Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jovana Rajkovic | -- | 3041 | 2023-12-20 23:34:18 | | | |
| 2 | Lindsay Dong | + 1 word(s) | 3042 | 2023-12-22 02:58:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gostimirovic, M.; Rajkovic, J.; Bukarica, A.; Simanovic, J.; Gojkovic-Bukarica, L. Resveratrol and Gut Microbiota Synergy. Encyclopedia. Available online: https://encyclopedia.pub/entry/52995 (accessed on 16 January 2026).
Gostimirovic M, Rajkovic J, Bukarica A, Simanovic J, Gojkovic-Bukarica L. Resveratrol and Gut Microbiota Synergy. Encyclopedia. Available at: https://encyclopedia.pub/entry/52995. Accessed January 16, 2026.
Gostimirovic, Milos, Jovana Rajkovic, Ana Bukarica, Jovana Simanovic, Ljiljana Gojkovic-Bukarica. "Resveratrol and Gut Microbiota Synergy" Encyclopedia, https://encyclopedia.pub/entry/52995 (accessed January 16, 2026).
Gostimirovic, M., Rajkovic, J., Bukarica, A., Simanovic, J., & Gojkovic-Bukarica, L. (2023, December 20). Resveratrol and Gut Microbiota Synergy. In Encyclopedia. https://encyclopedia.pub/entry/52995
Gostimirovic, Milos, et al. "Resveratrol and Gut Microbiota Synergy." Encyclopedia. Web. 20 December, 2023.
Copy Citation
Resveratrol (RSV) exerts beneficial properties in the modulation of cardiovascular, metabolic, and post-COVID-19-related disorders. In healthy individuals, it maintains an ergogenic capacity, prevents oxidative stress, and modulates the inflammatory response. Overall, it improves quality of life. The RSV–gut-microbiota interaction is beneficial in terms of maintaining human health. Along with physical activity, it is key for the prevention of chronic noncommunicable diseases.
resveratrol
Mediterranean diet
gut microbiota
cardiovascular diseases
1. Resveratrol, Mediterranean Diet, and the Human Gut Microbiome
The knowledge from basic research that scientists have gained regarding the role of resveratrol (RSV) in preventing and treating various diseases has been applied in different fields of medicine; hence today, the literature offers many clinical trials in which RSV has proved its efficacy and/or has opened new horizons of research. Although its concentrations may vary among dozens of natural sources, grape berry skin represents the most important one. Having rich and available sources of RSV on one hand and health-improving effects on the other hand, one may speculate that this substance has a potential for far-reaching health benefits, which is why it is still extensively studied. However, a stumbling block in its usage is its inconvenient pharmacokinetics [1], mostly due to the extensive phase II metabolism in the liver, which causes low bioavailability after an oral intake and limited permeation through the blood–brain barrier (BBB) compared to other polyphenols (e.g., epigallocatechin) [2].
In order to reduce the impact of an unfavorable pharmacokinetic profile on the expression of its full therapeutic potential and wider clinical use, several strategies are being applied [3]. For example, simultaneous administration with modulators of glucuronidation, methylation of RSV analogs, or RSV’s binding with its endogenous ligands (albumin, LDL lipoprotein, fibrinogen and hemoglobin) have been suggested. In addition, new methods are being investigated to improve the tissue delivery of RSV—a combination with dimethyl beta cyclodextrins (DM-β-CDs), the use of microparticles and vesicles with the potential to bind hydrophilic and hydrophobic substances, and recently, nanosponge formulations (a type of nanoparticle delivery system) [4][5]. Together with food-compatible ways of RSV delivery (for example, peanut oil), many sophisticated methods are represented, and recently, attention has been paid to its analogs with more favorable kinetics, too.
The Mediterranean diet (MD) represents the consumption of specific food consisting of proper, balanced, and polyphenol-rich substances and is highly recommended by experts for everyday use. The core food of this diet, whole grains, fruits, vegetables, beans, herbs and spices (coriander, rosemary, lavender, saffron, mint, etc.), nuts, red wine, and olive oil, not only maintains overall health and improves quality of life but also ameliorates the burden of many diseases. This is especially important in the time of the massive depopulation seen in the post-COVID-19 pandemic, where the integrity of an overwhelmed country’s health system may be protected or even enhanced by massive preventive policies such as the promotion of a healthy diet. Therefore, the healthy habit of enriching the diet with the concepts of MD is encouraged in every population group, especially in light of the benefits of the interaction between the metabolized constituents of MD and human gut microbiota.
The human gut microbiome represents a complex ecosystem of trillions of microorganisms including bacteria, fungi, and different types of viruses, mostly bacteriophages. Its composition remains comparatively permanent throughout one’s life and is unique to each individual but shows certain temporary fluctuations depending on the food that is consumed [6].
Upon oral intake, RSV is subjected to extensive and dynamic metabolism in the upper gastrointestinal tract (GIT; stomach and small intestine), whereby the activity of different enzymes is converted to more water-soluble absorbable forms. However, it is known today that even unabsorbed portions of RSV play an important role in its overall biological activity, since the majority of RSV-derived metabolites reach the lower GIT, where they interact with gut microbiota. Since the human microbiome is considered the main homeostatic regulator of many processes, this interaction could be useful, especially in ameliorating metabolic syndrome and cardiovascular diseases but also in diseases associated with microbial dysbiosis, such as inflammatory bowel disease, tumors, and obesity [7].
Regarding metabolic diseases, obese subjects who underwent several dietary interventions had the most significant phyla and species changes in microbiota, which revealed the role of Firmicutes/Bacteroides spp. in the obesity phenotype. As this concept frequently tends to be misleading due to the lack of association with the host’s lifestyle factors, probably the most useful therapeutic approach represents microbiota changes in combination with a tailored diet and drugs to treat obesity [8]. Figure 1 represents the aspects of RSV’s beneficial effects in relation to the health consequences of a high-fat unbalanced diet.
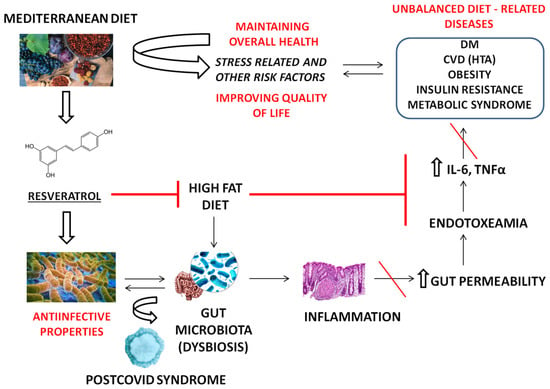
Figure 1. An overview of the properties of RSV on the most common dietary-related diseases. A high-fat diet (HFD) may deregulate the gut microbiome and increase local and systemic inflammation. This contributes to the development of HFD-related diseases, including CVD (cardiovascular diseases). When the gut barriers are impaired, lipopolysaccharides (LPS) of G-negative bacteria bind to Toll-like receptors on the surface of immune cells, additionally triggering inflammation, endotoxemia, and increased gut permeability. As a major component of Mediterranean diet, RSV attenuates the influence of HFD in the pathophysiology of CVD. IL—interleukin, TNF—tumor necrosis factor, HTA—hypertension.
2. RSV and Gut Microbiota Synergy in Pathological Conditions
2.1. Resveratrol, Gut Microbiota, and Cardiovascular Diseases
Gut microbiota produce biologically active metabolites with distinguished physiological features; thus, any change in its composition may lead to multiple diseases, including CVD [9]. It was shown that a major causative metabolite was TMA—trimethylamine [10], a derivative of dietary nutrients abundantly present in a western diet (lecithin, choline, carnitine, betaine) [11]. Upon digestion, TMA is oxidized through hepatic flavin monooxygenases (FMO) to trimethylamine N-oxide (TMAO), which promotes thrombogenesis, vascular inflammation, kidney fibrosis, and end-organ damage. Notably, the mechanisms of thrombosis include enhanced responsiveness to submaximal stimulation by agonists (thrombin, collagen, ADP) and stimulation of the tissue factor, an activator of the extrinsic clotting pathway [12]. Overall, higher levels of TMAO predict a higher risk for adverse cardiac events. This was proved in a meta-analysis of 25,000 people, where every 10 µmol/L of TMOA was associated with nearly 8% increase in all-cause mortality [13]. Another important metabolite, PAG—phenylacetylglutamine [14], is produced by microbial metabolism of phenylalanine and shows prothrombogenic properties through the stimulation of adrenergic receptors, which are involved in platelet and heart functions [15].
During the 1990s, the number of papers about RSV’s effects on CVDs started to grow. In the last ten years, more than 100 research papers have been published annually regarding RSV’s impacts on CVDs. During the search for a molecule that can prolong life and improve its quality, RSV has stood out. However, the treatment of CVDs remains a major problem, despite health care improvements, because of a sedentary lifestyle, the low quality of nutrition, and lack of physical activity, which outweigh the benefits that medicine and science have brought.
Out of several other herbal supplements (beetroot juice, bergamot extracts, barberry, pycnogenol), RSV represents a phenolic compound with proven blood-pressure (BP) lowering effects, which is recommended to be included in the nutrition of people with hypertension [16]. However, its appropriate dosage is still debatable [17]. A positive association between systolic-BP lowering RSV activity and body-mass index (BMI) at the baseline has been shown especially when used at a high dose (≥300 mg/day) and in diabetic patients [18]. This has been confirmed for both systolic and diastolic pressure in a subsequent novel study [19].
2.2. Resveratrol, Gut Microbiota, and Diabetes Mellitus
Alterations in microbiota composition can enhance heightened adiposity, which is linked to development of atherosclerosis, HTA, dyslipidemia, and type 2 diabetes mellitus (T2DM). Obesity is the most preventable, debilitating, and serious diet-related risk factor for the development of T2DM and CVD. It is still an important public health issue with an alarming incidence and tendency for further growth. However, results from many epidemiological studies suggest that dietary fibers significantly reduce the risk for obesity and associated chronic diseases, directly affecting the digestion, absorption, and appetite and indirectly through the microbiome [20].
Moreover, modern lifestyles and nutrition along with genetic predisposition have increased the number of patients with T2DM in the last few decades. It is classified as an age-related disease and can affect the life span. Although it is not possible to avoid the development of T2DM in some cases, our habits may impact the onset of its appearance, for instance, the consumption of foods enriched in bioactive compounds and nutraceuticals, which means taking fruits, vegetables, and whole grains in increasing proportion during daily meals. It was observed that in some regions people were healthier and lived longer; thus, researchers’ attention was directed to the food they consumed and habits they had [21]. Among them, Mediterranean cuisine was particularly interesting, and consequential studies have indicated that these active nutrients had other biological properties, antioxidant, antiproliferative, antithrombogenic, anti-inflammatory, antiaging, antimicrobial, estrogenic, cardio-protective, and most importantly, antidiabetic. This effect was observed through increasing glucose metabolism and improving vascular function, as well as in reducing insulin resistance and the HbA1c level [22]. The glycemia-lowering effect of RSV was confirmed in both types of diabetes mellitus (type 1 and 2).
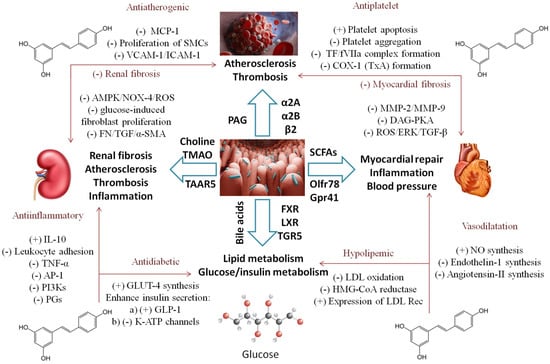
In a randomized, double-blind, and placebo-controlled trial of 56 patients with T2DM and coronary heart disease [23], a 4-week supplementation with 500 mg RSV daily turned out to be beneficial on glycemic control, HDL cholesterol, and the total-/HDL-cholesterol ratio, as well as in the total antioxidant capacity and a significant reduction in malondialdehyde levels [24]. In patients with T2DM, however, RSV failed to reduce waist circumference and triglyceride and HDL-cholesterol levels, according to the meta-analysis of 19 trials involving over 1100 patients [19]. The observed effects on the lipid profile are similar to those of the trials conducted on patients with metabolic syndrome, where, additionally, total serum cholesterol was significantly reduced. Also, there was no change in the liver enzyme activity, since the concentrations of alanine aminotransferase (ALT) [25] and aspartate aminotransferase (AST) [26] remained unchanged [27]. The lack of effect on liver enzyme levels, additionally including alkaline phosphatase (ALP), bilirubin, and gamma-glutamyl transferase (GGT) have also been confirmed in a pooled analysis of 15 randomized trials including over 700 participants [28]. On the other hand, in patients with nonalcoholic fatty liver disease (NAFLD), ALT and AST were significantly reduced [29], mostly in studies that lasted over 3 months and in patients with mean age < 45 years and BMI < 30kg/m2. RSV supplementation did not affect other metabolic indicators such as leptin and adiponectin levels [30]. Figure 2 represents phases in the pathogenesis of common CND where the RSV–gut-microbiota interaction could change the disease course and potential outcome.

Figure 2. Synergistic action of the gut microbiome and RSV in the various processes of common CND (from Section 3.1 and Section 3.2). MCP-1—monocyte chemoattractant protein-1, SMC-smooth muscle cells, VCAM-1—vascular cell adhesion molecule-1, ICAM-1—intercellular cell adhesion molecule-1, TF—tissue factor, f VIIa—activated clotting factor VII, COX-1—cyclooxygenase-1, TxA—thromboxane A, AMPK—AMP-activated protein kinase, NOX-4—nicotinamide adenine dinucleotide phosphate oxidase 4, ROS—reactive oxygen species, α-SMA—α-smooth muscle actin, FN—cellular fibronectin, IL-10—interleukin 10, TNF—tumor necrosis factor, AP-1—activator protein 1, PI3Ks—phosphatidylinositol 3-kinases, PGs—prostaglandins, GLUT-4—glucose transporter 4, GLP-1—glucagon like peptide 1, K-ATP—ATP sensitive K channels, LDL—low density lipoprotein, HMG-CoA—3-Hydroxy-3-methylglutaryl-coenzyme A, NO—nitric oxide, MMP—matrix metalloproteinase, DAG-PKA—diacylglycerol-protein kinase A pathway, ERK—extracellular signal-regulated kinases, TGFβ—transforming growth factor beta, SCFAs—short-chain fatty acids, FXR/LXR—farnesoid/liver X receptors, TGR5—G protein coupled bile acid receptor, TAAR5—trace amine-associated receptor 5, Olfr-78—olfactory receptor-78, Gpr41—free fatty acid receptor, PAG—phenylacetylglutamine, TMAO—trimethylamine N-oxide, α/β—adrenergic receptors. (+) stimulates, (-) inhibits.
2.3. Resveratrol, Gut Microbiota, and COVID-19 Infection in Patients with Preexisting Comorbidities
COVID-19 infection occurs in a wide clinical spectrum, ranging from asymptomatic patients to severe viral pneumonia, acute respiratory distress syndrome (ARDS), septic shock, and multiple organ failure [31]. Besides the ingested viral load, the presence of virulence factors, and previous prophylaxis, the favorable clinical outcome of the COVID-19 disease is associated mostly with the health status of a susceptible host; thus, a clinical course may vary among people with common comorbidities and previously healthy individuals. As a matter of fact, it seems that the hallmark of emerging diseases in the 2019–2022 period is represented by the interplay between viral particles and the metabolic and immunologic properties of a susceptible host. Finding a preferable treatment option for the complexity of this link sometimes requires the synergistic use of modern pharmacological treatment and traditional medicine–nutraceuticals, dietary fibers, and herbal supplements.
During the pandemic, it was observed that increased mortality and worse outcomes took place among patients with comorbidities, especially with CVD and metabolic disorders caused by lifestyle (obesity, diabetes mellitus, etc.). Researchers’ logical option was RSV because it inhibited the release of proinflammatory cytokines, ameliorated vascular thrombosis, upregulated eNOS (endothelial NO-synthetase), enhanced endothelial NO production, reduced endothelin-1 synthesis, and inhibited oxidative stress—all of which could influence the complications.
RSV was included in clinical trials as supplementation for patients with a poor prognosis [32]. Additionally, RSV’s effects include inhibition of TLR4 (Toll-Like receptor 4) activation and inhibitions of proinflammatory transcription factor NF-κB and Th17 helper T-cells, which was promising in combating the COVID-19-mediated activation of TLR4 and the stimulation of proinflammatory cytokines (IL-1, IL-6, CCL-5 (chemotactic chemokine ligand 5)) and TNF-α [33][34].
The main target of RSV distribution is the gastrointestinal tract; so, there is proof that RSV influences the gut microbiota, too. In eighteen studies (in vivo, ex vivo, in vitro) investigating the immune response, prevention of thromboembolic complications, and gene therapy after RSV supplementation, it was found that, upon administration, RSV changed the genetic expression in the microbial community. This mechanism could be suggested and applied in the treatment of disorders associated with microbial dysbiosis, like obesity, DM, degenerative diseases, and metabolic syndrome, as previously noted [7]. All of this can decrease the impact of morbidity (especially of CVD) in acute COVID-19 infection and in the second phase (post-COVID-19 syndrome) as well [32].
2.4. Resveratrol, Gut Microbiota, and Post-COVID-19 Syndrome
It is known that people with obesity/hypertension, metabolic syndrome, and/or immunodeficiency had worse outcomes regarding the consequences of the COVID-19 pandemic and a higher mortality rate [35][36]. This diverted investigation of the novel therapeutic strategies into dietary polyphenols and, shortly after, the gut microbiome.
RSV was shown to modulate several steps in acute COVID-19 infection. The antiviral properties of RSV include the inhibition of viral replication through downregulation of several transcription factors and signaling pathways responsible for viral gene expression, nucleic acid, and protein synthesis. These activities have been proven for the treatment of several respiratory viruses, including SARS-CoV-2. The fact that low micromolar concentrations of RSV caused a 60–98% reduction in replication upon viral entry suggested that RSV interfered with the early phase of viral infection. When recovered from the acute disease, however, some patients might experience long-term symptoms that could last for months, in a so called ‘post-COVID-19 syndrome’. These include dyspnea, myalgia, fatigue, insomnia, cognitive disorders, and by far the most common, gastrointestinal disturbances (heartburn, abdominal pain, diarrhea, constipation) [37].
3. Resveratrol and Gut Microbiota in Healthy Individuals
3.1. Resveratrol, Gut Microbiota, and Physical Activity
Studies regarding RSV’s association with metabolic and cellular changes during physical activity, ergogenic properties, and an athlete’s performances are heterogeneous. RSV has been described as a calorie restriction mimetic; thus, its usage during physical activity may improve exercise performance [38]. Interestingly, an animal study with Wistar rats showed that the consumption of beverages rich in RSV during physical exercise improved health despite the consumption of a high-fat diet [39]. Other studies in mice [40][41][42] confirmed RSV’s effects on physical performance by significantly increasing the aerobic capacity. In a pilot randomized clinical trial of 60 elderly, it was indicated that the combination of RSV and exercise in this population is safe and may improve the mitochondrial function of skeletal muscle and mobility-related indices of physical function. Another double blind placebo-controlled clinical trial of 36 young untrained males indicated that supplementation with RSV before effective training had an impact on muscle pain reduction, making the recovery of the anaerobic capacity faster and reducing the frequency of muscle damage [43].
3.2. Resveratrol, Gut Microbiota, and Quality of Life
Proper and continuous implementation of measures and activities within the domain of primary health care contributes to the reduction in numerous chronic and stress-related diseases but also improves quality of life (QoL) and overall psychosomatic public health. The measures of primary prevention include compliance with the principles of proper nutrition, good quality sleep, and regular moderate physical activity [44]. In addition, the presence of chronic diseases is not associated with increased mortality per se but with significant morbidity and alterations in daily activities. Those diseases include, for example, neurodegenerative diseases, chronic fatigue syndrome, fibromyalgia, inflammatory bowel disease, multiple sclerosis, rheumatoid arthritis, and, especially in younger people and adolescents, mild respiratory symptoms like allergic rhinitis and the common cold. RSV has the ability to enhance the effects of primary prevention measures, as well as to prevent or reduce the progression of diseases within different severity ranges, from mild to severe neurodegenerative diseases. Although there were discrepancies between animal studies and clinical trials [23] (since the initial studies showed no clear improvement in the cognitive function, mood, and sleep quality) in healthy, young humans [10], newer clinical trials succeeded in demonstrating that a single dose of 14 mg taken before sleep significantly improved non-REM sleep and the feeling of being well-rested compared to a placebo [45]. Some RSV-containing products even have been administered to patents in Japan for sleep improvement.
4. Conclusions
Based upon the available literature, the consumption of a diet enriched in plant polyphenols, especially RSV may redirect the natural course of noncommunicable diseases and act synergistically with other multimodal measures in enhancing overall health, including interference with human gut microbiota. Therefore, the daily use of a balanced Mediterranean diet is very useful, especially in predisposed people, with both modifiable and nonmodifiable risk factors. In that regard, this synergy can be helpful as well for the treatment of the long, deteriorating, and exhausting consequences of people recovering from COVID-19 infection, mostly due to multistep actions in complex post-COVID-19 syndrome. However, it is important to state that despite promising data, most of these are observational and insufficient. Further research on RSV-derived metabolites and gut microbiota changes is necessary in order to evaluate a deeper link between the products of this synergy, the immune system, and disease development, especially in immunocompromised people and patients with multiple comorbidities.
References
- Robertson, I.; Wai Hau, T.; Sami, F.; Sajid Ali, M.; Badgujar, V.; Murtuja, S.; Saquib Hasnain, M.; Khan, A.; Majeed, S.; Tahir Ansari, M. The science of resveratrol, formulation, pharmacokinetic barriers and its chemotherapeutic potential. Int. J. Pharm. 2022, 618, 121605.
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of Blood-Brain Barrier Permeability of Polyphenols, Anthocyanins, and Their Metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686.
- Jeandet, P.; Sobarzo-Sánchez, E.; Uddin, M.S.; Bru, R.; Clément, C.; Jacquard, C.; Nabavi, S.F.; Khayatkashani, M.; Batiha, G.E.; Khan, H.; et al. Resveratrol and cyclodextrins, an easy alliance: Applications in nanomedicine, green chemistry and biotechnology. Biotechnol. Adv. 2021, 53, 107844.
- Li, C.; Wang, Z.; Lei, H.; Zhang, D. Recent progress in nanotechnology-based drug carriers for resveratrol delivery. Drug Deliv. 2023, 30, 2174206.
- Saleem, Z.; Rehman, K.; Hamid Akash, M.S. Role of Drug Delivery System in Improving the Bioavailability of Resveratrol. Curr. Pharm. Des. 2022, 28, 1632–1642.
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-derived metabolites as drivers of gut-brain communication. Gut Microbes 2022, 14, 2102878.
- Inchingolo, A.D.; Malcangi, G.; Inchingolo, A.M.; Piras, F.; Settanni, V.; Garofoli, G.; Palmieri, G.; Ceci, S.; Patano, A.; De Leonardis, N.; et al. Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature. Int. J. Mol. Sci. 2022, 23, 4027.
- Caputo, M.; Pigni, S.; Antoniotti, V.; Agosti, E.; Caramaschi, A.; Antonioli, A.; Aimaretti, G.; Manfredi, M.; Bona, E.; Prodam, F. Targeting microbiota in dietary obesity management: A systematic review on randomized control trials in adults. Crit. Rev. Food Sci. Nutr. 2022, 8, 1–33.
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570.
- Wightman, E.L.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The effects of chronic trans-resveratrol supplementation on aspects of cognitive function, mood, sleep, health and cerebral blood flow in healthy, young humans. Br. J. Nutr. 2015, 114, 1427–1437.
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585.
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124.
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 2017, 38, 2948–2956.
- Davinelli, S.; Scapagnini, G.; Marzatico, F.; Nobile, V.; Ferrara, N.; Corbi, G. Influence of equol and resveratrol supplementation on health-related quality of life in menopausal women: A randomized, placebo-controlled study. Maturitas 2017, 96, 77–83.
- Nemet, I.; Saha, P.P.; Gupta, N.; Zhu, W.; Romano, K.A.; Skye, S.M.; Cajka, T.; Mohan, M.L.; Li, L.; Wu, Y.; et al. A Cardiovascular Disease-Linked Gut Microbial Metabolite Acts via Adrenergic Receptors. Cell 2020, 180, 862–877.e22.
- Nunan, D.; Mahtani, K.R.; Roberts, N.; Heneghan, C. Physical activity for the prevention and treatment of major chronic disease: An overview of systematic reviews. Syst. Rev. 2013, 2, 56.
- Lipert, A.; Szadkowska, I.; Matusiak-Wieczorek, E.; Kochan, E. The Effect of Herbal Supplements on Blood Pressure: Systematic Review and Meta-Analysis. Antioxidants 2022, 11, 1419.
- Fogacci, F.; Tocci, G.; Presta, V.; Fratter, A.; Borghi, C.; Cicero, A.F.G. Effect of resveratrol on blood pressure: A systematic review and meta-analysis of randomized, controlled, clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1605–1618.
- Gu, W.; Geng, J.; Zhao, H.; Li, X.; Song, G. Effects of Resveratrol on Metabolic Indicators in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. J. Clin. Pr. 2022, 2022, 9734738.
- Waddell, I.S.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2022, 63, 8752–8767.
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290.
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human Study and Clinical Trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379.
- Khorshidi, F.; Poljak, A.; Liu, Y.; Lo, J.W.; Crawford, J.D.; Sachdev, P.S. Resveratrol: A “miracle” drug in neuropsychiatry or a cognitive enhancer for mice only? A systematic review and meta-analysis. Ageing Res. Rev. 2021, 65, 101199.
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Ž.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051.
- Baltaci, S.B.; Mogulkoc, R.; Baltaci, A.K. Resveratrol and exercise. Biomed. Rep. 2016, 5, 525–530.
- Baldassarre, M.E.; Di Mauro, A.; Labellarte, G.; Pignatelli, M.; Fanelli, M.; Schiavi, E.; Mastromarino, P.; Capozza, M.; Panza, R.; Laforgia, N. Resveratrol plus carboxymethyl-β-glucan in infants with common cold: A randomized double-blind trial. Heliyon 2020, 6, e03814.
- Akbari, M.; Tamtaji, O.R.; Lankarani, K.B.; Tabrizi, R.; Dadgostar, E.; Haghighat, N.; Kolahdooz, F.; Ghaderi, A.; Mansournia, M.A.; Asemi, Z. The effects of resveratrol on lipid profiles and liver enzymes in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2020, 19, 25.
- Darand, M.; Farrokhzad, A.; Ghavami, A.; Hadi, A.; Karimi, E.; Fadel, A.; Askari, G. Effects of resveratrol supplementation on liver enzymes: A systematic review and meta-analysis of randomised controlled trials. Int. J. Clin. Pract. 2021, 75, e13692.
- Wei, S.; Yu, X. Efficacy of resveratrol supplementation on liver enzymes in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Complement Ther. Med. 2021, 57, 102635.
- Tabrizi, R.; Tamtaji, O.R.; Lankarani, K.B.; Akbari, M.; Dadgostar, E.; Dabbaghmanesh, M.H.; Kolahdooz, F.; Shamshirian, A.; Momen-Heravi, M.; Asemi, Z. The effects of resveratrol intake on weight loss: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 375–390.
- Wu, T.; Zuo, Z.; Kang, S.; Jiang, L.; Luo, X.; Xia, Z.; Liu, J.; Xiao, X.; Ye, M.; Deng, M. Multi-organ Dysfunction in Patients with COVID-19: A Systematic Review and Meta-analysis. Aging Dis. 2020, 11, 874–894.
- Gligorijević, N.; Stanić-Vučinić, D.; Radomirović, M.; Stojadinović, M.; Khulal, U.; Nedić, O.; Ćirković Veličković, T. Role of Resveratrol in Prevention and Control of Cardiovascular Disorders and Cardiovascular Complications Related to COVID-19 Disease: Mode of Action and Approaches Explored to Increase Its Bioavailability. Molecules 2021, 26, 2834.
- McCreary, M.R.; Schnell, P.M.; Rhoda, D.A. Randomized double-blind placebo-controlled proof-of-concept trial of resveratrol for outpatient treatment of mild coronavirus disease (COVID-19). Sci. Rep. 2022, 12, 10978.
- Giordo, R.; Zinellu, A.; Eid, A.H.; Pintus, G. Therapeutic Potential of Resveratrol in COVID-19-Associated Hemostatic Disorders. Molecules 2021, 26, 856.
- SeyedAlinaghi, S.; Karimi, A.; Barzegary, A.; Mojdeganlou, H.; Vahedi, F.; Mirghaderi, S.P.; Shobeiri, P.; Ramezani, M.; Yousefi Konjdar, P.; Mirzapour, P.; et al. COVID-19 mortality in patients with immunodeficiency and its predictors: A systematic review. Eur. J. Med. Res. 2022, 27, 195.
- Sanchis-Gomar, F.; Lavie, C.J.; Mehra, M.R.; Henry, B.M.; Lippi, G. Obesity and Outcomes in COVID-19: When an Epidemic and Pandemic Collide. Mayo Clin. Proc. 2020, 95, 1445–1453.
- Batiha, G.E.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Welson, N.N. Pathophysiology of Post-COVID syndromes: A new perspective. Virol. J. 2022, 19, 158.
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites-Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143.
- da Fonseca Cardoso, L.M.; de Souza Monnerat, J.A.; de Medeiros Silva, I.W.S.; da Silva Ferreira Fiochi, R.; da Matta Alvarez Pimenta, N.; Mota, B.F.; Dolisnky, M.; do Carmo, F.L.; Barroso, S.G.; da Costa, C.A.S.; et al. Beverages Rich in Resveratrol and Physical Activity Attenuate Metabolic Changes Induced by High-Fat Diet. J. Am. Coll. Nutr. 2021, 40, 485–495.
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122.
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342.
- Murase, T.; Haramizu, S.; Ota, N.; Hase, T. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology 2009, 10, 423–434.
- Huang, C.C.; Lee, M.C.; Ho, C.S.; Hsu, Y.J.; Ho, C.C.; Kan, N.W. Protective and Recovery Effects of Resveratrol Supplementation on Exercise Performance and Muscle Damage following Acute Plyometric Exercise. Nutrients 2021, 13, 3217.
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261.
- Chan, V.; Lo, K. Efficacy of dietary supplements on improving sleep quality: A systematic review and meta-analysis. Postgrad. Med. J. 2022, 98, 285–293.
More
Information
Subjects:
Pharmacology & Pharmacy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
564
Revisions:
2 times
(View History)
Update Date:
22 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




