Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jamir Pitton Rissardo | -- | 2559 | 2023-12-22 01:35:04 | | | |
| 2 | Sirius Huang | -1 word(s) | 2558 | 2023-12-22 01:57:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Rissardo, J.P.; Caprara, A.L.F. Deep Brain Stimulation for Refractory Neurological Disorder Management. Encyclopedia. Available online: https://encyclopedia.pub/entry/53050 (accessed on 07 February 2026).
Rissardo JP, Caprara ALF. Deep Brain Stimulation for Refractory Neurological Disorder Management. Encyclopedia. Available at: https://encyclopedia.pub/entry/53050. Accessed February 07, 2026.
Rissardo, Jamir Pitton, Ana Letícia Fornari Caprara. "Deep Brain Stimulation for Refractory Neurological Disorder Management" Encyclopedia, https://encyclopedia.pub/entry/53050 (accessed February 07, 2026).
Rissardo, J.P., & Caprara, A.L.F. (2023, December 22). Deep Brain Stimulation for Refractory Neurological Disorder Management. In Encyclopedia. https://encyclopedia.pub/entry/53050
Rissardo, Jamir Pitton and Ana Letícia Fornari Caprara. "Deep Brain Stimulation for Refractory Neurological Disorder Management." Encyclopedia. Web. 22 December, 2023.
Copy Citation
Deep brain stimulation (DBS) has been extensively studied due to its reversibility and significantly fewer side effects. DBS is mainly a symptomatic therapy, but the stimulation of subcortical areas by DBS is believed to affect the cytoarchitecture of the brain, leading to adaptability and neurogenesis. The neurological disorders most commonly studied with DBS were Parkinson’s disease, essential tremor, obsessive-compulsive disorder, and major depressive disorder.
DBS
movement disorder
brain stimulation
electrical stimulation
IPG
Parkinson’s disease
tremor
symptomatic
neurosurgery
neurology
1. Introduction
Deep brain stimulation (DBS), according to the National Institute of Neurological Disorders and Stroke (NINDS), is a surgical method used to manage various neurological conditions that do not effectively respond to conventional therapy. It comprises a neurostimulator surgically implanted battery-powered gadget, which resembles a cardiac pacemaker, that provides electrical stimulation to the appropriate location to block aberrant nerve signals [1]. The first studies with electrical stimulation of the cortex were designed at the end of the 19th century. Still, the main devices were only developed in the mid-20th century, following the scientific and technological achievements of the information age (Table 1).
Table 1. Timeline of the deep brain stimulation development.
| Year | Description |
|---|---|
| 1874 | Electrical stimulation of the human cortex was performed by American physician Robert Bartholow |
| 1947 | The stereotactic frame was developed for human neurosurgery. Ernest A. Spiegel developed a stereotactic frame, which was followed in 1949 by the arc-based Leksell frame |
| 1948 | J. Lawrence Pool performed the first chronic DBS implantation using an electrode connected to an induction coil |
| 1952 | The first stereotactic atlas with coronal photographs of the brain was published |
| 1954 | Acute thalamic DBS to target chronic pain. It is considered one of the first functional applications for DBS |
| Acute DBS used in pre-lesion targeting for psychiatric disorder | |
| 1958 | The first definitive cardiac pacemaker was implanted. The first temporary transcutaneous cardiac pacing device was made in 1952 |
| 1960 | Acute DBS is used to identify lesion targets in essential tremor |
| Frequency-dependent effects of DBS reported | |
| 1961 | The first human intraoperative microelectrode recordings |
| 1963 | José Manuel Rodríguez Delgado used a “stimoceiver” to inhibit the aggressive behavior of a bull |
| 1968 | Medtronic implantable pulse generator. Also, the first spinal cord stimulator was commercially available |
| 1970s | Computed tomography is used for stereotactic targeting |
| Radiofrequency control on an “external” transmitter on DBS systems | |
| 1972 | The first chronic DBS implant for PD |
| 1973 | Thalamic DBS for denervation pain |
| 1977 | Periventricular DBS for pain |
| 1980s | MRI is used for stereotactic targeting |
| The first fully intracranial DBS devices were available. Also, the long-lasting implantable lithium batteries greatly extend implant life and the maintenance of the device | |
| 1980 | DBS for multiple sclerosis tremor |
| 1987 | DBS of the ventral intermediate nucleus of the thalamus was effective in the management of tremor in individuals with PD |
| DBS therapy for the management of tremors was successfully reported by Alim-Louis Benabid | |
| 1990 | Refinement of battery-driven pacemakers |
| DBS reverses motor symptoms in MPTP-induced parkinsonism in monkeys | |
| 1994 | DBS of the subthalamic nucleus is used in the management of tremors in patients with PD |
| 1997 | The FDA approves DBS of the ventral intermediate nucleus of the thalamus for the management of essential tremors |
| 1999 | DBS of the anterior limb of the internal capsule was first used to manage obsessive-compulsive disorder |
| Visser-Vanderwalle reported the effective use of DBS of the medial thalamus in three patients with Tourette’s syndrome | |
| Globus pallidus internus DBS for the management of refractory dystonia | |
| Implantable pulse generators with dual-channel technology, which was developed after the creation of dual chamber cardiac pacing in 1998 | |
| 2000s | DBS therapy is refined for treating essential tremors, PD, and dystonia |
| 2002 | US FDA approves DBS in PD |
| Quadripolar electrodes are commercially available | |
| 2003 | The US FDA approves DBS for dystonia |
| 2004 | Computer models of DBS |
| 2005 | DBS is used to treat depression |
| 2007 | DBS is used to treat minimally conscious states |
| 2009 | DBS of the bilateral anterior limb of the internal capsule for the management of obsessive-compulsive disorder received a humanitarian device exemption from the FDA |
| Rechargeable DBS batteries are available | |
| 2010 | Sin Alzheimer’s pilot trial evaluates the DBS of the fornix |
| 2011 | Close-loop stimulation for epilepsy management |
| 2013 | DBS device capable of simultaneous stimulation and recording activities of the local field potential signal processing. |
| DBS of the subcallosal cingulate gyrus in an anorexia pilot trial | |
| A closed-loop, responsive DBS system was introduced to treat epilepsy. These devices need to have neural activity sensitivity, leading to a decreased number of side effects and a longer battery life | |
| 2015 | The emergence of directional DBS leads can lead to an adjustment of the electrical field along the lead axis |
| 2018 | The US FDA has approved DBS as an add-on treatment for drug-resistant epilepsy in adults |
| 2020 | The US FDA approves a DBS device capable of neurosensitivity and directional leads |
| Wireless devices with three Tesla MRI compatibility |
Abbreviations: DBS, deep brain stimulation; MRI, magnetic resonance imaging; PD, Parkinson’s disease; US FDA, US Food and Drug Administration.
The first condition approved to be managed with DBS was essential tremor in 1997 by the US Food and Drug Administration (FDA). In the following years, clinical trials were published showing the efficacy of DBS therapy for managing other movement disorders. In this context, DBS for Parkinson’s disease (PD) was approved by the FDA in 2002, and dystonia in 2003 [2]. The current guidelines recommend DBS rather than ablative surgical methods for treating drug-resistant PD. Additionally, DBS has shown promise in managing other neuropsychiatric conditions, such as substance-related and addictive disorders, aggressive behavior, eating disorders, major depressive disorder, obsessive-compulsive disorder, and refractory Gilles de la Tourette syndrome [3].
DBS implantation was based on lesioning operations performed in the last century to improve neurological symptoms, which resulted in a high percentage of undesired side effects [4]. DBS was considered a safer alternative when compared to lesioning procedures due to fewer adverse events, leading to active research and further investigation of neuromodulation approaches for various neurological disorders [5].
Although DBS has been extensively used in managing tremor in individuals with PD, the exact mechanism of action for improving the symptoms in the neural circuitry is not fully understood. It is believed that stimulating the main nerve tracts while inhibiting the nearby neurons may facilitate the movement (Figure 1). The zones of uncertainty and cerebellar-thalamic pathways, which decrease tremor and increase dopamine, are also implicated [6]. Moreover, the nosological entity with adequate stimulation parameters and the cytoarchitecture of the brain structure (typically subcortical) are probably related to the efficacy of DBS therapy [7]. In this way, some authors proposed that the leads of DBS can inhibit the structures rich in cell bodies or disinhibit a specific collection of axons, leading to the synchronization of an abnormal pattern, which can facilitate movement or inhibit unusual neuronal activity [8]. Some individuals show a progressive improvement in motor symptoms, suggesting a possible change in the cytoarchitecture of the central nervous system and neuroplasticity [9].
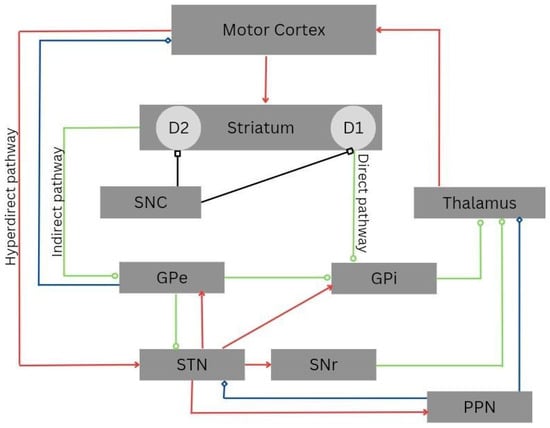
Figure 1. Cortico-basal-ganglia-thalamo-cortical circuitry. The direct, indirect, and hyperdirect pathways are indicated. Green lines denote inhibitory connections (GABAergic), red lines denote excitatory connections (glutamatergic), black lines denote dopaminergic pathways, and blue lines denote mixed cholinergic connections. Notably, the pedunculopontine nucleus (PPN) exhibits anatomic projections to the striatum and cortex. Abbreviations: D1, dopamine receptor D1; D2, dopamine receptor D2; GPe, external globus pallidus; GPi, internal globus pallidus; PPN, pedunculopontine nucleus; SNC, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus.
2. Surgical Techniques
The surgical procedure for implanting DBS devices involves several key elements and can vary in approach and technique. A meticulous preoperative airway assessment is necessary since the patient’s head will be immobilized in a stereotactic headframe during the DBS procedure. Monitored anesthesia care with sedation is the most commonly used anesthesia technique during lead implantation for most patients. This leads to minimal effects of anesthetic agents on neuronal background and spike activity during microelectrode recording localization [10].
The primary components of a fully implanted DBS system include the precise implantation of an intracranial electrode, which involves surgically placing an intracranial electrode into the specific target area within the brain where stimulation is intended. The implant lead extension connects the intracranial electrode to the power-generating and programming sources. An internal pulse generator that generates electrical pulses for stimulation is implanted under the skin, typically in the chest or abdomen [11].
The surgical procedure for DBS can differ among medical facilities and centers. The most common approach for implanting the device is general anesthesia. On the other hand, local anesthetics are applied for device maintenance that does not involve lead manipulations such as battery changes [12]. A Leksell stereotactic frame is sometimes securely attached to the patient’s head under local anesthesia. This frame is used for precise targeting during the surgery. After securing the frame, stereotactic imaging is performed to aid in planning the electrode’s target and trajectory. Various software packages are available for this purpose, and they can employ coordinate frame-based, frameless, or robotic stereotaxic procedures [13].
Overall, the choice of approach and surgical technique may depend on the specific patient, the target area within the brain, and the preferences and expertise of the medical facility performing the procedure.
The surgical procedure for DBS involves the following steps: The patient is positioned semi-recumbent, and the scalp is prepared by clipping the hair and applying betadine solution to ensure sterility. Then, a coronally oriented incision is typically made, spanning Kocher’s point bilaterally on the scalp. However, alternative incision techniques can be used. The scalp is opened to expose the skull’s frontal bone. A hole, or trephination, is made approximately 1 cm anterior to the coronal suture and at least 2 cm lateral from the midline of the skull. The dura mater is coagulated and carefully incised. Special care is taken to minimize cerebrospinal fluid loss. A guide cannula is inserted into the brain, typically about 1.0 to 1.5 cm above the intended target area. Microelectrode recording is used to identify the electrophysiological characteristics of the target structure and determine its dorsal-ventral boundaries. This helps with the precise placement of the macrolectrodes. Once a suitable tract is identified, the microelectrodes are removed, and a permanent macroelectrode is inserted into the target structure [14].
Stimulation tests are conducted at each electrode contact point to evaluate for adverse effects and clinical efficacy. This ensures that the stimulation is effective and safe. The proper placement of the DBS electrode is verified through intra-operative fluoroscopy [15]. If placement is confirmed, the electrode is affixed to the skull. The incision is closed, completing the surgical procedure. These steps ensure the precise placement of the DBS electrode in the intended target area within the brain while minimizing complications. It is a delicate and highly specialized procedure performed by neurosurgeons with expertise in DBS. In some DBS centers, microelectrodes are advanced through the cannula for recording or stimulation. The microelectrode stimulation can define the anatomical location of the electrode, which can be further assessed with directional leads and changing the voltage and current. This is important for the evaluation of possible side effects related to the localization of the leads, such as paresthesia, muscle contractions, and flashes of light [16]. Also, during the insertion of the DBS lead, fluoroscopy can be used to confirm the location of the lead in a two-dimensional view (Table 2).
Table 2. Stimulus-induced side effects in DBS surgical procedures.
| Adverse Event | DBS Target | Region Related to the Side Effect | Correctional | Reference |
|---|---|---|---|---|
| Dyskinesias | GPe, GPi, STN | Excessive modulation of the indirect pathway | Decrease frequency. Removal of leads | [17] |
| Dysphonia, dysarthria | STN, GPi | Internal capsule and associate circuits of basal ganglia | If possible, change the hemisphere | [18] |
| Muscle contractions | STN, GPi, VOP | Corticospinal tract of the internal capsule | Move posterior | [19] |
| Mood changes, risky behavior | GPi, STN | Associative and limbic circuits of the basal ganglia | Move dorsal | [20] |
| Oculomotor disturbances | GPi, STN | Internal capsule for conjugate eye deviation Third nerve medial to STN for ipsilateral eye movements |
Move medial; Move lateral | [21] |
| Paresthesia | Vim, STN, VOP, PPN | Lemniscal fibers | Move anterior | [22] |
| Phosphenes | GPi | Optic tract | Move medial | [23] |
| Sadness, depression | STN | Ventromedial STN, substantia nigra pars reticularis | Move dorsal | [24] |
| Verbal fluency, working memory | GPi, STN | Associative circuits of the basal ganglia | Move dorsal | [25] |
| Weight gain | STN, GPi | Normalization of energy metabolism | Increase physical activity | [26] |
Abbreviations: DBS, deep brain stimulation; GPe, globus pallidus externus; GPi, globus pallidus internus; PPN, pedunculopontine nucleus; STN, subthalamic nucleus; Vim, ventral intermediate nucleus of the thalamus; VOP, posterior ventral oral nucleus.
Following the initial procedure, the patient undergoes general anesthesia, and the intracranial electrodes are connected to extension wires. These wires are placed under the skin, behind the ear, and down to the chest through a tunnel. An additional incision is made below the clavicle to create a pocket for the internal pulse generator. Then, the extension wires are connected to the internal pulse generator, and the system’s impedances are checked to ensure proper function [27]. Patients are usually admitted for observation for one night after the procedure. After, an outpatient appointment is scheduled within eight weeks of the procedure for device activation and programming, ensuring the DBS system is optimized for their specific needs. When treating Parkinson’s disease, programmers often begin with a monopolar configuration to stimulate the brain. In this configuration, a contact electrode is the cathode with a negative voltage. In contrast, the outer casing of the implantable pulse generator serves as the anode with a positive voltage. If adverse effects arise at higher voltages, a bipolar stimulation configuration can be used. In this configuration, one contact serves as the cathode and another as the anode, limiting current spread into adjacent brain regions that cause side effects. This technique is useful in ensuring that therapy remains within the therapeutic range and does not induce any side effects (Figure 2) [28].
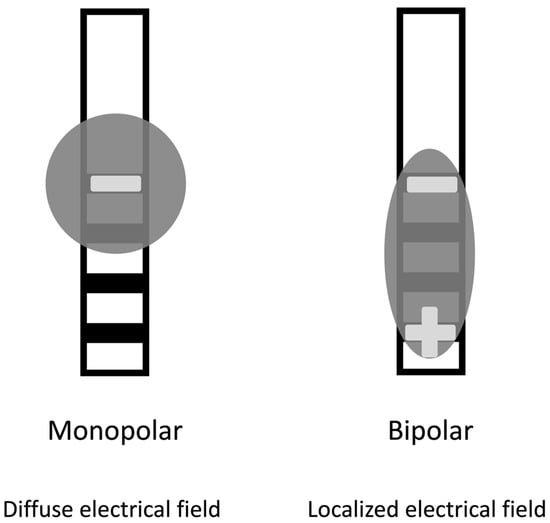
Figure 2. Modes of stimulation. The monopolar (cathodic) stimulation has a spreading negative current in all directions. In the bipolar, the electrode has both anodic and cathodic contact points, with a narrower and more intense flow of current between them.
The implantable pulse generators contain a battery, power module, central processing unit, program memory, and a microprocessor. They are the DBS system’s active components and control the devices’ functions, including activation, deactivation, pulsing parameters, internal diagnostics, and communication with external devices. Features of implantable pulse generators for deep brain stimulation are described in Table 3 [29].
Table 3. Features of implantable pulse generators for deep brain stimulation.
| Model | No. of Chambers | Weight (g) | Size (mm) | Rechargeable Cell | Frequency Range (Hz) | Pulse Width (μs) | Temporal Fractionation | Current Fractionation | Directional Lead | Magnetic Resonance Safety | Local Field Potential |
|---|---|---|---|---|---|---|---|---|---|---|---|
| St. Jude (Abbott) Infinity 5 a | 2 | 49 | 56 × 50 × 13 | No | 2–240 | 20–500 | Multi-stim set | Coactivation | Yes | Conditional: more than 1.5T requires specific conditions | No |
| St. Jude (Abbott) Infinity 7 a | 2 | 58 | 67 × 50 × 14 | No | 2–240 | 20–500 | Multi-stim set | Coactivation | Yes | Conditional: more than 1.5T requires specific conditions | No |
| Boston Scientific Vercise PC b | 2 | 55 | 71 × 50 × 11 | No | 2–255 | 10–450 | Areas | Multiple independent current controls | Yes | Unsafe failure of the equipment | No |
| Boston Scientific Vercise Gevia b | 2 | 26 | 51 × 46 × 11 | Yes | 2–255 | 20–450 | Areas | Multiple independent current controls | Yes | Conditional | No |
| Boston Scientific Vercise Genus P8/P16 b | 1 or 2 | 58 | 72 × 50 × 12 | No | 2–255 | 20–450 | Areas | Multiple independent current controls | Yes | Conditional | No |
| Boston Scientific Vercise Genus R16 b | 2 | 27 | 52 × 46 × 11 | Yes | 2–255 | 20–450 | Areas | Multiple independent current controls | Yes | Conditional | No |
| Medtronic Activa PC c | 2 | 67 | 65 × 49 × 15 | No | 2–250 | 60–450 | Interleaving | No | No | Conditional, certain requirements | No |
| Medtronic Activa RC c | 2 | 40 | 54 × 54 × 9 | Yes | 2–250 | 60–450 | Interleaving | No | No | Conditional, 1.5T MRI | No |
| Medtronic Activa SC c | 1 | 44 | 55 × 60 × 11 | No | 3–250 | 60–450 | Interleaving | No | No | Conditional, but not eligible for full-body MRI | No |
| Medtronic Perpcept PC c | 2 | 61 | 68 × 51 × 12 | No | 2–250 | 20–450 | Interleaving | No | No | Conditional, 3T, and 1.5T MRI | Yes |
a https://www.neuromodulation.abbott/us/en/parkinsons/infinity-for-deep-brain-stimulation.html, accessed on 15 October 2023. b https://www.bostonscientific.com/en-US/products/deep-brain-stimulation-systems.html, accessed on 15 October 2023. c https://www.medtronic.com/us-en/patients/treatments-therapies/deep-brain-stimulation-parkinsons-disease/about-dbs-therapy/dbs-products.html, accessed on 15 October 2023.
References
- Ramirez-Zamora, A.; Giordano, J.; Gunduz, A.; Alcantara, J.; Cagle, J.N.; Cernera, S.; Difuntorum, P.; Eisinger, R.S.; Gomez, J.; Long, S.; et al. Proceedings of the Seventh Annual Deep Brain Stimulation Think Tank: Advances in Neurophysiology, Adaptive DBS, Virtual Reality, Neuroethics and Technology. Front. Hum. Neurosci. 2020, 14, 54.
- Deng, H.; Yue, J.K.; Wang, D.D. Trends in Safety and Cost of Deep Brain Stimulation for Treatment of Movement Disorders in the United States: 2002–2014. Br. J. Neurosurg. 2021, 35, 57–64.
- Naesström, M.; Blomstedt, P.; Bodlund, O. A Systematic Review of Psychiatric Indications for Deep Brain Stimulation, with Focus on Major Depressive and Obsessive-Compulsive Disorder. Nord. J. Psychiatry 2016, 70, 483–491.
- Alshaikh, J.; Fishman, P.S. Revisiting Bilateral Thalamotomy for Tremor. Clin. Neurol. Neurosurg. 2017, 158, 103–107.
- Sharma, V.D.; Patel, M.; Miocinovic, S. Surgical Treatment of Parkinson’s Disease: Devices and Lesion Approaches. Neurotherapeutics 2020, 17, 1525–1538.
- Herrington, T.M.; Cheng, J.J.; Eskandar, E.N. Mechanisms of Deep Brain Stimulation. J. Neurophysiol. 2016, 115, 19–38.
- Warren, A.E.L.; Dalic, L.J.; Bulluss, K.J.; BAppSci, A.R.; Thevathasan, W.; Archer, J.S. The Optimal Target and Connectivity for Deep Brain Stimulation in Lennox-Gastaut Syndrome. Ann. Neurol. 2022, 92, 61–74.
- Chiken, S.; Nambu, A. Mechanism of Deep Brain Stimulation: Inhibition, Excitation, or Disruption? Neuroscientist 2016, 22, 313–322.
- Little, S.; Brown, P. Debugging Adaptive Deep Brain Stimulation for Parkinson’s Disease. Mov. Disord. 2020, 35, 555–561.
- Dallapiazza, R.F.; De Vloo, P.; Fomenko, A.; Lee, D.J.; Hamani, C.; Munhoz, R.P.; Hodaie, M.; Lozano, A.M.; Fasano, A.; Kalia, S.K. Considerations for Patient and Target Selection in Deep Brain Stimulation Surgery for Parkinson’s Disease. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4.
- Ostrem, J.L.; Ziman, N.; Galifianakis, N.B.; Starr, P.A.; Luciano, M.S.; Katz, M.; Racine, C.A.; Martin, A.J.; Markun, L.C.; Larson, P.S. Clinical Outcomes Using ClearPoint Interventional MRI for Deep Brain Stimulation Lead Placement in Parkinson’s Disease. J. Neurosurg. 2016, 124, 908–916.
- Chakrabarti, R.; Ghazanwy, M.; Tewari, A. Anesthetic Challenges for Deep Brain Stimulation: A Systematic Approach. N. Am. J. Med. Sci. 2014, 6, 359–369.
- Piano, C.; Bove, F.; Mulas, D.; Bentivoglio, A.R.; Cioni, B.; Tufo, T. Frameless Stereotaxy in Subthalamic Deep Brain Stimulation: 3-Year Clinical Outcome. Neurol. Sci. 2021, 42, 259–266.
- Krauss, J.K.; Lipsman, N.; Aziz, T.; Boutet, A.; Brown, P.; Chang, J.W.; Davidson, B.; Grill, W.M.; Hariz, M.I.; Horn, A.; et al. Technology of Deep Brain Stimulation: Current Status and Future Directions. Nat. Rev. Neurol. 2021, 17, 75–87.
- Smith, A.P.; Bakay, R.A.E. Frameless Deep Brain Stimulation Using Intraoperative O-Arm Technology. Clinical Article. J. Neurosurg. 2011, 115, 301–309.
- Benabid, A.L.; Benazzouz, A.; Hoffmann, D.; Limousin, P.; Krack, P.; Pollak, P. Long-Term Electrical Inhibition of Deep Brain Targets in Movement Disorders. Mov. Disord. 1998, 13, 119–125.
- Lozano, A.M.; Lang, A.E.; Levy, R.; Hutchison, W.; Dostrovsky, J. Neuronal Recordings in Parkinson’s Disease Patients with Dyskinesias Induced by Apomorphine. Ann. Neurol. 2000, 47, 141–146.
- Chudy, D.; Raguž, M.; Vuletić, V.; Rački, V.; Papić, E.; Nenadić Baranašić, N.; Barišić, N. GPi DBS Treatment Outcome in Children with Monogenic Dystonia: A Case Series and Review of the Literature. Front. Neurol. 2023, 14, 1151900.
- Zhuang, P.; Li, Y.; Hallett, M. Neuronal Activity in the Basal Ganglia and Thalamus in Patients with Dystonia. Clin. Neurophysiol. 2004, 115, 2542–2557.
- Okun, M.S.; Wu, S.S.; Fayad, S.; Ward, H.; Bowers, D.; Rosado, C.; Bowen, L.; Jacobson, C.; Butson, C.; Foote, K.D. Acute and Chronic Mood and Apathy Outcomes from a Randomized Study of Unilateral STN and GPi DBS. PLoS ONE 2014, 9, 114140.
- Patel, S.; Fitzgerald, J.J.; Antoniades, C.A. Oculomotor Effects of Medical and Surgical Treatments of Parkinson’s Disease. Prog. Brain Res. 2019, 249, 297–305.
- Franzini, A.; Cordella, R.; Messina, G.; Marras, C.E.; Romito, L.M.; Albanese, A.; Rizzi, M.; Nardocci, N.; Zorzi, G.; Zekaj, E.; et al. Targeting the Brain: Considerations in 332 Consecutive Patients Treated by Deep Brain Stimulation (DBS) for Severe Neurological Diseases. Neurol. Sci. 2012, 33, 1285–1303.
- Abou Khzam, R.; El Jalbout, N.D.; Seif, R.; Sadaka, A. An Unusual Presentation of Convergence Insufficiency in a Patient with Parkinson’s Disease Stimulated by Deep Brain Stimulation. Am. J. Ophthalmol. Case Rep. 2022, 26, 101531.
- Strutt, A.M.; Simpson, R.; Jankovic, J.; York, M.K. Changes in Cognitive-Emotional and Physiological Symptoms of Depression Following STN-DBS for the Treatment of Parkinson’s Disease. Eur. J. Neurol. 2012, 19, 121–127.
- Pillon, B.; Ardouin, C.; Damier, P.; Krack, P.; Houeto, J.L.; Klinger, H.; Bonnet, A.M.; Pollak, P.; Benabid, A.L.; Agid, Y. Neuropsychological Changes between “off” and “on” STN or GPi Stimulation in Parkinson’s Disease. Neurology 2000, 55, 411–418.
- Rieu, I.; Derost, P.; Ulla, M.; Marques, A.; Debilly, B.; De Chazeron, I.; Chéreau, I.; Lemaire, J.J.; Boirie, Y.; Llorca, P.M.; et al. Body Weight Gain and Deep Brain Stimulation. J. Neurol. Sci. 2011, 310, 267–270.
- Nazzaro, J.M.; Lyons, K.E.; Pahwa, R.; Ridings, L.W. The Importance of Testing Deep Brain Stimulation Lead Impedances before Final Lead Implantation. Surg. Neurol. Int. 2011, 2, 131.
- Hancu, I.; Boutet, A.; Fiveland, E.; Ranjan, M.; Prusik, J.; Dimarzio, M.; Rashid, T.; Ashe, J.; Xu, D.; Kalia, S.K.; et al. On the (Non-) Equivalency of Monopolar and Bipolar Settings for Deep Brain Stimulation fMRI Studies of Parkinson’s Disease Patients. J. Magn. Reson. Imaging 2019, 49, 1736–1749.
- Sarica, C.; Iorio-Morin, C.; Aguirre-Padilla, D.H.; Najjar, A.; Paff, M.; Fomenko, A.; Yamamoto, K.; Zemmar, A.; Lipsman, N.; Ibrahim, G.M.; et al. Implantable Pulse Generators for Deep Brain Stimulation: Challenges, Complications, and Strategies for Practicality and Longevity. Front. Hum. Neurosci. 2021, 15, 708481.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
939
Entry Collection:
Neurodegeneration
Revisions:
2 times
(View History)
Update Date:
22 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




