
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Perla D. Maldonado | -- | 3643 | 2023-12-22 21:29:57 | | | |
| 2 | Lindsay Dong | Meta information modification | 3643 | 2023-12-25 02:17:10 | | |
Video Upload Options
Stroke represents one of the main causes of death and disability in the world; despite this, pharmacological therapies against stroke remain insufficient. Ischemic stroke is the leading etiology of stroke. Different molecular mechanisms, such as excitotoxicity, oxidative stress, and inflammation, participate in cell death and tissue damage. At a preclinical level, different garlic compounds have been evaluated against these mechanisms. Additionally, there is evidence supporting the participation of garlic compounds in other mechanisms that contribute to brain tissue recovery, such as neuroplasticity. After ischemia, neuroplasticity is activated to recover cognitive and motor function. Some garlic-derived compounds and preparations have shown the ability to promote neuroplasticity under physiological conditions and, more importantly, in cerebral damage models.
1. Introduction
2. Stroke
2.1. Stroke Epidemiology and Risk Factors
2.2. Damage Mechanisms in Ischemic Stroke
2.3. Neuroprotective Mechanisms in Ischemic Stroke: Neuroplasticity
2.3.1. Synaptogenesis
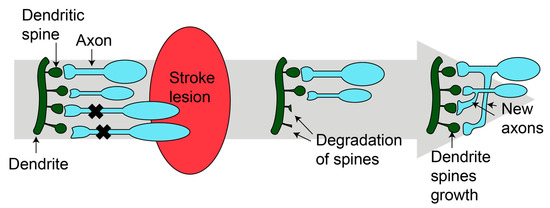
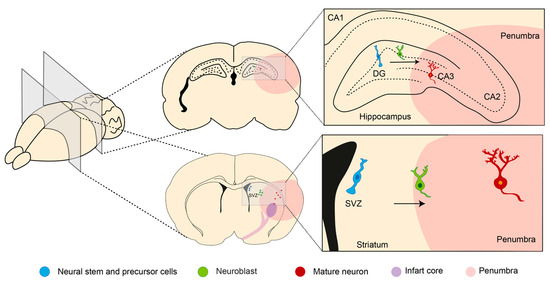
2.3.2. Neurogenesis
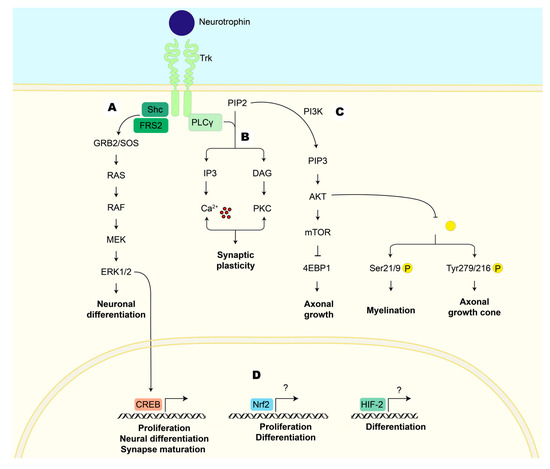
2.3.3. Neurotrophic Factors
- (1)
-
NTs promote neuronal survival, neuronal differentiation, axonal and dendritic growth, synaptic plasticity, and synaptogenesis [26]. Some examples are nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin-3 (NT-3).
- (2)
-
Members of the transforming growth factor family (TGF) stimulate astrocyte proliferation, migration, and transformation to the axon growth-supportive phenotype [27]. They stimulate neural cell proliferation and differentiation and the synthesis of NGF in astrocytes [27]. After stroke, they promote neurogenesis, angiogenesis, and provide oligodendrocyte protection [28], e.g., glia-derived neurotrophic factor (GDNF).
- (3)
-
Neurokines, such as interleukin 6 (IL6), play critical roles in immunity, brain-regulating neurodevelopment, food intake, body temperature, learning, and memory [29].
- (4)
2.4. Treatments for Ischemic Stroke
Intravenous Thrombolysis
3. Garlic
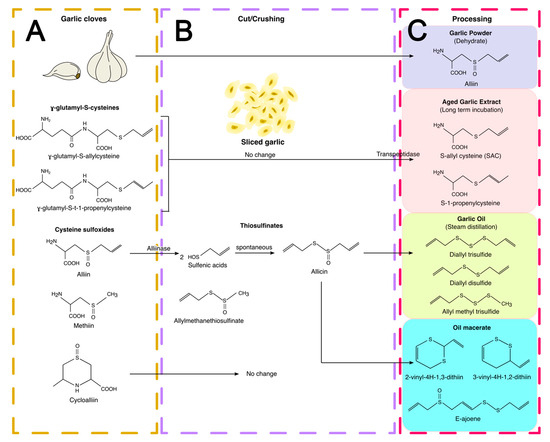
3.1. Garlic Preparations
3.2. Garlic Compounds as Treatment for Ischemic Stroke
3.3. Garlic Preparations as Treatment for Ischemic Stroke
3.4. Garlic Compounds and Neuroplasticity
4. Conclusions
Different garlic OSCs and preparations have been extensively utilized in preclinical studies for treating stroke. Their protective properties are principally attributed to their antioxidant and anti-inflammatory capacities assessed during short periods of ischemia and/or reperfusion. However, the mechanisms activated over longer periods, such as neuroplasticity, that are essential for effective patient recovery have not been studied. Despite this, both garlic compounds and preparations can stimulate neuroplasticity in healthy animals and models of neurological damage, suggesting that garlic compounds and preparations might stimulate neuroplasticity in ischemic stroke. Although this is a process that occurs after ischemic stroke, it requires an antioxidant and anti-inflammatory environment to ensure the survival of the new neurons and the proper functioning of connections between pre-existing neurons.
References
- Wang, X.; Wang, Y.; Ding, Z.J.; Yue, B.; Zhang, P.Z.; Chen, X.D.; Chen, X.; Chen, J.; Chen, F.Q.; Chen, Y.; et al. The role of RIP3 mediated necroptosis in ouabain-induced spiral ganglion neurons injuries. Neurosci. Lett. 2014, 2014, 111–116.
- Avan, A.; Digaleh, H.; di Napoli, M.; Stranges, S.; Behrouz, R.; Shojaeianbabaei, G.; Amiri, A.; Tabrizi, R.; Mokhber, N.; Spence, J.D.; et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019, 17, 191.
- Godwin, K.M.; Wasserman, J.; Ostwald, S.K. Cost associated with stroke: Outpatient rehabilitative services and medication. Top. Stroke Rehabil. 2011, 18, 676–684.
- Cowled, P.; Fitridge, R. Pathophysiology of reperfusion injury. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists, 1st ed.; Fitridge, R., Matthew, T., Eds.; University of Adelaide Press: Adelaide, Australia, 2011; pp. 331–350.
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581.
- Roy-O’Reilly, M.A.; Ahnstedt, H.; Spychala, M.S.; Munshi, Y.; Aronowski, J.; Sansing, L.H.; McCullough, L.D. Aging exacerbates neutrophil pathogenicity in ischemic stroke. Aging 2020, 12, 436–461.
- Yousufuddin, M.; Young, N. Aging and ischemic stroke. Aging 2019, 11, 2542–2544.
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P.; Lloyd-Sherlock, P.; Epping-Jordan, J.E.; Peeters, G.M.E.E.; Mahanani, W.R.; et al. The World report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154.
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion-from mechanism to translation. Nat. Med. 2011, 17, 1391–1401.
- Palop, J.J.; Chin, J.; Mucke, L. A network dysfunction perspective on neurodegenerative diseases. Nature 2006, 443, 768–773.
- Zhao, H.; Jaffer, T.; Eguchi, S.; Wang, Z.; Linkermann, A.; Ma, D. Role of necroptosis in the pathogenesis of solid organ injury. Cell Death Dis. 2015, 6, e1975.
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881.
- Domercq, M.; Matute, C. Excitotoxicity therapy for stroke patients still alive. EBioMedicine 2019, 39, 3–4.
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188.
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke 2009, 4, 461–470.
- Crack, P.J.; Taylor, J.M. Reactive oxygen species and the modulation of stroke. Free Radic. Biol. Med. 2005, 38, 1433–1444.
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutierrez, R.; Matamala, J.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative Stress and Pathophysiology of Ischemic Stroke: Novel Therapeutic Opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714.
- Yang, J. The role of reactive oxygen species in angiogenesis and preventing tissue injury after brain ischemia. Microvasc. Res. 2019, 123, 62–67.
- Burke, S.; Barnes, C. Neural plasticity in the ageing brain. Nat. Rev. Neurosci. 2006, 7, 30–40.
- Brown, C.E.; Li, P.; Boyd, J.D.; Delaney, K.R.; Murphy, T.H. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J. Neurosci. 2007, 27, 4101–4109.
- Brown, C.E.; Wong, C.; Murphy, T.H. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke 2008, 39, 1286–1291.
- Cotman, C.W. Axon Sprouting and Reactive Synaptogenesis. In Basic Neurochemistry: Molecular, Cellular and Medical Aspects; Siegel, G.J., Agranoff, B.W., Albers, R.W., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1999. Available online: https://www.ncbi.nlm.nih.gov/books/NBK28183/ (accessed on 17 July 2023).
- Koh, S.H.; Park, H.H. Neurogenesis in Stroke Recovery. Transl. Stroke Res. 2017, 8, 3–13.
- Ming, G.-L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2012, 70, 687–702.
- Rocha, N.P.; Teixeira, A.L. Neurotrophic Factors in Aging. In Encyclopedia of Geropsychology; Pachana, N.A., Ed.; Springer: Singapore, 2017; pp. 1628–1638.
- Al-Qudah, M.A.; Al-Dwairi, A. Mechanisms and regulation of neurotrophin synthesis and secretion. Neurosciences 2016, 21, 306–313.
- White, R.E.; Rao, M.; Gensel, J.C.; Mctigue, D.M.; Kaspar, B.K.; Jakeman, L.B. Transforming Growth Factor α Transforms Astrocytes to a Growth-Supportive Phenotype after Spinal Cord Injury. J. Neurosci. 2011, 31, 15173–15187.
- Dai, X.; Chen, J.; Xu, F.; Zhao, J.; Cai, W.; Sun, Z.; Hitchens, T.K.; Foley, L.M.; Leak, R.K.; Chen, J.; et al. TGFα preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J. Cereb. Blood Flow Metab. 2020, 40, 639–655.
- Sarver, D.C.; Lei, X.; Wong, G.W. FAM19A (TAFA): An Emerging Family of Neurokines with Diverse Functions in the Central and Peripheral Nervous System. ACS Chem. Neurosci. 2021, 12, 945–958.
- Lanfranconi, S.; Locatelli, F.; Corti, S.; Candelise, L.; Comi, G.P.; Baron, P.L.; Strazzer, S.; Bresolin, N.; Bersano, A. Growth factors in ischemic stroke. J. Cell. Mol. Med. 2011, 15, 1645–1687.
- Zacchigna, S.; Lambrechts, D.; Carmeliet, P. Neurovascular signalling defects in neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 169–181.
- Pramanik, S.; Yanuar, I.; Sulistio, A.; Heese, K. Neurotrophin Signaling and Stem Cells-Implications for Neurodegenerative Diseases and Stem Cell Therapy. Mol. Neurobiol. 2017, 54, 7401–7459.
- Minichiello, L. Long-term potentiation Synaptic plasticity TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009, 10, 850–860.
- Ehrlich, D.E.; Josselyn, S.A. Plasticity-related genes in brain development and amygdala-dependent learning. Genes Brain Behav. 2016, 15, 125–143.
- Prabhakaran, S.; Ruff, I.; Bernstein, R.S. Acute Stroke Intervention: A Systematic Review. JAMA 2015, 313, 1451–1462.
- Craig, L.E.; Middleton, S.; Hamilton, H.; Cudlip, F.; Swatzell, V.; Alexandrov, A.V.; Lightbody, E.; Watkins, D.C.; Philip, S.; Cadilhac, D.A.; et al. Does the Addition of Non-Approved Inclusion and Exclusion Criteria for rtPA Impact Treatment Rates? Findings in Australia, the UK, and the USA. Interv. Neurol. 2020, 8, 1–12.
- Demaerschalk, B.M.; Kleindorfer, D.O.; Adeoye, O.M.; Demchuk, A.M.; Fugate, J.E.; Grotta, J.C.; Khalessi, A.A.; Levy, E.I.; Palesch, Y.Y.; Prabhakaran, S.; et al. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, 581–641.
- Caprio, F.Z.; Sorond, F.A. Cerebrovascular Disease Primary and Secondary Stroke Prevention. Med. Clin. North Am. 2019, 103, 295–308.
- Petrovska, B.; Cekovska, S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010, 4, 106–110.
- Rivlin, R.S. Historical perspective on the use of garlic. J Nutr. 2001, 131, 951–954.
- Ekşi, G.; Mine, A.; Özkan, G.; Koyuncu, M. Garlic and onions: An eastern tale. J. Ethnopharmacol. 2020, 253, 112675.
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H.B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246.
- Omar, S.H.; Al-Wabel, N.A. Organosulfur compounds and possible mechanism of garlic in cancer. Saudi Pharm. J. 2010, 18, 51–58.
- Amagase, H. Clarifying the Real Bioactive Constituents of Garlic. J. Nutr. 2006, 136, 716–725.
- Ribeiro, M.; Alvarenga, L.; Cardozo, L.F.M.F.; Chermut, T.R.; Sequeira, J.; de Souza Gouveia Moreira, L.; Teixeira KT, R.; Shiels, P.G.; Stenvinkel, P.; Mafra, D. From the distinctive smell to therapeutic effects: Garlic for cardiovascular, hepatic, gut, diabetes and chronic kidney disease. Clin. Nutr. 2021, 40, 4807–4819.
- Abe, K.; Hori, Y.; Myoda, Y. Volatile compounds of fresh and processed garlic. Exp. Ther. Med. 2020, 19, 1585–1593.
- Aviello, G.; Abenavoli, L.; Borrelli, F.; Capasso, R.; Izzo, A.A.; Lembo, F.; Romano, B.; Capasso, F. Garlic: Empiricism or science? Nat. Prod. Commun. 2009, 4, 1785–1796.
- Lawson, L.D. Garlic: A Review of Its Medicinal Effects and Indicated Active Compounds. Avicenna J. Phytomed. 2014, 4, 1–14.
- Yoo, M.; Lee, S.; Kim, S.; Hwang, J.B.; Choe, J.; Shin, D. Composition of organosulfur compounds from cool- and warm-type garlic (Allium sativum L.) in Korea. Food Sci. Biotechnol. 2014, 23, 337–344.
- Yamaguchi, Y.; Kumagai, H. Characteristics, biosynthesis, decomposition, metabolism and functions of the garlic odour precursor, S-allyl-L-cysteine sulfoxide. Exp. Ther. Med. 2020, 19, 1528–1535.
- Borek, C. Antioxidant Health Effects of Aged Garlic Extract. J. Nutr. 2001, 131, 1010–1015.
- Xu, X.; Miao, Y.; Chen, J.Y.; Zhang, Q.; Wang, J. Effective production of S-allyl-L-cysteine through a homogeneous reaction with activated endogenous γ-glutamyltranspeptidase in garlic (Allium Sativum). J. Food Sci. Technol. 2015, 52, 1724–1729.
- Kim, J.M.; Lee, J.C.; Chang, N.; Chun, H.S.; Kim, W.K. S-Allyl-l-cysteine attenuates cerebral ischemic injury by scavenging peroxynitrite and inhibiting the activity of extracellular signal-regulated kinase. Free Radic. Res. 2006, 40, 827–835.
- Maldonado, P.D.; Alvarez-Idaboy, J.R.; Aguilar-González, A.; Lira-Rocha, A.; Jung-Cook, H.; Medina-Campos, O.N.; Pedraza-Chaverrí, J.; Galano, A. Role of allyl group in the hydroxyl and peroxyl radical scavenging activity of S-allylcysteine. J. Phys. Chem. B 2011, 115, 13408–13417.
- Kärkkäinen, V.; Pomeshchik, Y.; Savchenko, E.; Dhungana, H.; Kurronen, A.; Lehtonen, S.; Naumenko, N.; Tavi, P.; Levonen, A.L.; Yamamoto, M.; et al. Nrf2 Regulates Neurogenesis and Protects Neural Progenitor Cells Against Aβ Toxicity. Stem Cells 2014, 32, 1904–1916.
- Chen, C.; Pung, D.; Leong, V.; Hebbar, V.; Shen, G.; Nair, S.; Li, W.; Tony Kong, A.N. Induction of detoxifying enzymes by garlic organosulfur compounds through transcription factor Nrf2: Effect of chemical structure and stress signals. Free Radic. Biol. Med. 2004, 37, 1578–1590.
- Fisher, C.D.; Augustine, L.M.; Maher, J.M.; Nelson, D.M.; Slitt, A.L.; Klaassen, C.D.; Lehman-McKeeman, L.D.; Cherrington, N.J. Induction of Drug-Metabolizing Enzymes by Garlic and Allyl Sulfide Compounds via Activation of Constitutive Androstane Receptor and Nuclear Factor E2-Related Factor 2. Drug Metab. Dispos. 2007, 35, 995–1000.
- Shi, H.; Jing, X.; Wei, X.; Perez, R.G.; Ren, M.; Zhang, X.; Lou, H. S-allyl cysteine activates the Nrf2-dependent antioxidant response and protects neurons against ischemic injury in vitro and in vivo. J. Neurochem. 2015, 133, 298–308.
- Arreola, R.; Quintero-Fabián, S.; Lopez-Roa, R.L.; Flores-Gutierrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuno-Sahagun, D. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. J. Immunol. Res. 2015, 2015, 401630.
- Moutia, M.; Habti, N.; Badou, A. In Vitro and In Vivo Immunomodulator Activities of Allium sativum L. Evid. Based Complement. Alternat. Med. 2018, 2018, 4984659.
- Kim, J.M.; Hyun, J.C.; Kim, W.K.; Chang, N.; Hyang, S.C. Structure−Activity Relationship of Neuroprotective and Reactive Oxygen Species Scavenging Activities for Allium Organosulfur Compounds. J. Agric. Food Chem. 2006, 54, 6547–6553.
- Lin, J.J.; Chang, T.; Cai, W.K.; Zhang, Z.; Yang, Y.X.; Sun, C.; Li, X.Y.; Li, W.X. Post-injury administration of allicin attenuates ischemic brain injury through sphingosine kinase 2: In vivo and in vitro studies. Neurochem. Int. 2015, 89, 92–100.
- Atif, F.; Yousuf, S.; Agrawal, S.K. S-Allyl L-cysteine diminishes cerebral ischemia-induced mitochondrial dysfunctions in hippocampus. Brain Res. 2009, 1265, 128–137.
- Ashafaq, M.; Khan, M.M.; Shadab Raza, S.; Ahmad, A.; Khuwaja, G.; Javed, H.; Khan, A.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. S-allyl cysteine mitigates oxidative damage and improves neurologic deficit in a rat model of focal cerebral ischemia. Nutr. Res. 2012, 32, 133–143.
- Numagami, Y.; Sato, S.; Ohnishi, S.T. Attenuation of rat ischemic brain damage by aged garlic extracts: A possible protecting mechanism as antioxidants. Neurochem. Int. 1996, 29, 135–143.
- Batirel, H.F.; Aktan, S.; Aykut, C.; Yeǧen, B.C.; Coşkun, T. The effect of aqueous garlic extract on the levels of arachidonic acid metabolites (leukotriene C4 and prostaglandin E2) in rat forebrain after ischemia-reperfusion injury. Prostaglandins Leukot. Essent. Fat. Acids 1996, 54, 289–291.
- Gupta, R.; Singh, M.; Sharma, A. Neuroprotective effect of antioxidants on ischaemia and reperfusion-induced cerebral injury. Pharmacol. Res. 2003, 48, 209–215.
- Aguilera, P.; Chánez-Cárdenas, M.E.; Ortiz-Plata, A.; León-Aparicio, D.; Barrera, D.; Espinoza-Rojo, M.; Villeda-Hernández, J.; Sánchez-García, A.; Maldonado, P.D. Aged garlic extract delays the appearance of infarct area in a cerebral ischemia model, an effect likely conditioned by the cellular antioxidant systems. Phytomedicine 2010, 17, 241–247.
- Colín-González, A.L.; Ortiz-Plata, A.; Villeda-Hernández, J.; Barrera, D.; Molina-Jijón, E.; Pedraza-Chaverrí, J.; Maldonado, P.D. Aged Garlic Extract Attenuates Cerebral Damage and Cyclooxygenase-2 Induction after Ischemia and Reperfusion in Rats. Plant Foods Hum. Nut. 2011, 66, 348–354.
- Gomez, C.D.; Aguilera, P.; Ortiz-Plata, A.; López, F.N.; Chánez-Cárdenas, M.E.; Flores-Alfaro, E.; Ruiz-Tachiquín, M.E.; Espinoza-Rojo, M. Aged garlic extract and S-allylcysteine increase the GLUT3 and GCLC expression levels in cerebral ischemia. Adv. Clin. Exp. Med. 2019, 28, 1609–1614.
- Saleem, S.; Ahmad, M.; Ahmad, A.S.; Yousuf, S.; Ansari, M.A.; Khan, M.B.; Ishrat, T.; Islam, F. Behavioral and Histologic Neuroprotection of Aqueous Garlic Extract After Reversible Focal Cerebral Ischemia. J. Med. Food 2007, 9, 537–544.
- Cervantes, M.I.; De Oca Balderas, P.M.; de Jesús Gutiérrez-Baños, J.; Orozco-Ibarra, M.; Fernández-Rojas, B.; Medina-Campos, O.M.; Espinoza-Rojo, M.; Ruiz-Tachiquín, M.; Ortiz-Plata, A.; Salazar, M.I.; et al. Comparison of antioxidant activity of hydroethanolic fresh and aged garlic extracts and their effects on cerebral ischemia. Food Chem. 2013, 140, 343–352.
- Moriguchi, T.; Matsuura, H.; Kodera, Y.; Itakura, Y.; Katsuki, H.; Saito, H.; Nishiyama, N. Neurotrophic activity of organosulfur compounds having a thioallyl group on cultured rat hippocampal neurons. Neurochem. Res. 1997, 22, 1449–1452.
- Hashimoto, M.; Nakai, T.; Masutani, T.; Unno, K.; Akao, Y. Improvement of Learning and Memory in Senescence-Accelerated Mice by S-Allylcysteine in Mature Garlic Extract. Nutrients 2020, 12, 1834.
- Reyes-Soto, C.Y.; Rangel-López, E.; Galván-Arzate, S.; Colín-González, A.L.; Silva-Palacios, A.; Zazueta, C.; Pedraza-Chaverri, J.; Ramírez, J.; Chavarria, A.; Túnez, I.; et al. S-Allylcysteine Protects Against Excitotoxic Damage in Rat Cortical Slices Via Reduction of Oxidative Damage, Activation of Nrf2/ARE Binding, and BDNF Preservation. Neurotox. Res. 2020, 38, 929–940.
- Nam, S.M.; Yoo, Y.; Kim, W.; Yoo, M.; Kim, D.W.; Won, M.H.; Hwang, I.K.; Yoon, Y.S. Effects of S-Allyl-L-Cysteine on Cell Proliferation and Neuroblast Differentiation in the Mouse Dentate Gyrus. J. Vet. Med. Sci. 2011, 73, 1071–1075.
- Javed, H.; Khan, M.M.; Khan, A.; Vaibhav, K.; Ahmad, A.; Khuwaja, G.; Ahmed, M.E.; Raza, S.S.; Ashafaq, M.; Tabassum, R.; et al. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2011, 1389, 133–142.
- Zarezadeh, M.; Baluchnejadmojarad, T.; Kiasalari, Z.; Afshin-Majd, S.; Roghani, M. Garlic active constituent s-allyl cysteine protects against lipopolysaccharide-induced cognitive deficits in the rat: Possible involved mechanisms. Eur. J. Pharmacol. 2017, 795, 13–21.
- Xiang, Q.; Li, X.; Yang, B.; Fang, X.; Jia, J.; Ren, J.; Dong, Y.; Ou-Yang, C.; Wang, G. Allicin attenuates tunicamycin-induced cognitive deficits in rats via its synaptic plasticity regulatory activity. Iran. J. Basic Med. Sci. 2017, 20, 676–682.
- Yamada, N.; Hattori, A.; Hayashi, T.; Nishikawa, T.; Fukuda, H.; Fujino, T. Improvement of scopolamine-induced memory impairment by Z-ajoene in the water maze in mice. Pharmacol. Biochem. Behav. 2004, 78, 787–791.
- Jung, H.Y.; Lee, K.Y.; Yoo, D.Y.; Kim, J.W.; Yoo, M.; Lee, S.; Yoo, K.Y.; Yoon, Y.S.; Hoon Choi, J.; Hwang, I.K. Essential oils from two Allium species exert effects on cell proliferation and neuroblast differentiation in the mouse dentate gyrus by modulating brain-derived neurotrophic factor and acetylcholinesterase. BMC Complement. Med. Ther. 2016, 16, 431.
- Huang, Y.J.; Lu, K.H.; Lin, Y.E.; Panyod, S.; Wu, H.Y.; Chang, W.T.; Sheen, Y. Garlic essential oil mediates acute and chronic mild stress-induced depression in rats via modulation of monoaminergic neurotransmission and brain-derived neurotrophic factor levels. Food Funct. 2019, 10, 8094–8105.
- Alipour, F.; Bideskan, A.E.; Fazel, A.; Sadeghi, A.; Hami, J.; Kheradmand, H.; Haghir, H. Protective effects of ascorbic acid and garlic extract against neurogenesis inhibition caused by developmental lead exposure in the dentate gyrus of rat. Comp. Clin. Pathol. 2014, 23, 1681–1687.
- Semuyaba, I.; Alao Safiriyu, A.; Ayikobua Tiyo, E.; Figueredo Niurka, R. Memory Improvement Effect of Ethanol Garlic (A. sativum) Extract in Streptozotocin-Nicotinamide Induced Diabetic Wistar Rats Is Mediated through Increasing of Hippocampal Sodium-Potassium ATPase, Glutamine Synthetase, and Calcium ATPase Activities. Evid. Based Complement. Alternat. Med. 2017, 2017, 3720380.




