
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maxime Mourer | -- | 2172 | 2023-12-22 10:50:19 | | | |
| 2 | Lindsay Dong | Meta information modification | 2172 | 2023-12-25 01:49:42 | | | | |
| 3 | Lindsay Dong | Meta information modification | 2172 | 2023-12-29 09:27:18 | | |
Video Upload Options
Since the discovery of polyphenolic resins 150 years ago, the study of polymeric compounds named calix[n]arene has continued to progress, and those skilled in the art perfectly know now how to modulate this phenolic ring. Consequently, calix[n]arenes are now used in a large range of applications and notably in therapeutic fields. In particular, the calix[4]arene exhibits multiple possibilities for regioselective polyfunctionalization on both of its rims and offers researchers the possibility of precisely tuning the geometry of their structures. Thus, in the crucial research of new antibacterial active ingredients, the design of calixarenes finds its place perfectly. Out of all the work of the community, there are some excellent activities emerging that could potentially place these original structures in a very good position for the development of new active ingredients.
1. The Structure of Calixarenes

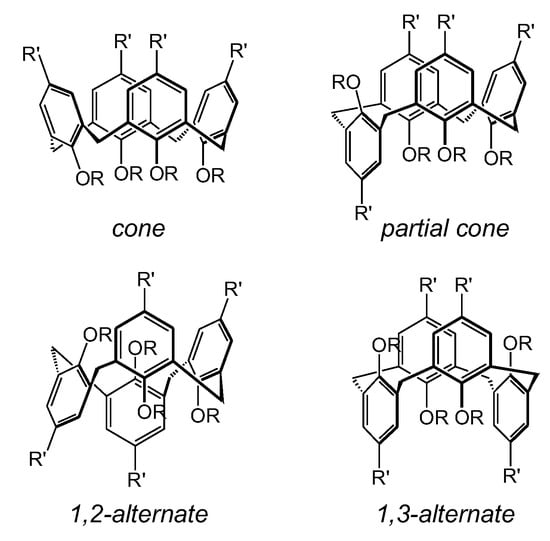
2. The Functionalization of Calix[4]arene
3. Cytotoxicity of Calix[n]arenes
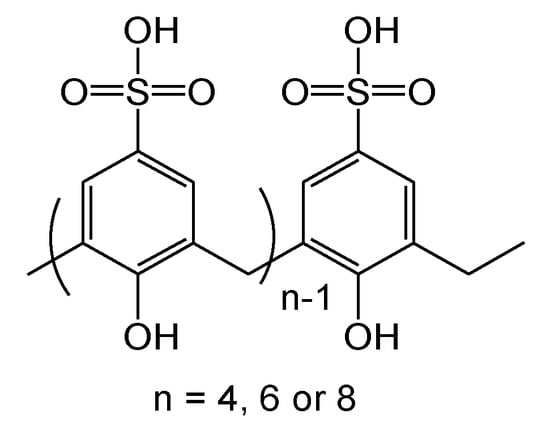
4. Antibacterial Activities of Calix[n]arenes
4.1. Intrinsically Active Calixarenes
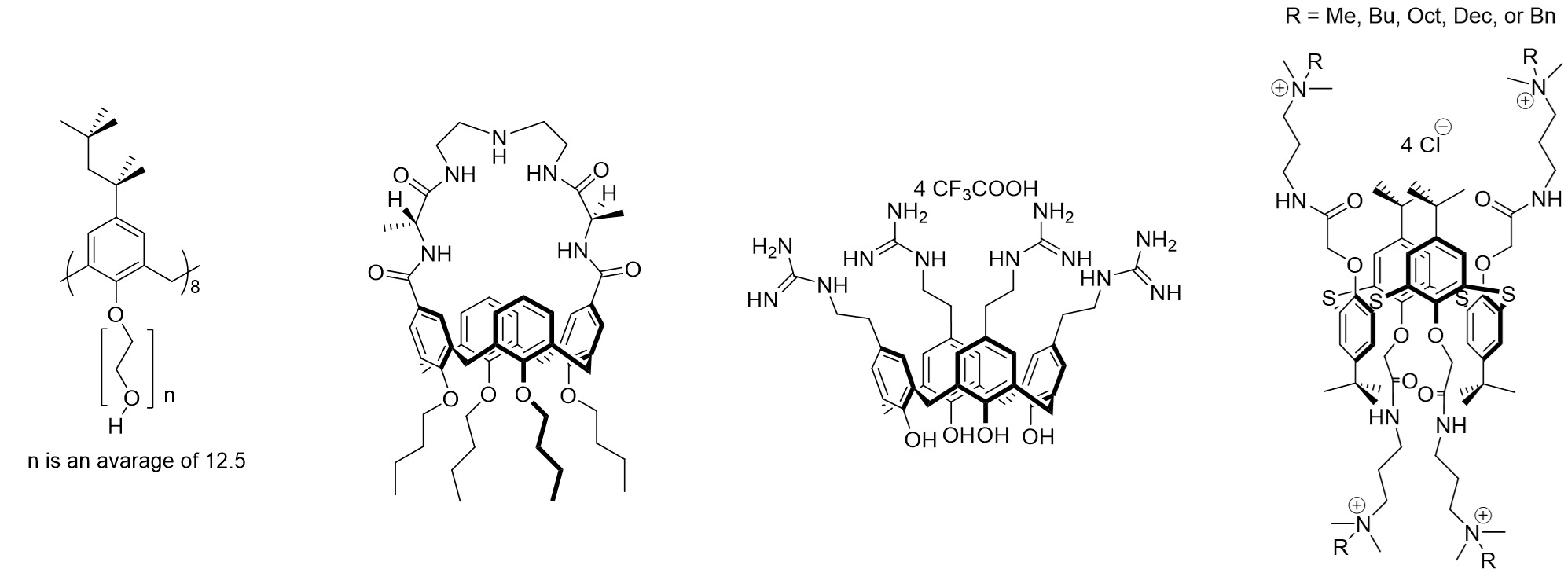
Subsequently, very good results appeared, for example, for peptidocalix[4]arenes (Figure 4) with good activity against MRSA (methicillin-resistant S. aureus) (4 to 8 µg/mL) [57][58][59]. From 2006, calix[4]arene positively charged by the introduction of guanidinium functions showed excellent activities on reference bacterial strains (Figure 4). These last introduced on the upper rim displays a significant antibacterial activity (MIC <8 µg/mL) against the bacterial reference strains E. coli, S. aureus, E. faecalis and P. aeruginosa [18][60], but also on the clinical isolates penicillinase producing E. coli, methicillin-resistant S. aureus, vancomycin-resistant E. faecium, vancomycin- and teicoplanin-resistant E. faecalis, and P. aeruginosa overexpressing efflux pumps [61]. This guanidinium calixarene and its derivatives also have very good activity against a particular strain: Mycobacterium tuberculosis [60][62].
Another examples concern a water-soluble macromolecule based on calix[4]arene and morpholine units [63], appears to have an excellent activity profile with the inhibitions of growth were measured at 4 µg/mL against gram-positive and gram-negative species, but also a serie of azo compounds [64] showing very good activities through CMI measurements on five Gram-positive bacterial strains (B. subtilis, S. aureus, methicillin-resistant S. aureus (MRSA), S. epidermidis, and E. faecalis) (<10 µg/mL).
Finally, a recent study confirms the interest of calixarenes carrying cationic functions but also of the impact of conformation [65] (Figure 4) as described in a previous study [60]. Indeed, the ammonium derivatives concerned exhibit activities close to 1 µg/ml against Gram-positive and Gram-negative strains.
4.2. Molecular Carrier/Drug Delivery
These studies propose, for example, the introduction of penicillin via amide or ester functions, which should release the biologically active free acid and amine after hydrolysis by esterases [66][67] (Figure 5). The same strategy allows the synthesis of bis-quinolone derivatives [68][69] and a study of the anti-bacterial activity against Gram-positive reference ATCC strains, S. aureus and E. faecalis, as well as against two Gram-negative reference strains E. coli and P. aeruginosa. Designed as hydrophobic compounds, these structures are not really easy for in vitro standard evaluation as antimicrobial agents. Thus, an additional study proposes the development of water-soluble compounds via the introduction of ammonium functions [70]. Other researchers have also obtained promising results through the synthesis and biological evaluation of penicillins V and X clustered by a calixarene scaffold [71] and the corresponding cephalosporine derivatives [72] (Figure 5). The impact of cyclotetramerization was demonstrated by a notable increase in activity on all penicillin cyclotetramer strains versus monomers.
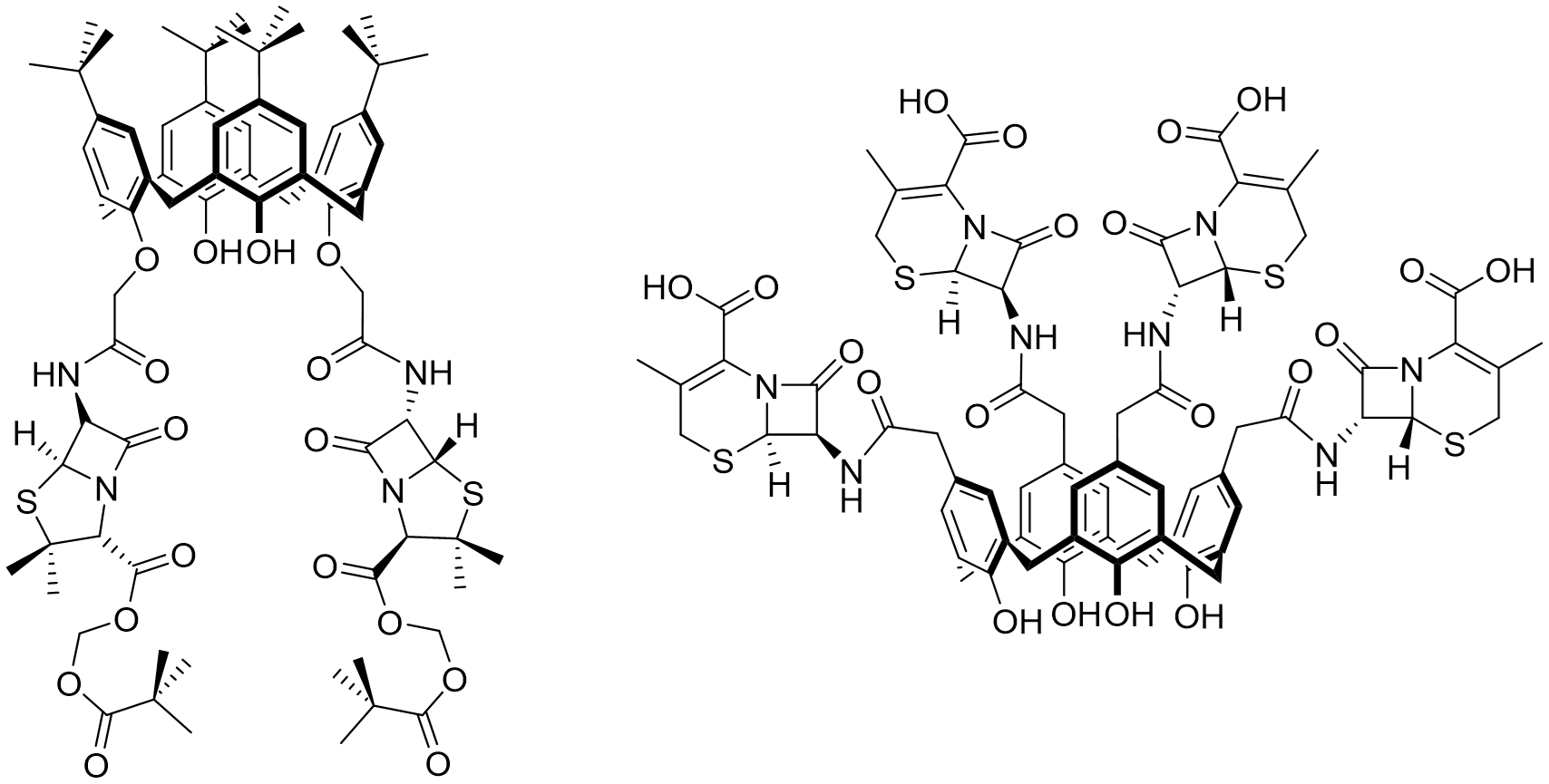
Figure 5. Examples of molecular carriers/drug delivery: penicillin (left) or cephalosporine (right)
4.3. Complexes
In this sense, researchers have developed complex structures of mononuclear copper [73][74] (Figure 6) for their antibacterial activities against Gram-positive (B. subtilis, B. cereus, E. faecalis (clinic sample), S. aureus) and Gram-negative bacteria (E. coli, E. coli, P. aeruginosa, Proteus vulgaris) or binuclear [75] (Figure 6) active against Gram-positive S. albus and Gram-negative E. coli. On the other hand, the thiosemicarbazide moieties and their thiosemicarbazone derivatives known for their ability to coordinate with transition metals by bonding with sulfur and nitrogen atoms [167,168] allowed the development of cobalt (II), nickel (II), copper (II), or zinc (II) complexes.
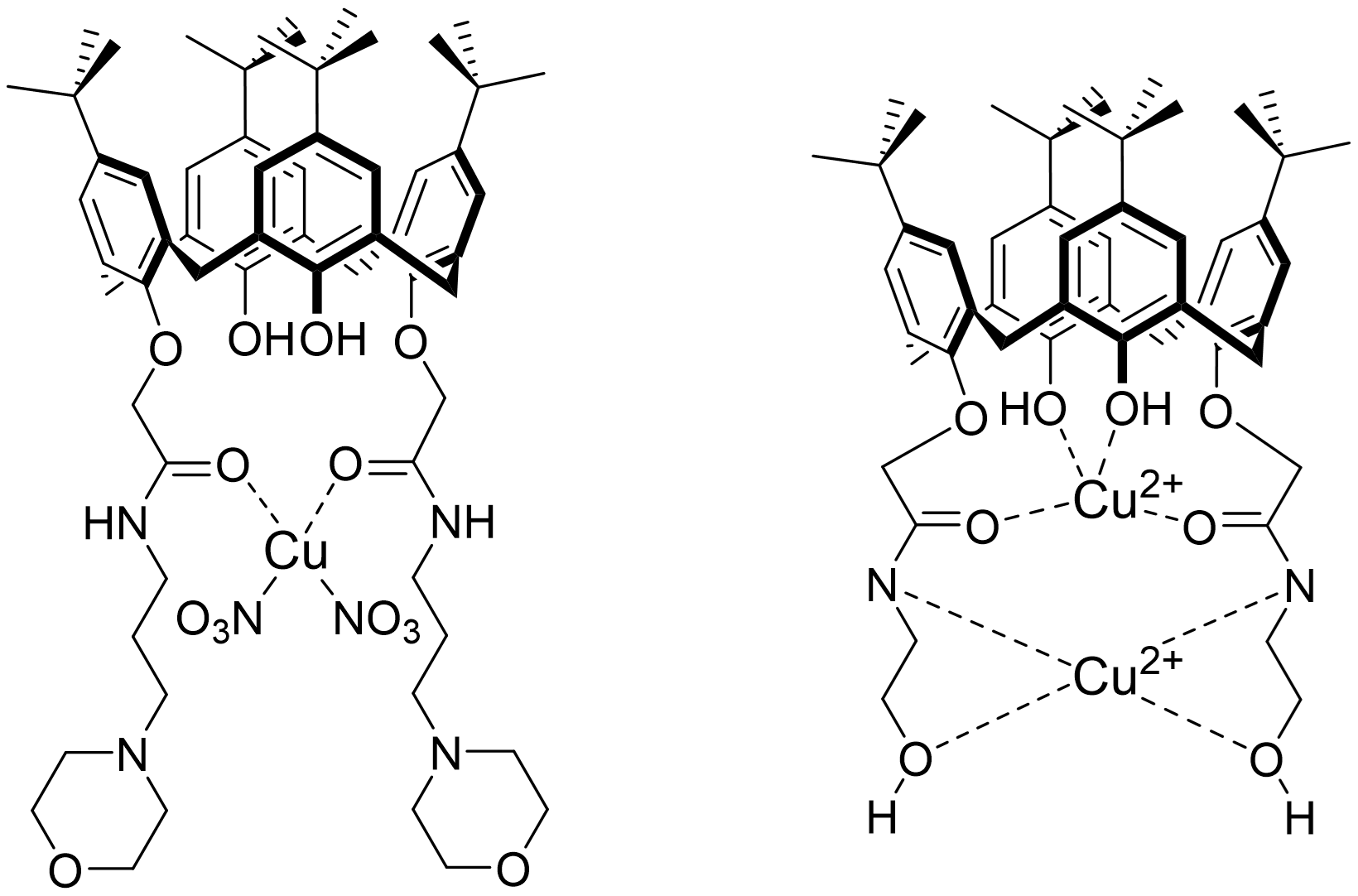
Figure 6. Examples of mononuclear copper (left) and binuclear copper (right) complexes
5. Conclusions
References
- Baeyer, A. Ueber Die Verbindungen Der Aldehyde Mit Den Phenolen. Berichte Der Dtsch. Chem. Ges. 1872, 5, 280–282.
- Baeyer, A. Ueber Die Verbindungen Der Aldehyde Mit Den Phenolen Und Aromatischen Kohlenwasserstoffen. Berichte Der Dtsch. Chem. Ges. 1872, 5, 1094–1100.
- Niederl, J.B.; Vogel, H.J. Aldehyde—Resorcinol Condensations1. J. Am. Chem. Soc. 1940, 62, 2512–2514.
- Zinke, A.; Ziegler, E. Zur Kenntnis Des Härtungsprozesses von Phenol-Formaldehyd-Harzen. V. Mitteilung. Berichte Der Dtsch. Chem. Ges. (A B Ser.) 1941, 74, 541–545.
- Gutsche, C.D.; Muthukrishnan, R. Calixarenes. 1. Analysis of the Product Mixtures Produced by the Base-Catalyzed Condensation of Formaldehyde with Para-Substituted Phenols. J. Org. Chem. 1978, 43, 4905–4906.
- Gutsche, C.D. Calixarenes Revisited; Royal Society of Chemistry: Cambridge, UK, 1998.
- Arduini, A.; Pochini, A.; Raverberi, S.; Ungaro, R. P-t-Butyl-Calix[4]Arene Tetracarboxylic Acid. A Water Soluble Calixarene in a Cone Structure. J. Chem. Soc. Chem. Commun. 1984, 15, 981–982.
- Gutsche, C.D.; Alam, I. Calixarenes. 23. the Complexation and Catalytic Properties of Water Soluble Calixarenes. Tetrahedron 1988, 44, 4689–4694.
- Shinkai, S.; Mori, S.; Tsubaki, T.; Sone, T.; Manabe, O. New Water-Soluble Host Molecules Derived from Calix[6]Arene. Tetrahedron Lett. 1984, 25, 5315–5318.
- Shinkai, S.; Araki, K.; Tsubaki, T.; Arimura, T.; Manabe, O. New Syntheses of Calixarene-p-Sulphonates and p-Nitrocalixarenes. J. Chem. Soc. Perkin Trans. 1987, 1, 2297–2299.
- Seiji, S.; Seiichi, M.; Hiroshi, K.; Takayuki, T.; Osamu, M. Hexasulfonated Calix[6]Arene Derivatives: A New Class of Catalysts, Surfactants, and Host Molecules. J. Am. Chem. Soc. 1986, 108, 2409–2416.
- Shinkai, S.; Mori, S.; Koreishi, H.; Tsubaki, T.; Manabe, O. Chloromethylation of Calixarenes and Synthesis of New Water Soluble Macrocyclic Hosts. Tetrahedron 1989, 45, 2177–2182.
- Gutsche, C.D.; Iqbal, M.; Nam, K.S.; See, K.; Alam, I. Conformational and Complexational Characteristics of Calixarenes. Pure Appl. Chem. 1988, 60, 483–488.
- Gutsche, C.D.; Nam, K. Chun. Calixarenes. 22. Synthesis, Properties, and Metal Complexation of Aminocalixarenes. J. Am. Chem. Soc. 1988, 110, 6153–6162.
- Ohseto, F.; Murakami, H.; Araki, K.; Shinkai, S. Substitution of OH with NH2 Calix[4]Arenes: An Approach to the Synthesis of Aminocalixarenes. Tetrahedron Lett. 1992, 33, 1217–1220.
- Aleksiuk, O.; Grynszpan, F.; Biali, S.E. Spirodienone Route for Aminodehydroxylation: Monoaminotrihydroxy-p-Tert-Butylcalix[4]Arene. J. Org. Chem. 1993, 58, 1994–1996.
- Dudic, M.; Colombo, A.; Sansone, F.; Casnati, A.; Donofrio, G.; Ungaro, R. A General Synthesis of Water Soluble Upper Rim Calix[n]Arene Guanidinium Derivatives Which Bind to Plasmid DNA. Tetrahedron 2004, 60, 11613–11618.
- Mourer, M.; Duval, R.E.; Finance, C.; Regnouf-de-Vains, J.-B. Functional Organisation and Gain of Activity: The Case of the Antibacterial Tetra-Para-Guanidinoethyl-Calix[4]Arene. Bioorganic Med. Chem. Lett. 2006, 16, 2960–2963.
- Gutsche, C.D.; Pagoria, P.F. Calixarenes. 16. Functionalized Calixarenes: The Direct Substitution Route. J. Org. Chem. 1985, 50, 5795–5802.
- Conner, M.; Janout, V.; Regen, S.L. Synthesis and Alkali Metal Binding Properties of “Upper Rim” Functionalized CalixArenes. J. Org. Chem. 1992, 57, 3744–3746.
- Verboom, W.; Durie, A.; Egberink, R.J.M.; Asfari, Z.; Reinhoudt, D.N. Ipso Nitration of P-Tert-ButylcalixArenes. J. Org. Chem. 1992, 57, 1313–1316.
- Redon, S.; Li, Y.; Reinaud, O. Unprecedented Selective Ipso-Nitration of Calixarenes Monitored by the O-Substituents. J. Org. Chem. 2003, 68, 7004–7008.
- Shinkai, S.; Mori, S.; Tsubaki, T.; Sone, T.; Manabe, O. New Water-Soluble Host Molecules Derived from CalixArene. Tetrahedron Lett. 1984, 25, 5315–5318.
- Seiji, S.; Seiichi, M.; Hiroshi, K.; Takayuki, T.; Osamu, M. Hexasulfonated CalixArene Derivatives: A New Class of Catalysts, Surfactants, and Host Molecules. J. Am. Chem. Soc. 1986, 108, 2409–2416.
- Marcillac, A.; Choquard, P.; Vains, J.-B.R.D.; Lamartine, R. Process for the Dealkylating Sulfonation of P-Alkyl Calixarenes. CA2258904A1, 31 December 1997. Available online: https://patents.google.com/patent/CA2258904A1/en (accessed on 21 September 2023).
- Arduini, A.; Manfredi, G.; Pochini, A.; Sicuri, A.R.; Ungaro, R. Selective Formylation of CalixArenes at the ‘Upper Rim’ and Synthesis of New Cavitands. J. Chem. Soc. Chem. Commun. 1991, 14, 936–937.
- Van Loon, J.D.; Arduini, A.; Coppi, L.; Verboom, W.; Pochini, A.; Ungaro, R.; Harkema, S.; Reinhoudt, D.N. Selective Functionalization of CalixArenes at the Upper Rim. J. Org. Chem. 1990, 55, 5639–5646.
- Van Loon, J.-D.; Arduini, A.; Verboom, W.; Ungaro, R.; van Hummel, G.J.; Harkema, S.; Reinhoudt, D.N. Selective Functionalization of CalixArenes at the Upper Rim. Tetrahedron Lett. 1989, 30, 2681–2684.
- Gutsche, C.D.; Dhawan, B.; Levine, J.A.; Hyun No, K.; Bauer, L.J. Calixarenes 9: Conformational Isomers of the Ethers and Esters of CalixArenes. Tetrahedron 1983, 39, 409–426.
- Araki, K.; Iwamoto, K.; Shinkai, S.; Matsuda, T. “pKa” of Calixarenes and Analogs in Nonaqueous Solvents. BCSJ 1990, 63, 3480–3485.
- Van Loon, J.D.; Kraft, D.; Ankone, M.J.K.; Verboom, W.; Harkema, S.; Vogt, W.; Boehmer, V.; Reinhoudt, D.N. CalixArenes Bridged at the Lower Rim. J. Org. Chem. 1990, 55, 5176–5179.
- Arduini, A.; Casnati, A.; Dodi, L.; Pochini, A.; Ungaro, R. Selective 1,2-Functionalization of CalixArenes at the Lower Rim. Synthesis of a New Type of Bis-Calixcrown Ether. J. Chem. Soc., Chem. Commun. 1990, 22, 1597–1598.
- Groenen, L.C.; Ruël, B.H.M.; Casnati, A.; Timmerman, P.; Verboom, W.; Harkema, S.; Pochini, A.; Ungaro, R.; Reinhoudt, D.N. Syn-1,2-Dialkylated CalixArenes: General Intermediates in the NaH/DMF Tetraalkylation of CalixArenes. Tetrahedron Lett. 1991, 32, 2675–2678.
- See, K.A.; Fronczek, F.R.; Watson, W.H.; Kashyap, R.P.; Gutsche, C.D. Calixarenes. 26. Selective Esterification and Selective Ester Cleavage of CalixArenes. J. Org. Chem. 1991, 56, 7256–7268.
- Iwamoto, K.; Araki, K.; Shinkai, S. Syntheses of All Possible Conformational Isomers of O-Alkyl-p-t-ButylcalixArenes. Tetrahedron 1991, 47, 4325–4342.
- Brunink, J.A.J.; Verboom, W.; Engbersen, J.F.J.; Reinhoudt, D.N.; Harkema, S. Synthesis and Cation Complexation Selectivity of Bis(Syn-Proximally) Functionalized CalixArenes. Recl. Des Trav. Chim. Des Pays Bas 1992, 111, 511–516.
- Iqbal, M.; Mangiafico, T.; Gutsche, C.D. Calixarenes 21: The Conformations and Structures of the Products of Aroylation of the, CalixArenes. Tetrahedron 1987, 43, 4917–4930.
- Bottino, F.; Giunta, L.; Pappalardo, S. CalixArenes with Pyridine Pendant Groups. Regioselective Proximal Alkylation at the “Lower Rim”. J. Org. Chem. 1989, 54, 5407–5409.
- Iwamoto, K.; Fujimoto, K.; Matsuda, T.; Shinkai, S. Remarkable Metal Template Effects on Selective Syntheses of P-t-ButylcalixArene Conformers. Tetrahedron Lett. 1990, 31, 7169–7172.
- Gutsche, C.D.; Reddy, P.A. Calixarenes. 25. Conformations and Structures of the Products of Arylmethylation of CalixArenes. J. Org. Chem. 1991, 56, 4783–4791.
- Perret, F.; Lazar, A.N.; Coleman, A.W. Biochemistry of the Para-Sulfonato-CalixArenes. Chem. Commun. 2006, 23, 2425–2438.
- Perret, F.; Coleman, A.W. Biochemistry of Anionic CalixArenes. Chem. Commun. 2011, 47, 7303–7319.
- Bahojb Noruzi, E.; Molaparast, M.; Zarei, M.; Shaabani, B.; Kariminezhad, Z.; Ebadi, B.; Shafiei-Irannejad, V.; Rahimi, M.; Pietrasik, J. Para-SulfonatocalixArene-Based Biomaterials: Recent Progress in Pharmaceutical and Biological Applications. Eur. J. Med. Chem. 2020, 190, 112121.
- Da Silva, E.; Shahgaldian, P.; Coleman, A.W. Haemolytic Properties of Some Water-Soluble Para-Sulphonato-Calix-[n]-Arenes. Int. J. Pharm. 2004, 273, 57–62.
- Paclet, M.-H.; Rousseau, C.F.; Yannick, C.; Morel, F.; Coleman, A.W. An Absence of Non-Specific Immune Response towards Para-Sulphonato-Calix[n]Arenes. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 353–357.
- Wheate, N.J.; Abbott, G.M.; Tate, R.J.; Clements, C.J.; Edrada-Ebel, R.; Johnston, B.F. Side-on Binding of p-Sulphonatocalix[4]Arene to the Dinuclear Platinum Complex Trans-[{PtCl(NH3)2}2μ-Dpzm]2+ and Its Implications for Anticancer Drug Delivery. J. Inorg. Biochem. 2009, 103, 448–454.
- Geller, C.; Fontanay, S.; Mourer, M.; Dibama, H.M.; Regnouf-de-Vains, J.-B.; Finance, C.; Duval, R.E. Antiseptic Properties of Two Calix[4]Arenes Derivatives on the Human Coronavirus 229E. Antivir. Res. 2010, 88, 343–346.
- Lalor, R.; Baillie-Johnson, H.; Redshaw, C.; Matthews, S.E.; Mueller, A. Cellular Uptake of a Fluorescent Calix[4]Arene Derivative. J. Am. Chem. Soc. 2008, 130, 2892–2893.
- Da Silva, E.; Shahgaldian, P.; Coleman, A.W. Haemolytic Properties of Some Water-Soluble Para-Sulphonato-Calix--Arenes. Int. J. Pharm. 2004, 273, 57–62.
- Mammen, M.; Choi, S.-K.; Whitesides, G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2754–2794.
- Fasting, C.; Schalley, C.A.; Weber, M.; Seitz, O.; Hecht, S.; Koksch, B.; Dernedde, J.; Graf, C.; Knapp, E.-W.; Haag, R. Multivalency as a Chemical Organization and Action Principle. Angew. Chem. Int. Ed. 2012, 51, 10472–10498.
- Haag, R. Multivalency as a Chemical Organization and Action Principle. Beilstein J. Org. Chem. 2015, 11, 848–849.
- Giuliani, M.; Morbioli, I.; Sansone, F.; Casnati, A. Moulding Calixarenes for Biomacromolecule Targeting. Chem. Commun. 2015, 51, 14140–14159.
- Cornforth, J.W.; Hart, P.D.; Nicholls, G.A.; Rees, R.J.W.; Stock, J.A. Antituberculous Effects of Certain Surface-Active Polyoxyethylene Ethers. Br. J. Pharmacol. Chemother. 1955, 10, 73–86.
- Cornforth, J.W.; Morgan, E.D.; Potts, K.T.; Rees, R.J.W. Preparation of Antituberculous Polyoxyethylene Ethers of Homogeneous Structure. Tetrahedron 1973, 29, 1659–1667.
- Loiseau, F.A.; Hill, A.M.; (Mimi) Hii, K.K. Preparation of Macrocyclon Analogues: Calix[8]Arenes with Extended Polyethylene Glycol Chains. Tetrahedron 2007, 63, 9947–9959.
- Casnati, A.; Fabbi, M.; Pelizzi, N.; Pochini, A.; Sansone, F.; Ungaro, R.; Di Modugno, E.; Tarzia, G. Synthesis, Antimicrobial Activity and Binding Properties of Calix[4]Arene Based Vancomycin Mimics. Bioorganic Med. Chem. Lett. 1996, 6, 2699–2704.
- Frish, L.; Sansone, F.; Casnati, A.; Ungaro, R.; Cohen, Y. Complexation of a Peptidocalix[4]Arene, a Vancomycin Mimic, with Alanine-Containing Guests by NMR Diffusion Measurements. J. Org. Chem. 2000, 65, 5026–5030.
- Casnati, A.; Sansone, F.; Ungaro, R. Peptido- and Glycocalixarenes: Playing with Hydrogen Bonds around Hydrophobic Cavities. Acc. Chem. Res. 2003, 36, 246–254.
- Mourer, M.; Duval, R.E.; Constant, P.; Daffé, M.; Regnouf-de-Vains, J.-B. Impact of Tetracationic Calix[4]Arene Conformation—From Conic Structure to Expanded Bolaform—On Their Antibacterial and Antimycobacterial Activities. ChemBioChem 2019, 20, 911–921.
- Grare, M.; Mourer, M.; Fontanay, S.; Regnouf-de-Vains, J.-B.; Finance, C.; Duval, R.E. In Vitro Activity of Para-Guanidinoethylcalix[4]Arene against Susceptible and Antibiotic-Resistant Gram-Negative and Gram-Positive Bacteria. J. Antimicrob. Chemother. 2007, 60, 575–581.
- Mourer, M.; Massimba Dibama, H.; Constant, P.; Daffé, M.; Regnouf-de-Vains, J.-B. Anti-Mycobacterial Activities of Some Cationic and Anionic Calix[4]Arene Derivatives. Bioorganic Med. Chem. 2012, 20, 2035–2041.
- Memon, S.; Soomro, A.M.; Oad, R.K.; Qureshi, I. Bioactivity Assessment of Water Soluble Calix[4]Arene Derivative. Pak. J. Anal. Environ. Chem. 2012, 13, 39.
- Ali, Y.; Muhamad Bunnori, N.; Susanti, D.; Muhammad Alhassan, A.; Abd Hamid, S. Synthesis, in-Vitro and in Silico Studies of Azo-Based Calix[4]Arenes as Antibacterial Agent and Neuraminidase Inhibitor: A New Look Into an Old Scaffold. Front. Chem. 2018, 6, 210.
- Padnya, P.L.; Terenteva, O.S.; Akhmedov, A.A.; Iksanova, A.G.; Shtyrlin, N.V.; Nikitina, E.V.; Krylova, E.S.; Shtyrlin, Y.G.; Stoikov, I.I. Thiacalixarene Based Quaternary Ammonium Salts as Promising Antibacterial Agents. Bioorganic Med. Chem. 2021, 29, 115905.
- Ben Salem, A.; Regnouf-de-Vains, J.-B. Synthesis and Characterisation of a New Podand Based on a Calixarene and a β-Lactam. Tetrahedron Lett. 2001, 42, 7033–7036.
- Korchowiec, B.; Salem, A.B.; Corvis, Y.; Regnouf-de-Vains, J.-B.; Korchowiec, J.; Rogalska, E. Calixarenes in a Membrane Environment: A Monolayer Study on the Miscibility of Three p-Tert-Butylcalix[4]Arene β-Lactam Derivatives with 1,2-Dimyristoyl-Sn-Glycero-3-Phosphoethanolamine. J. Phys. Chem. B 2007, 111, 13231–13242.
- Ben Salem, A.; Regnouf-de-Vains, J.-B. Towards a New Family of Calixarene-Based Podands Incorporating Quinolone Arms. An Example Using Nalidixic Acid. Tetrahedron Lett. 2003, 44, 6769–6771.
- Korchowiec, B.; Orlof, M.; Sautrey, G.; Ben Salem, A.; Korchowiec, J.; Regnouf-de-Vains, J.-B.; Rogalska, E. The Mechanism of Metal Cation Binding in Two Nalidixate Calixarene Conjugates. A Langmuir Film and Molecular Modeling Study. J. Phys. Chem. B 2010, 114, 10427–10435.
- Dibama, H.M.; Clarot, I.; Fontanay, S.; Salem, A.B.; Mourer, M.; Finance, C.; Duval, R.E.; Regnouf-de-Vains, J.-B. Towards Calixarene-Based Prodrugs: Drug Release and Antibacterial Behaviour of a Water-Soluble Nalidixic Acid/Calix[4]Arene Ester Adduct. Bioorganic Med. Chem. Lett. 2009, 19, 2679–2682.
- Pur, F.N.; Dilmaghani, K.A. Calixpenams: Synthesis, Characterization, and Biological Evaluation of Penicillins V and X Clustered by Calixarene Scaffold. Turk. J. Chem. 2014, 38, 288–296.
- Pur, F.N.; Dilmaghani, K.A. Calixcephems: Clustered cephalosporins analogous to calixpenams as novel potential anti-MRSA agents. Turk. J. Chem. 2014, 38, 850–858.
- Özkan, Ş.Ç.; Yilmaz, A.; Arslan, E.; Açık, L.; Sayın, Ü.; Mutlu, E.G. Novel Copper(II) Complexes of p-Tert-Butylcalix[4]Arene Diamide Derivatives: Synthesis, Antimicrobial and DNA Cleavage Activities. Supramol. Chem. 2015, 27, 255–267.
- Akkuş, G.U.; Al, E.; Korcan, S.E. Selective Extraction of Toxic Heavy Metals and Biological Activity Studies Using Pyrimidylthioamide Functionalised Calix[4]Arene. Supramol. Chem. 2015, 27, 522–526.
- Memon, S.; Chandio, A.A.; Memon, A.A.; Panhwar, Q.K.; Nizamani, S.M.; Bhatti, A.A.; Brohi, N.A. Synthesis, Characterization, and Exploration of Antimicrobial Activity of Copper Complex of Diamide Derivative of p-Tert-Butylcalix[4]Arene. Polycycl. Aromat. Compd. 2017, 37, 362–374.
- Memon, S.; Chandio, A.A.; Memon, A.A.; Panhwar, Q.K.; Nizamani, S.M.; Bhatti, A.A.; Brohi, N.A. Synthesis, Characterization, and Exploration of Antimicrobial Activity of Copper Complex of Diamide Derivative of p-Tert-Butylcalix[4]Arene. Polycycl. Aromat. Compd. 2017, 37, 362–374.




