6% of lesions, similar to US and MRI but higher than FFDM.
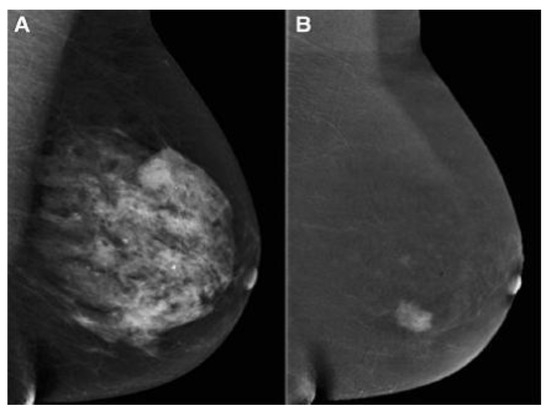
Figure 2. (A) FFDM showing a dense breast and (B) the same breast on CEM showing breast cancer [43]. “Reprinted with permission from Ref. [41]. 2020, Springer”. More details on “Copyright and Licensing” are available via the following link: https://link.springer.com/article/10.1007/s10549-020-05881-2 (accessed on 1 December 2023).
In malignancies, the contrast agent is absorbed more than in normal tissue due to high angiogenesis. Therefore, methods such as DCE-MRI and CEM have been given attention. Contrast-enhanced mammography is superior to DM and DBT in terms of accuracy and is comparable to DCE-MRI in evaluating breast malignancy [43]. Based on Huang et al. [44], benign and malignant lesions showed the highest contrast enhancement at 3 and 2 min, respectively. Therefore, to observe the maximum contrast enhancement of BC in CEM, 2 min is the best interval to differentiate between benign and malignant breast lesions.
About BC, it should be noted that not completely removing cancer cells leads to the creation of disease-resistant cells. These cells are undetectable and can unpredictably lead to recurrence and metastasis. Such drug resistance prevents anti-cancer treatments, so it is necessary to improve diagnostic methods [45]. Studies have been conducted that investigate the ability of different imaging methods in the diagnosis of residual disease. For example, the study of Molly P. Hogan et al. has shown that CEM is an acceptable alternative to breast MRI for the diagnosis of residual disease after neoadjuvant treatment [46].
Despite all the mentioned advantages of CEM in patients with lesions, it is not suitable in patients with spreading of unifocal disease, ductal carcinoma in situ histotypes, lesion size less than 10 mm, and index lesion with micro-calcification [47]. This method also has some disadvantages, such as the need to inject contrast agents, which can lead to allergic reactions, and CEM-guided biopsy is unavailable. There may be a low rate of false positive and false negative results, and benign tissues can be associated with contrast enhancement, which leads to unnecessary imaging and biopsy [48]. CEM does not have sufficient sensitivity to detect poorly advanced cancers. In addition, it does not show cancers with increased parenchyma in the background or near the chest wall [49].
1.4. Nanoparticles in Mammography
The use of nanoparticles (NPs) in medical imaging and mammography is fast becoming a key instrument in cancer detection and treatment. A large and growing body of literature has investigated the use of different NPs in mammography. Surveys such as that conducted by Naha et al. [50] have shown that gold–silver alloy NPs (GSAN) can be used as a contrast agent for cancer detection with dual-energy mammography and computed tomography (CT). It has been demonstrated that [51] a high biocompatibility of silver telluride NPs (Ag2Te NPs) results in their use in mammography and as a CT contrast agent for cancer detection. Karunamuni et al. [52] found that silver NPs are an effective contrast agent for cancer detection and screening with dual-energy mammography. In a study that set out to increase the sensitivity and specificity of mammography for cancer detection, Cole et al. [53] found that bisphosphonate-functionalized gold NPs (BP-Au NPs) improved sensitivity and specificity for the detection of microcalcifications. In another study, Cole et al. [54] points out that BP-Au NPs can be used for dense mammary tissue imaging with high sensitivity and specificity.
2. Ultrasound Imaging (US)
Ultrasound is a screening method that does not require ionizing radiation or intravenous contrast. US has advantages such as its portability, lower cost than MG, and versatility. It is the perfect imaging tool for biopsy, as it distinguishes cystic masses from solid masses. Choudhery et al. [55] investigated the adequacy of the US to detect masses. According to their study, most masses recalled from DBT screening can be evaluated with just an US. A diagnostic MG should be performed if the recalled mass is not seen. A handheld US (HHUS) or an automatic breast US (ABUS) can be used [56]. Both have 100% sensitivity, and their specificity is 85% and 95%, respectively. One of the advantages of the automatic type is its higher diagnostic accuracy than the manual type [57], and it overcomes limitations such as being operator-dependent, time-consuming, and unrepeatable [58]. It has been made clear that the US has low specificity and false positive risk [59], and a double reading of the ABUS can solve this problem. This was investigated in a study by Lee et al. [60]. The study showed that double reading could increase diagnostic efficiency and decrease false positives. The authors also stated that adding ABUS can improve the recall rate of FFDM and DBT screening. Based on a meta-analysis by Rupali et al. [61], the US has a sensitivity and specificity of 80.1% and 88.4% for BC diagnosis; hence, the authors showed that it incorporates a high potential for BC diagnosis and can be utilized as an early diagnosis tool. Even though the studies the authors checked were heterogeneous, this demonstrated a limited impact on their conclusions. In another study, Badu-Peprah et al. [62] showed that the sensitivity of the clinical diagnosis is 50.5%, MG 73.0%, and US 100%, and the specificity of MG and US is 80.0% and 80.4%, respectively. In this manner, they proposed that the US be utilized as the primary line of imaging for diagnosis. In addition, a study conducted by Harada-Shoji et al. [63] compared the sensitivity of MG and US and found that the sensitivity of MG and US alone is lower than the combined sensitivity of these methods, that is, if US is utilized as a supplement to MG, the sensitivity increases. In addition, the authors indicated that the adjuvant US increases the detection of early invasive cancers in dense and non-DB.
In a study by Yi et al. [64], the authors investigated the value of adding US after DM/DBT. According to their research, the addition of the US led to the detection of three extra cancers in 925 women with negative DM/DBT results, and all other cancers were detected in women with DB. Still, US screening in women with non-DB who experienced DM/DBT is useless. Another study compared the utilization of US after DM and after DMT in women with DB. This study showed no difference in diagnosis when US is utilized after DM or after DMT, and the utilization of DMT does not remove the additional US in women with DB [65]. Comparing CEM and US showed that axillary and lymph node lesions might not be seen in CEM, but the US can show these regions’ anomalies [66]. Moreover, a study by Lu et al. [67] compared the performance of CEM and US in patients with DB. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of CEM were 93.8%, 88.1%, 88.2%, 93.7%, and 90%, respectively, and 90.6%, 82.1%, 82.9%, 90.8%, and 86.3% for the US. The authors stated that the ability of the US to detect benign lesions is higher than that of CEM, and misdiagnosis with CEM can delay the treatment of benign lesions. A comparison between MG and US also showed that MG is not a viable diagnostic method for DB [68], because dense tissue and BC are seen as white in MG, whereas in US, dense tissue is echogenic, and BC is hypoechoic (Figure 3). It has been found that the addition of US screening can increase the BC detection rate by 1.9–4.2% [69].
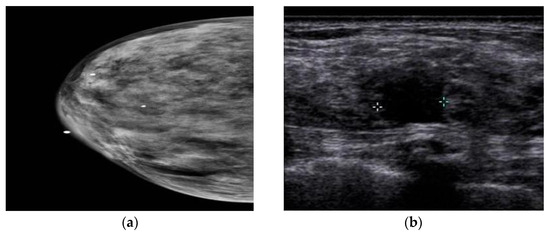
Figure 3. (a) DB is white in MG like BC. (b) DF is echogenic, but BC is hypoechoic in US [69]. More details on “Copyright and Licensing” are available via the following link: Creative Commons—Attribution 4.0 International—CC BY 4.0.
The drawbacks of the US profoundly depend on the experience of the radiologist [70]. It has unsatisfactory false positives and false negatives in asymptomatic women [71]. The trouble in recognizing between a cyst and a solid tumor can be remedied by Doppler and Power Doppler methods [72]. In women with a background of BC, there is a possibility of recurrence on the same side of the breast or chest wall, regional lymph nodes, or far-off organs; because MG has a limited field of view, the diagnosis of regional recurrences in MG is debated. Therefore, the US can be utilized as a complementary screening method. This has been investigated by Shin et al. [73]. Their study showed that axillary recurrence after BC and axillary treatment is rare in asymptomatic women with negative MG results. US screening of the whole breast after surgery does not assist in detecting axillary recurrence. Kim et al. [74] showed that complementary US had a lower interpretation rate of abnormalities and higher specificity (in women aged 50 years and older and in women two years after surgery) in women with a personal history of BC compared to women without a personal history of BC. Even though anti-hormonal treatment or aromatase inhibitors can decrease benign breast disease and false positive findings in the US [75], they did not affect the results of this study.
Nanoparticles in US
The low specificity of breast US for cancer detection is a classic problem that requires the use of a contrast agent [76]. Recent evidence suggests that NPs can be used for cancer detection and treatment as an US contrast agent. In an analysis of mesoporous silica NPs (MSNs) functionalized with the monoclonal antibody Herceptin®, Milgroom et al. [76] showed the potential of MSNs as a stable, biocompatible, and effective therapeutic and diagnostic (“theranostic”) agent for US-based breast cancer imaging, diagnosis, and treatment. Another study [77] has considered the usage of metal oxide NPs for cancer detection with US. In a major advance in 2021, Cao et al. [78] developed nanocarriers for sonodynamic therapy (SDT) of breast cancer.
3. Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging has advantages such as its high sensitivity and specificity, and it is suitable for patients who have breast-conserving surgery. Its limitations include the high cost and time of scanning [79], false positive results, its limited use in patients with claustrophobia, and its hypersensitivity to contrast agents. Also, this method provides false positive results for extensive screening and the ideal BC stage [6]. According to the guidelines, MRI screening for high-risk populations is recommended, such as for women with BRCA mutations, women with Li-Fraumeni and other high-risk syndromes, women who received chest radiation between the ages of 10 and 30 years, and women with 20–25% or greater lifetime risk of developing breast cancer [80][81]. One of the critical issues with high-density breast imaging, particularly for small tumors, is reducing sensitivity below 40% [82]. However, magnetic resonance mammography (MRMG) has high sensitivity in diagnosing BC regardless of breast density [83]. The cost-effectiveness of MRMG compared to MG in patients with high breast density was evaluated by Kaiser et al. [84]. Their preliminary study showed that MRMG is a more accurate and less expensive modality than MG in patients with an intermediate risk of BC. They indicated that MR techniques such as parallel imaging and abbreviated protocols offer assistance in diminishing time and increase cost-effectiveness. According to their study, two-year screening with MRMG can be cost-effective for patients with DB tissue. In a study by Sippo et al. [85], the screening performance of breast MRI was evaluated. The authors’ research showed no difference in breast MR imaging screening performance for cancer detection rates among women with BRCA mutation or history of chest radiation therapy, women with a personal history of breast cancer, and women with a history of high-risk lesions. Women with a family history of breast cancer were found to have a lower cancer detection rate and positive predictive value compared to those with a BRCA mutation or previous chest radiation. Kim [86] conducted a retrospective study to compare abbreviated breast MRI with full-protocol MRI. According to their investigation, abbreviated MRI has higher sensitivity and specificity than full-protocol MRI in women with an individual history of BC.
According to the obtained results, the sensitivity of breast MRI for breast carcinoma is between 88 and 100% in the diagnostic environment, and the characteristics of breast MRI reach 87% in the screening environment [87][88]. In other words, breast MRI is more sensitive than MG, US, or physical examination.
In the study of Monika Graeser et al. [89], the ability of US and MRI to determine residual tumor size was investigated. The results of the study showed that in hormone receptor (HR)+/human epidermal growth factor receptor 2(HER2)+ and HR+/-HER2 breast cancer, MRI is less prone to underestimation than ultrasound, and ultrasound is associated with a lower risk of overestimating the size of the tumor.
Recently, positron emission tomography/magnetic resonance imaging (PET/MRI) has been considered a promising imaging method for breast cancer evaluation. Cancer is a very heterogeneous disease, and moreover, each patient is unique in terms of disease behavior and prognosis. Therefore, imaging methods that provide morphological data as well as functional data are very valuable. In the study of Valeria Romeo et al. [90], the role of PET/MRI in the evaluation of breast cancer was investigated. In this study, technical aspects of hybrid PET/MRI, new developments in MRI and PET, descriptions of new PET detectors, and clinical applications of hybrid PET/MRI of the breast are described. In this study, it is stated that despite the high costs and limited availability of PET/MRI, this imaging method is useful for morphological and functional assessment of breast cancer. Furthermore, in a study by Janna Morawitz et al. [91], the results of CT, MRI, and [18F]-fluorodeoxyglucose positron emission tomography ([18F]-FDG PET/MRI) in determining the correct status of nodes in axillary (level I–III), supraclavicular, and internal mammary lymph nodes in patients with newly diagnosed breast cancer were compared. The results of this study showed that PET/MRI performs better in diagnosing lymph node metastasis, with higher speed and accuracy in all lymph node stations than CT or MRI. It has the highest sensitivity, and CT has the lowest sensitivity.
3.1. Dynamic Contrast-Enhanced MRI
The DCE-MRI technique is a non-invasive and three-dimensional imaging technique that can show tumor angiogenesis and lymph node metastasis in BC [92]. Unlike MG, this technique is not limited by breast tissue density, but the main limitation is its non-specificity [93]. Other disadvantages are its long imaging time, high cost, high false positive rates, poor patient tolerance, contraindications such as a pacemaker or claustrophobia, the worry of gadolinium deposition in the brain [94][95][96], and the overlap between morphological features and kinetic patterns of benign and malignant lesions [97]. Also, the menstrual cycle can lead to a non-specific increase in breast parenchyma in DCE-MRI and, thus, false positives. Therefore, it is better to perform DCE-MRI between days 7 and 13 of the menstrual cycle [98]. In DCE-MRI, a pre-contrast T1-weighted image is first taken, and then a sequence of T1-weighted images after contrast is taken.
Although DCE-MRI has a high sensitivity for detecting BC, using gadolinium-based contrast agents is still a concern, so using non-contrast MR-based conductivity imaging has been considered. The result of Suh’s et al. study [99] showed that the current performance of this method is lower compared to T2WI, DWI, and MG. Still, conductivity imaging can reduce biopsy caused by DCE-MRI due to low conductivity values in benign lesions. In a study by Jochelson et al. [38], a comparison was made between bilateral CEM, conventional DM, and MRI in women with BC. The study showed that the DCE contrast agent persisted for at least 10 min after the infusion was complete, in contrast to the quick washout seen with MRI. In this manner, the arrangement in which the images are obtained is not essential. The authors demonstrated, moreover, that DCE could show lesions regardless of size; despite being sensitive to MR imaging, it presents fewer blunders.
3.2. Diffusion-Weighted Imaging (DWI)
Although DCE-MRI is recommended for breast screening, this method is costly and time-consuming and requires the injection of a contrast agent. An abbreviated breast MRI can reduce time and cost, but a contrast agent still needs to be injected. Therefore, the potential of DW as a screening tool has been investigated [93]. The findings of this study showed that although DW is less sensitive than DCE MRI but higher than MG and US, it works better than MG and US and can be effective as a method for identifying malignancies hidden by MG. A study by Moy et al. [100] showed that DCE-MRI has been found to have a higher resolution in soft tissues than MRDW, and the positive predictive value of DCE-MRI is higher than the positive predictive value of MRI alone. Also, it is demonstrated that the sensitivity of DWI in diagnosing malignancy is higher compared to MRS and DCE, and it can also detect malignancy in all cases of indeterminate DCE [97].
It has been found that MRI often fails to distinguish between malignant and benign breast lesions. In contrast, DWI can identify breast lesions better than conventional MRI. The only significant issue related to DWI is finding the appropriate ADC value for diagnosing malignant and benign breast lesions. The value of the apparent diffusion coefficient (ADC) in normal tissue is higher than that in benign tissue, and in benign tissue, it is more heightened than in malignant tissue, so tissues can be diagnosed using MRDW and ADC measurement [101]. According to a meta-analysis study [102], the average ADC of benign breast lesions is more than 1.00 × 10−3 mm2/s, and most malignant lesions have ADC values less than 2.0 × 10−3 mm2/s. The authors of the study stated that 1.00 × 10−3 mm2/s could be used as a threshold value to distinguish malignant and benign breast lesions. The main limitation of DWI is that small cancer foci may not be seen on ADC maps [103].
In a study [104], the use of DWI as an independent parameter and multiparametric (mpMRI) using DCE-MRI and DWI for breast cancer diagnosis was investigated. Based on the authors’ findings, DWI cannot be used as an independent and alternative parameter of DCE-MRI, because its spatial resolution is still very low. mpMRI has high sensitivity and specificity in diagnosis. The authors also showed that DCE-MRI is the most sensitive method for breast cancer diagnosis. Research has shown that DWI with a reduced field of view can create images with better quality and higher resolution than typical bilateral DWI, and this can also be used instead of DCE-MRI [105]. It should be noted that bilateral DWI has disadvantages such as magnetic susceptibility, chemical shifts, low signal-to-noise ratio, and low resolution [106].
3.3. Magnetic Resonance Spectroscopy
In MRS, increased choline-containing compounds (the peak of Choline is 3.23 ppm [107]) in malignant breast lesions differentiate these from benign lesions and increase MRI specificity [108]. A study investigated in vivo 1H-MRS to distinguish malignant from benign breast lesions using the high choline (Cho) peak. Based on the findings of this study, the choline peak has a suitable sensitivity and specificity for detecting malignant breast lesions. This study showed that malignant tumors with a Cho-positive peak were significantly larger than Cho-negative tumors. It was also stated that the sensitivity and specificity of the Cho peak are considerably lower than the multi-parametric MRMG, but placing the spectra located in the tissue around the tumor and the analysis of lipid peaks can increase this [109].
In some studies, MRS was compared with mpMRI. For example, in a retrospective study by Uma Sharma, multi-parametric MR combining DCE-MRI, DWI, and MRS data was evaluated to increase the sensitivity of breast lesion detection. The authors’ research showed that mpMRI could improve the detection of breast malignancies, and the approaches could complement each other. They also showed that the sensitivity to detect malignancy was the highest for DWI compared to MRS and DCE-MRI [97]. In another study by Sodano et al. [110], the use of MRS for suspicious lesions in mpMRI was investigated. The study showed that the quantitative assessment of tCho from 1H-MRS can diagnose malignancy in breast lesions that are considered suspect by evaluating mp breast MRI using DCE, T2W, and diffusion-weighted images. They also stated that a low concentration of tCho indicates the absence of metastasis to the lymph nodes.
3.4. Magnetic Resonance Elastography
The MRE imaging technique is a non-invasive method used to measure tissues’ stiffness or elasticity. This method uses sound waves in the range of 100 to 1000 Hz, and the imaging is performed using motion-sensitive MRI sequences. Breast MRE is a cross-sectional imaging method that quantifies the viscoelastic properties of breast tissues [111][112]. Due to the increased number of cells, collagen, and proteoglycans, BC has a higher stiffness than the surrounding normal tissues and benign lesions [112]. Manual touch lacks specificity and sensitivity, and MRE can overcome this limitation [113]. The most critical limit of MRE in BC is low spatial resolution and the detection of small focal lesions [114].
Today, MRE of the breast is in the research stage, and efforts are being made to reduce the scanning time and improve the spatial resolution. Also, the use of MRE to evaluate breast cancer and its ability to be used as a marker in malignant lesions needs more studies [113].
References
- Nikolova, N.K. Microwave imaging for breast cancer. IEEE Microw. Mag. 2011, 12, 78–94.
- Løberg, M.; Lousdal, M.L.; Bretthauer, M.; Kalager, M. Benefits and harms of mammography screening. Breast Cancer Res. 2015, 17, 63.
- Dibden, A.; Offman, J.; Duffy, S.W.; Gabe, R. Worldwide review and meta-analysis of cohort studies measuring the effect of mammography screening programmes on incidence-based breast cancer mortality. Cancers 2020, 12, 976.
- Hendrick, R.E. Radiation Doses and Risks in Breast Screening. J. Breast Imaging 2020, 2, 188–200.
- Zeeshan, M.; Salam, B.; Khalid, Q.S.B.; Alam, S.; Sayani, R. Diagnostic accuracy of digital mammography in the detection of breast cancer. Cureus 2018, 10, e2448.
- He, Z.; Chen, Z.; Tan, M.; Elingarami, S.; Liu, Y.; Li, T.; Deng, Y.; He, N.; Li, S.; Fu, J. A review on methods for diagnosis of breast cancer cells and tissues. Cell Prolif. 2020, 53, e12822.
- Mandelson, M.T.; Oestreicher, N.; Porter, P.L.; White, D.; Finder, C.A.; Taplin, S.H.; White, E. Breast density as a predictor of mammographic detection: Comparison of interval-and screen-detected cancers. J. Natl. Cancer Inst. 2000, 92, 1081–1087.
- Seeram, E. Full-Field Digital Mammography. In Digital Radiography: Physical Principles and Quality Control; Seeram, E., Ed.; Springer: Singapore, 2019; pp. 111–123.
- Song, S.Y.; Park, B.; Hong, S.; Kim, M.J.; Lee, E.H.; Jun, J.K. Comparison of Digital and Screen-Film Mammography for Breast-Cancer Screening: A Systematic Review and Meta-Analysis. J. Breast Cancer 2019, 22, 311–325.
- Farber, R.; Houssami, N.; Wortley, S.; Jacklyn, G.; Marinovich, M.L.; McGeechan, K.; Barratt, A.; Bell, K. Impact of Full-Field Digital Mammography Versus Film-Screen Mammography in Population Screening: A Meta-Analysis. JNCI J. Natl. Cancer Inst. 2020, 113, 16–26.
- Pisano, E.D.; Gatsonis, C.; Hendrick, E.; Yaffe, M.; Baum, J.K.; Acharyya, S.; Conant, E.F.; Fajardo, L.L.; Bassett, L.; D’Orsi, C.; et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N. Engl. J. Med. 2005, 353, 1773–1783.
- Posso, M.; Louro, J.; Sánchez, M.; Román, M.; Vidal, C.; Sala, M.; Baré, M.; Castells, X.; Group, B.S. Mammographic breast density: How it affects performance indicators in screening programmes? Eur. J. Radiol. 2019, 110, 81–87.
- Kerlikowske, K.; Hubbard, R.A.; Miglioretti, D.L.; Geller, B.M.; Yankaskas, B.C.; Lehman, C.D.; Taplin, S.H.; Sickles, E.A.; Consortium, B.C.S. Comparative effectiveness of digital versus film-screen mammography in community practice in the United States: A cohort study. Ann. Intern. Med. 2011, 155, 493–502.
- Korhonen, K.E.; Weinstein, S.P.; McDonald, E.S.; Conant, E.F. Strategies to increase cancer detection: Review of true-positive and false-negative results at digital breast tomosynthesis screening. Radiographics 2016, 36, 1954.
- Baker, J.A.; Lo, J.Y. Breast tomosynthesis: State-of-the-art and review of the literature. Acad. Radiol. 2011, 18, 1298–1310.
- Gennaro, G.; Bernardi, D.; Houssami, N. Radiation dose with digital breast tomosynthesis compared to digital mammography: Per-view analysis. Eur. Radiol. 2018, 28, 573–581.
- Georgian-Smith, D.; Obuchowski, N.A.; Lo, J.Y.; Brem, R.F.; Baker, J.A.; Fisher, P.R.; Rim, A.; Zhao, W.; Fajardo, L.L.; Mertelmeier, T. Can Digital Breast Tomosynthesis Replace Full-Field Digital Mammography? A Multireader, Multicase Study of Wide-Angle Tomosynthesis. Am. J. Roentgenol. 2019, 212, 1393–1399.
- Ali, E.A.; Adel, L. Study of role of digital breast tomosynthesis over digital mammography in the assessment of BIRADS 3 breast lesions. Egypt. J. Radiol. Nucl. Med. 2019, 50, 48.
- Østerås, B.H.; Martinsen, A.C.T.; Gullien, R.; Skaane, P. Digital Mammography versus Breast Tomosynthesis: Impact of Breast Density on Diagnostic Performance in Population-based Screening. Radiology 2019, 293, 60–68.
- Dang, P.A.; Wang, A.; Senapati, G.M.; Ip, I.K.; Lacson, R.; Khorasani, R.; Giess, C.S. Comparing Tumor Characteristics and Rates of Breast Cancers Detected by Screening Digital Breast Tomosynthesis and Full-Field Digital Mammography. Am. J. Roentgenol. 2019, 214, 701–706.
- Heindel, W.; Weigel, S.; Gerß, J.; Hense, H.-W.; Sommer, A.; Krischke, M.; Kerschke, L. Digital breast tomosynthesis plus synthesised mammography versus digital screening mammography for the detection of invasive breast cancer (TOSYMA): A multicentre, open-label, randomised, controlled, superiority trial. Lancet Oncol. 2022, 23, 601–611.
- You, C.; Zhang, Y.; Gu, Y.; Xiao, Q.; Liu, G.; Shen, X.; Yang, W.; Peng, W. Comparison of the diagnostic performance of synthesized two-dimensional mammography and full-field digital mammography alone or in combination with digital breast tomosynthesis. Breast Cancer 2020, 27, 47–53.
- Choi, J.S.; Han, B.-K.; Ko, E.Y.; Kim, G.R.; Ko, E.S.; Park, K.W. Comparison of synthetic and digital mammography with digital breast tomosynthesis or alone for the detection and classification of microcalcifications. Eur. Radiol. 2019, 29, 319–329.
- Choi, Y.; Woo, O.-h.; Shin, H.-s.; Cho, K.R.; Seo, B.K.; Choi, G.-Y. Quantitative analysis of radiation dosage and image quality between digital breast tomosynthesis (DBT) with two-dimensional synthetic mammography and full-field digital mammography (FFDM). Clin. Imaging 2019, 55, 12–17.
- Falomo, E.; Myers, K.; Reichel, K.F.; Carson, K.A.; Mullen, L.; Di Carlo, P.; Harvey, S. Impact of insurance coverage and socioeconomic factors on screening mammography patients’ selection of digital breast tomosynthesis versus full-field digital mammography. Breast J. 2018, 24, 1091–1093.
- Barca, P.; Lamastra, R.; Aringhieri, G.; Tucciariello, R.M.; Traino, A.; Fantacci, M.E. Comprehensive assessment of image quality in synthetic and digital mammography: A quantitative comparison. Australas. Phys. Eng. Sci. Med. 2019, 42, 1141–1152.
- Murakami, R.; Uchiyama, N.; Tani, H.; Yoshida, T.; Kumita, S. Comparative analysis between synthetic mammography reconstructed from digital breast tomosynthesis and full-field digital mammography for breast cancer detection and visibility. Eur. J. Radiol. Open 2020, 7, 100207.
- Singla, D.; Chaturvedi, A.K.; Aggarwal, A.; Rao, S.A.; Hazarika, D.; Mahawar, V. Comparing the diagnostic efficacy of full field digital mammography with digital breast tomosynthesis using BIRADS score in a tertiary cancer care hospital. Indian J. Radiol. Imaging 2018, 28, 115–122.
- Skaane, P.; Bandos, A.I.; Niklason, L.T.; Sebuødegård, S.; Østerås, B.H.; Gullien, R.; Gur, D.; Hofvind, S. Digital mammography versus digital mammography plus tomosynthesis in breast cancer screening: The Oslo Tomosynthesis Screening Trial. Radiology 2019, 291, 23–30.
- Yi, A.; Chang, J.M.; Shin, S.U.; Chu, A.J.; Cho, N.; Noh, D.-Y.; Moon, W.K. Detection of noncalcified breast cancer in patients with extremely dense breasts using digital breast tomosynthesis compared with full-field digital mammography. Br. J. Radiol. 2019, 92, 20180101.
- Alabousi, M.; Wadera, A.; Kashif Al-Ghita, M.; Kashef Al-Ghetaa, R.; Salameh, J.P.; Pozdnyakov, A.; Zha, N.; Samoilov, L.; Dehmoobad Sharifabadi, A.; Sadeghirad, B.; et al. Performance of Digital Breast Tomosynthesis, Synthetic Mammography, and Digital Mammography in Breast Cancer Screening: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2021, 113, 680–690.
- Khanani, S.; Hruska, C.; Lazar, A.; Hoernig, M.; Hebecker, A.; Obuchowski, N. Performance of Wide-Angle Tomosynthesis with Synthetic Mammography in Comparison to Full Field Digital Mammography. Acad. Radiol. 2022, 30, 3–13.
- Zuckerman, S.P.; Conant, E.F.; Keller, B.M.; Maidment, A.D.; Barufaldi, B.; Weinstein, S.P.; Synnestvedt, M.; McDonald, E.S. Implementation of synthesized two-dimensional mammography in a population-based digital breast tomosynthesis screening program. Radiology 2016, 281, 730.
- Bernardi, D.; Macaskill, P.; Pellegrini, M.; Valentini, M.; Fantò, C.; Ostillio, L.; Tuttobene, P.; Luparia, A.; Houssami, N. Breast cancer screening with tomosynthesis (3D mammography) with acquired or synthetic 2D mammography compared with 2D mammography alone (STORM-2): A population-based prospective study. Lancet Oncol. 2016, 17, 1105–1113.
- Tamam, N.; Salah, H.; Rabbaa, M.; Abuljoud, M.; Sulieman, A.; Alkhorayef, M.; Bradley, D.A. Evaluation of patients radiation dose during mammography imaging procedure. Radiat. Phys. Chem. 2021, 188, 109680.
- Ghaderi, K.F.; Phillips, J.; Perry, H.; Lotfi, P.; Mehta, T.S. Contrast-enhanced mammography: Current applications and future directions. Radiographics 2019, 39, 1907–1920.
- Diekmann, F.; Lawaczeck, R. Contrast Media in CEDM. In Contrast-Enhanced Digital Mammography (CEDM); Nori, J., Kaur, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 25–33.
- Jochelson, M.S.; Dershaw, D.D.; Sung, J.S.; Heerdt, A.S.; Thornton, C.; Moskowitz, C.S.; Ferrara, J.; Morris, E.A. Bilateral contrast-enhanced dual-energy digital mammography: Feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013, 266, 743–751.
- Lee, S.; Lee, Y. Performance evaluation of total variation (TV) denoising technique for dual-energy contrast-enhanced digital mammography (CEDM) with photon counting detector (PCD): Monte Carlo simulation study. Radiat. Phys. Chem. 2019, 156, 94–100.
- Li, L.; Roth, R.; Germaine, P.; Ren, S.; Lee, M.; Hunter, K.; Tinney, E.; Liao, L. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): A retrospective comparison in 66 breast lesions. Diagn. Interv. Imaging 2017, 98, 113–123.
- Phillips, J.; Miller, M.M.; Mehta, T.S.; Fein-Zachary, V.; Nathanson, A.; Hori, W.; Monahan-Earley, R.; Slanetz, P.J. Contrast-enhanced spectral mammography (CESM) versus MRI in the high-risk screening setting: Patient preferences and attitudes. Clin. Imaging 2017, 42, 193–197.
- Bozzini, A.; Nicosia, L.; Pruneri, G.; Maisonneuve, P.; Meneghetti, L.; Renne, G.; Vingiani, A.; Cassano, E.; Mastropasqua, M.G. Clinical performance of contrast-enhanced spectral mammography in pre-surgical evaluation of breast malignant lesions in dense breasts: A single center study. Breast Cancer Res. Treat. 2020, 184, 723–731.
- Chou, C.-P.; Lewin, J.M.; Chiang, C.-L.; Hung, B.-H.; Yang, T.-L.; Huang, J.-S.; Liao, J.-B.; Pan, H.-B. Clinical evaluation of contrast-enhanced digital mammography and contrast enhanced tomosynthesis—Comparison to contrast-enhanced breast MRI. Eur. J. Radiol. 2015, 84, 2501–2508.
- Huang, J.-S.; Pan, H.-B.; Yang, T.-L.; Hung, B.-H.; Chiang, C.-L.; Tsai, M.-Y.; Chou, C.-P. Kinetic patterns of benign and malignant breast lesions on contrast enhanced digital mammogram. PLoS ONE 2020, 15, e0239271.
- De Silva, F.; Alcorn, J. A tale of two cancers: A current concise overview of breast and prostate cancer. Cancers 2022, 14, 2954.
- Hogan, M.P.; Horvat, J.V.; Ross, D.S.; Sevilimedu, V.; Jochelson, M.S.; Kirstein, L.J.; Goldfarb, S.B.; Comstock, C.E.; Sung, J.S. Contrast-enhanced mammography in the assessment of residual disease after neoadjuvant treatment. Breast Cancer Res. Treat. 2023, 198, 349–359.
- Bicchierai, G.; Amato, F.; Vanzi, B.; De Benedetto, D.; Boeri, C.; Vanzi, E.; Di Naro, F.; Bianchi, S.; Cirone, D.; Cozzi, D. Which clinical, radiological, histological, and molecular parameters are associated with the absence of enhancement of known breast cancers with Contrast Enhanced Digital Mammography (CEDM)? Breast 2020, 54, 15–24.
- Patel, B.K.; Naylor, M.E.; Kosiorek, H.E.; Lopez-Alvarez, Y.M.; Miller, A.M.; Pizzitola, V.J.; Pockaj, B.A. Clinical utility of contrast-enhanced spectral mammography as an adjunct for tomosynthesis-detected architectural distortion. Clin. Imaging 2017, 46, 44–52.
- Mori, M.; Akashi-Tanaka, S.; Suzuki, S.; Daniels, M.I.; Watanabe, C.; Hirose, M.; Nakamura, S. Diagnostic accuracy of contrast-enhanced spectral mammography in comparison to conventional full-field digital mammography in a population of women with dense breasts. Breast Cancer 2017, 24, 104–110.
- Naha, P.C.; Lau, K.C.; Hsu, J.C.; Hajfathalian, M.; Mian, S.; Chhour, P.; Uppuluri, L.; McDonald, E.S.; Maidment, A.D.; Cormode, D.P. Gold silver alloy nanoparticles (GSAN): An imaging probe for breast cancer screening with dual-energy mammography or computed tomography. Nanoscale 2016, 8, 13740–13754.
- Nieves, L.M.; Hsu, J.C.; Lau, K.C.; Maidment, A.D.; Cormode, D.P. Silver telluride nanoparticles as biocompatible and enhanced contrast agents for X-ray imaging: An in vivo breast cancer screening study. Nanoscale 2021, 13, 163–174.
- Karunamuni, R.; Naha, P.C.; Lau, K.C.; Al-Zaki, A.; Popov, A.V.; Delikatny, E.J.; Tsourkas, A.; Cormode, D.P.; Maidment, A.D. Development of silica-encapsulated silver nanoparticles as contrast agents intended for dual-energy mammography. Eur. Radiol. 2016, 26, 3301–3309.
- Cole, L.E.; Vargo-Gogola, T.; Roeder, R.K. Contrast-enhanced X-ray detection of breast microcalcifications in a murine model using targeted gold nanoparticles. ACS Nano 2014, 8, 7486–7496.
- Cole, L.E.; Vargo-Gogola, T.; Roeder, R.K. Contrast-enhanced x-ray detection of microcalcifications in radiographically dense mammary tissue using targeted gold nanoparticles. ACS Nano 2015, 9, 8923–8932.
- Choudhery, S.; Axmacher, J.; Conners, A.L.; Geske, J.; Brandt, K. Masses in the era of screening tomosynthesis: Is diagnostic ultrasound sufficient? Br. J. Radiol. 2019, 92, 20180801.
- Vourtsis, A.; Kachulis, A. The performance of 3D ABUS versus HHUS in the visualisation and BI-RADS characterisation of breast lesions in a large cohort of 1886 women. Eur. Radiol. 2018, 28, 592–601.
- Lin, X.; Wang, J.; Han, F.; Fu, J.; Li, A. Analysis of eighty-one cases with breast lesions using automated breast volume scanner and comparison with handheld ultrasound. Eur. J. Radiol. 2012, 81, 873–878.
- Shin, H.J.; Kim, H.H.; Cha, J.H. Current status of automated breast ultrasonography. Ultrasonography 2015, 34, 165.
- Melnikow, J.; Fenton, J.J.; Whitlock, E.P.; Miglioretti, D.L.; Weyrich, M.S.; Thompson, J.H.; Shah, K. Supplemental Screening for Breast Cancer in Women With Dense Breasts: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 268–278.
- Lee, J.M.; Partridge, S.C.; Liao, G.J.; Hippe, D.S.; Kim, A.E.; Lee, C.I.; Rahbar, H.; Scheel, J.R.; Lehman, C.D. Double reading of automated breast ultrasound with digital mammography or digital breast tomosynthesis for breast cancer screening. Clin. Imaging 2019, 55, 119–125.
- Sood, R.; Rositch, A.F.; Shakoor, D.; Ambinder, E.; Pool, K.-L.; Pollack, E.; Mollura, D.J.; Mullen, L.A.; Harvey, S.C. Ultrasound for breast cancer detection globally: A systematic review and meta-analysis. J. Glob. Oncol. 2019, 5, 1–17.
- Badu-Peprah, A.; Adu-Sarkodie, Y. Accuracy of clinical diagnosis, mammography and ultrasonography in preoperative assessment of breast cancer. Ghana Med. J. 2018, 52, 133–139.
- Harada-Shoji, N.; Suzuki, A.; Ishida, T.; Zheng, Y.-F.; Narikawa-Shiono, Y.; Sato-Tadano, A.; Ohta, R.; Ohuchi, N. Evaluation of adjunctive ultrasonography for breast cancer detection among women aged 40–49 years with varying breast density undergoing screening mammography: A secondary analysis of a randomized clinical trial. JAMA Netw. Open 2021, 4, e2121505.
- Yi, A.; Jang, M.-j.; Yim, D.; Kwon, B.R.; Shin, S.U.; Chang, J.M. Addition of screening breast US to digital mammography and digital breast Tomosynthesis for breast cancer screening in women at average risk. Radiology 2021, 298, 568–575.
- Dibble, E.H.; Singer, T.M.; Jimoh, N.; Baird, G.L.; Lourenco, A.P. Dense Breast Ultrasound Screening After Digital Mammography Versus After Digital Breast Tomosynthesis. Am. J. Roentgenol. 2019, 213, 1397–1402.
- Choi, H.Y.; Park, M.; Seo, M.; Song, E.; Shin, S.Y.; Sohn, Y.-M. Preoperative axillary lymph node evaluation in breast cancer: Current issues and literature review. Ultrasound Q. 2017, 33, 6–14.
- Lu, Z.; Hao, C.; Pan, Y.; Mao, N.; Wang, X.; Yin, X. Contrast-enhanced spectral mammography versus ultrasonography: Diagnostic performance in symptomatic patients with dense breasts. Korean J. Radiol. 2020, 21, 442–449.
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.A.; Hislop, G.; Chiarelli, A.; Minkin, S. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 2007, 356, 227–236.
- Thigpen, D.; Kappler, A.; Brem, R. The Role of Ultrasound in Screening Dense Breasts—A Review of the Literature and Practical Solutions for Implementation. Diagnostics 2018, 8, 20.
- Berg, W.A.; Gutierrez, L.; NessAiver, M.S.; Carter, W.B.; Bhargavan, M.; Lewis, R.S.; Ioffe, O.B. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 2004, 233, 830–849.
- Teh, W.; Wilson, A. The role of ultrasound in breast cancer screening. A consensus statement by the European Group for Breast Cancer Screening. Eur. J. Cancer 1998, 34, 449–450.
- Iranmakani, S.; Mortezazadeh, T.; Sajadian, F.; Ghaziani, M.F.; Ghafari, A.; Khezerloo, D.; Musa, A.E. A review of various modalities in breast imaging: Technical aspects and clinical outcomes. Egypt. J. Radiol. Nucl. Med. 2020, 51, 57.
- Shin, S.U.; Chang, J.M.; Park, J.; Lee, H.B.; Han, W.; Moon, W.K. The Usefulness of Ultrasound Surveillance for Axillary Recurrence in Women With Personal History of Breast Cancer. J. Breast Cancer 2022, 25, 25–36.
- Kim, S.-Y.; Cho, N.; Kim, S.Y.; Choi, Y.; Kim, E.S.; Ha, S.M.; Lee, S.H.; Chang, J.M.; Moon, W.K. Supplemental Breast US Screening in Women with a Personal History of Breast Cancer: A Matched Cohort Study. Radiology 2020, 295, 54–63.
- Tan-Chiu, E.; Wang, J.; Costantino, J.P.; Paik, S.; Butch, C.; Wickerham, D.L.; Fisher, B.; Wolmark, N. Effects of tamoxifen on benign breast disease in women at high risk for breast cancer. J. Natl. Cancer Inst. 2003, 95, 302–307.
- Milgroom, A.; Intrator, M.; Madhavan, K.; Mazzaro, L.; Shandas, R.; Liu, B.; Park, D. Mesoporous silica nanoparticles as a breast-cancer targeting ultrasound contrast agent. Colloids Surf. B Biointerfaces 2014, 116, 652–657.
- Subhan, M.A. Advances with metal oxide-based nanoparticles as MDR metastatic breast cancer therapeutics and diagnostics. RSC Adv. 2022, 12, 32956–32978.
- Nguyen Cao, T.G.; Kang, J.H.; You, J.Y.; Kang, H.C.; Rhee, W.J.; Ko, Y.T.; Shim, M.S. Safe and Targeted Sonodynamic Cancer Therapy Using Biocompatible Exosome-Based Nanosonosensitizers. ACS Appl. Mater. Interfaces 2021, 13, 25575–25588.
- Morrow, M.; Waters, J.; Morris, E. MRI for breast cancer screening, diagnosis, and treatment. Lancet 2011, 378, 1804–1811.
- Daly, M.B.; Pilarski, R.; Berry, M.; Buys, S.S.; Farmer, M.; Friedman, S.; Garber, J.E.; Kauff, N.D.; Khan, S.; Klein, C. NCCN guidelines insights: Genetic/familial high-risk assessment: Breast and ovarian, version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 9–20.
- Gradishar, W.J.; Anderson, B.O.; Balassanian, R.; Blair, S.L.; Burstein, H.J.; Cyr, A.; Elias, A.D.; Farrar, W.B.; Forero, A.; Giordano, S.H.; et al. NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J. Natl. Compr. Cancer Netw. JNCCN 2017, 15, 433–451.
- Riedl, C.C.; Luft, N.; Bernhart, C.; Weber, M.; Bernathova, M.; Tea, M.K.; Rudas, M.; Singer, C.F.; Helbich, T.H. Triple-modality screening trial for familial breast cancer underlines the importance of magnetic resonance imaging and questions the role of mammography and ultrasound regardless of patient mutation status, age, and breast density. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1128–1135.
- Benndorf, M.; Baltzer, P.A.; Vag, T.; Gajda, M.; Runnebaum, I.B.; Kaiser, W.A. Breast MRI as an adjunct to mammography: Does it really suffer from low specificity? A retrospective analysis stratified by mammographic BI-RADS classes. Acta Radiol. 2010, 51, 715–721.
- Kaiser, C.G.; Dietzel, M.; Vag, T.; Froelich, M.F. Cost-effectiveness of MR-mammography vs. conventional mammography in screening patients at intermediate risk of breast cancer—A model-based economic evaluation. Eur. J. Radiol. 2021, 136, 109355.
- Sippo, D.A.; Burk, K.S.; Mercaldo, S.F.; Rutledge, G.M.; Edmonds, C.; Guan, Z.; Hughes, K.S.; Lehman, C.D. Performance of screening breast MRI across women with different elevated breast cancer risk indications. Radiology 2019, 292, 51–59.
- Kim, S.-Y.; Cho, N.; Hong, H.; Lee, Y.; Yoen, H.; Kim, Y.S.; Park, A.R.; Ha, S.M.; Lee, S.H.; Chang, J.M.; et al. Abbreviated Screening MRI for Women with a History of Breast Cancer: Comparison with Full-Protocol Breast MRI. Radiology 2022, 305, 36–45.
- Plana, M.N.; Carreira, C.; Muriel, A.; Chiva, M.; Abraira, V.; Emparanza, J.I.; Bonfill, X.; Zamora, J. Magnetic resonance imaging in the preoperative assessment of patients with primary breast cancer: Systematic review of diagnostic accuracy and meta-analysis. Eur. Radiol. 2012, 22, 26–38.
- Comstock, C.E.; Gatsonis, C.; Newstead, G.M.; Snyder, B.S.; Gareen, I.F.; Bergin, J.T.; Rahbar, H.; Sung, J.S.; Jacobs, C.; Harvey, J.A.; et al. Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection among Women With Dense Breasts Undergoing Screening. JAMA 2020, 323, 746–756.
- Graeser, M.; Schrading, S.; Gluz, O.; Strobel, K.; Herzog, C.; Umutlu, L.; Frydrychowicz, A.; Rjosk-Dendorfer, D.; Würstlein, R.; Culemann, R. Magnetic resonance imaging and ultrasound for prediction of residual tumor size in early breast cancer within the ADAPT subtrials. Breast Cancer Res. 2021, 23, 36.
- Romeo, V.; Helbich, T.H.; Pinker, K. Breast PET/MRI Hybrid imaging and targeted tracers. J. Magn. Reson. Imaging 2023, 57, 370–386.
- Morawitz, J.; Bruckmann, N.-M.; Dietzel, F.; Ullrich, T.; Bittner, A.-K.; Hoffmann, O.; Ruckhäberle, E.; Mohrmann, S.; Häberle, L.; Ingenwerth, M. Comparison of nodal staging between CT, MRI, and -FDG PET/MRI in patients with newly diagnosed breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 992–1001.
- Choi, E.J.; Choi, H.; Choi, S.A.; Youk, J.H. Dynamic contrast-enhanced breast magnetic resonance imaging for the prediction of early and late recurrences in breast cancer. Medicine 2016, 95, e5330.
- Amornsiripanitch, N.; Bickelhaupt, S.; Shin, H.J.; Dang, M.; Rahbar, H.; Pinker, K.; Partridge, S.C. Diffusion-weighted MRI for unenhanced breast cancer screening. Radiology 2019, 293, 504.
- Millet, I.; Pages, E.; Hoa, D.; Merigeaud, S.; Curros Doyon, F.; Prat, X.; Taourel, P. Pearls and pitfalls in breast MRI. Br. J. Radiol. 2012, 85, 197–207.
- Gulani, V.; Calamante, F.; Shellock, F.G.; Kanal, E.; Reeder, S.B. Gadolinium deposition in the brain: Summary of evidence and recommendations. Lancet Neurol. 2017, 16, 564–570.
- Layne, K.A.; Dargan, P.I.; Archer, J.R.; Wood, D.M. Gadolinium deposition and the potential for toxicological sequelae–A literature review of issues surrounding gadolinium-based contrast agents. Br. J. Clin. Pharmacol. 2018, 84, 2522–2534.
- Sharma, U.; Agarwal, K.; Hari, S.; Mathur, S.R.; Seenu, V.; Parshad, R.; Jagannathan, N.R. Role of diffusion weighted imaging and magnetic resonance spectroscopy in breast cancer patients with indeterminate dynamic contrast enhanced magnetic resonance imaging findings. Magn. Reson. Imaging 2019, 61, 66–72.
- Chotai, N.; Kulkarni, S. Breast Imaging Essentials; Springer: Berlin/Heidelberg, Germany, 2020.
- Suh, J.; Kim, J.-H.; Kim, S.-Y.; Cho, N.; Kim, D.-H.; Kim, R.; Kim, E.S.; Jang, M.-j.; Ha, S.M.; Lee, S.H.; et al. Noncontrast-Enhanced MR-Based Conductivity Imaging for Breast Cancer Detection and Lesion Differentiation. J. Magn. Reson. Imaging 2021, 54, 631–645.
- Moy, L.; Noz, M.E.; Maguire Jr, G.Q.; Melsaether, A.; Deans, A.E.; Murphy-Walcott, A.D.; Ponzo, F. Role of fusion of prone FDG-PET and magnetic resonance imaging of the breasts in the evaluation of breast cancer. Breast J. 2010, 16, 369–376.
- Rabasco, P.; Caivano, R.; Simeon, V.; Dinardo, G.; Lotumolo, A.; Gioioso, M.; Villonio, A.; Iannelli, G.; D’Antuono, F.; Zandolino, A. Can diffusion-weighted imaging and related apparent diffusion coefficient be a prognostic value in women with breast cancer? Cancer Investig. 2017, 35, 92–99.
- Surov, A.; Meyer, H.J.; Wienke, A. Can apparent diffusion coefficient (ADC) distinguish breast cancer from benign breast findings? A meta-analysis based on 13 847 lesions. BMC Cancer 2019, 19, 955.
- Sharma, U.; Jagannathan, N.R. Characterization of breast tissues by diffusion weighted MR imaging. Biomed. Spectrosc. Imaging 2014, 3, 1–13.
- Pinker, K.; Moy, L.; Sutton, E.J.; Mann, R.M.; Weber, M.; Thakur, S.B.; Jochelson, M.S.; Bago-Horvath, Z.; Morris, E.A.; Baltzer, P.A. Diffusion-weighted imaging with apparent diffusion coefficient mapping for breast cancer detection as a stand-alone-parameter: Comparison with dynamic contrast-enhanced and multiparametric magnetic resonance imaging. Investig. Radiol. 2018, 53, 587.
- Cho, E.; Lee, J.H.; Baek, H.J.; Ha, J.Y.; Ryu, K.H.; Park, S.E.; Moon, J.I.; Gho, S.-M.; Wakayama, T. Clinical Feasibility of Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging with Computed Diffusion-Weighted Imaging Technique in Breast Cancer Patients. Diagnostics 2020, 10, 538.
- Partridge, S.C.; Nissan, N.; Rahbar, H.; Kitsch, A.E.; Sigmund, E.E. Diffusion-weighted breast MRI: Clinical applications and emerging techniques. J. Magn. Reson. Imaging JMRI 2017, 45, 337–355.
- Galati, F.; Trimboli, R.M.; Pediconi, F. Special Issue “Advances in Breast MRI&rdquo. Diagnostics 2021, 11, 2297.
- Montemezzi, S.; Cavedon, C.; Camera, L.; Meliadò, G.; Caumo, F.; Baglio, I.; Sardanelli, F. 1H-MR spectroscopy of suspicious breast mass lesions at 3T: A clinical experience. La Radiol. Medica 2017, 122, 161–170.
- Prvulovic Bunovic, N.; Sveljo, O.; Kozic, D.; Boban, J. Is Elevated Choline on Magnetic Resonance Spectroscopy a Reliable Marker of Breast Lesion Malignancy? Front. Oncol. 2021, 11, 610354.
- Sodano, C.; Clauser, P.; Dietzel, M.; Kapetas, P.; Pinker, K.; Helbich, T.H.; Gussew, A.; Baltzer, P.A. Clinical relevance of total choline (tCho) quantification in suspicious lesions on multiparametric breast MRI. Eur. Radiol. 2020, 30, 3371–3382.
- Hawley, J.R.; Kalra, P.; Mo, X.; Raterman, B.; Yee, L.D.; Kolipaka, A. Quantification of breast stiffness using MR elastography at 3 Tesla with a soft sternal driver: A reproducibility study. J. Magn. Reson. Imaging JMRI 2017, 45, 1379–1384.
- Patel, B.K.; Samreen, N.; Zhou, Y.; Chen, J.; Brandt, K.; Ehman, R.; Pepin, K. MR Elastography of the Breast: Evolution of Technique, Case Examples, and Future Directions. Clin. Breast Cancer 2021, 21, e102–e111.
- Pepin, K.M.; Ehman, R.L.; McGee, K.P. Magnetic resonance elastography (MRE) in cancer: Technique, analysis, and applications. Prog. Nucl. Magn. Reson. Spectrosc. 2015, 90, 32–48.
- Lorenzen, J.; Sinkus, R.; Lorenzen, M.; Dargatz, M.; Leussler, C.; Röschmann, P.; Adam, G. MR elastography of the breast:preliminary clinical results. Rofo 2002, 174, 830–834.
More
Information
Subjects:
Others
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
657
Revisions:
2 times
(View History)
Update Date:
26 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




