You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammad Mobarak Hossain | -- | 7786 | 2023-12-20 16:38:09 | | | |
| 2 | Camila Xu | Meta information modification | 7786 | 2023-12-21 01:56:24 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Hossain, M.; Biswas, P.; Islam, R. Flash Flood and Cold Injury of Bangladesh Rice. Encyclopedia. Available online: https://encyclopedia.pub/entry/52987 (accessed on 22 December 2025).
Hossain M, Biswas P, Islam R. Flash Flood and Cold Injury of Bangladesh Rice. Encyclopedia. Available at: https://encyclopedia.pub/entry/52987. Accessed December 22, 2025.
Hossain, Mobarak, Partha Biswas, Rafiqul Islam. "Flash Flood and Cold Injury of Bangladesh Rice" Encyclopedia, https://encyclopedia.pub/entry/52987 (accessed December 22, 2025).
Hossain, M., Biswas, P., & Islam, R. (2023, December 20). Flash Flood and Cold Injury of Bangladesh Rice. In Encyclopedia. https://encyclopedia.pub/entry/52987
Hossain, Mobarak, et al. "Flash Flood and Cold Injury of Bangladesh Rice." Encyclopedia. Web. 20 December, 2023.
Copy Citation
Rice cultivation in the low-lying basin-like wetlands, known as the Haor, is often affected by early flash floods during the first two weeks of April. The flooding is mainly caused by heavy rainfall and water surging downstream from the Meghalaya hills in India. This flash flood poses a significant threat to rice production, risking the country’s food security. Dry winter (Boro) rice is the primary food source throughout the year in the Haor region. Flash floods are the most catastrophic, affecting about 80% or even the entire rice yield. In 2017, a loss of 0.88 million metric tons of Boro rice in Haor regions cost the nation USD 450 million.
flash flood
low temperature
cold injury
Haor
short duration
rice
1. Introduction
The low-lying saucer-shaped marshlands are known as the Haor basin (Figure 1). Haor is a unique wetland habitat in the northeastern part of Bangladesh (Figure 2). This country is home to 423 Haors. Haors occupy 0.86 million hectares of land, which is 43% of the total land of the Kishoreganj, Sunamganj, Netrokona, Habiganj, Moulvibazar, Brahmanbaria, and Sylhet districts (Table 1). Sunamganj has the highest number (133) of Haors, while Kishoreganj has the second most (122) followed by Netrokona (80) and Habiganj (38) [1]. The major Haors are the Tanguar, Hakaloki, Shanir, Mokhar, Dekhar, Gongajury, Kaoadighi, etc. The topographic distribution of a Haor is comprised of the high, medium–high, medium–low, low, and very low land types (Figure 3), of which 11% of areas are high to medium–high lands, and 89% areas are low-laying lands [2][3]. During April–November, the Haor basins experience standing water or sudden floods, while in December–March, they remain dry.
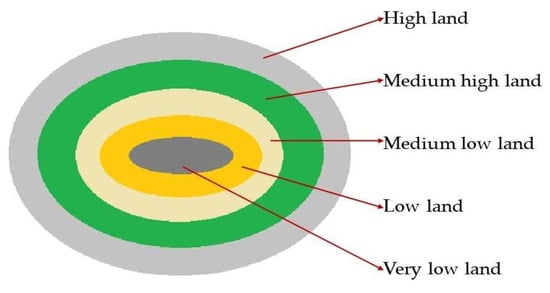
Figure 1. A conceptual model of Haor basin.
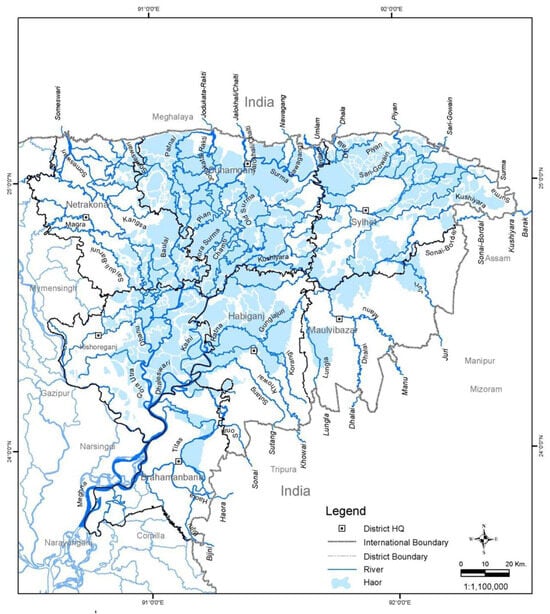
Figure 2. Haor areas of Bangladesh.
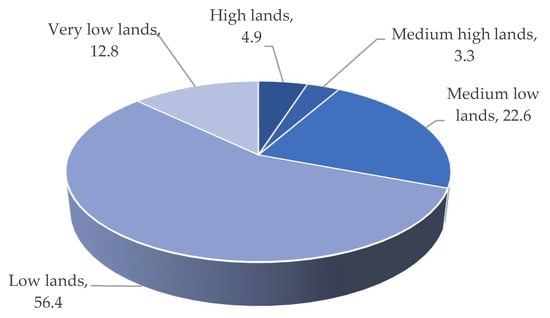
Figure 3. Topographic distribution of Hoar regions.
Table 1. Number and coverage of Haor areas in Bangladesh.
| Districts | Upazilas | Total Land Area (M ha) | No. of Haors | Total Haor Area (M ha) |
|---|---|---|---|---|
|
Nasirnagar and Sadar | 0.19 | 3 | 0.03 |
|
Ajmerigonj, Bahubal, and Sadar | 0.26 | 38 | 0.11 |
|
Austragram, Bazitpur, Bhairab, Itna, Karimgonj, Kuliarchar, Mithamoin, Nikli, and Tarail | 0.27 | 122 | 0.13 |
|
Kulaura, Rajnagar, Sadar, and Sreemangal | 0.28 | 4 | 0.05 |
|
Atpara, Barhatta, Kandua, Khaliajuri, Madan, and Mohongonj | 0.27 | 80 | 0.08 |
|
Bishambarpur, Chhatak, Derai, Dharmapasha, Jagannathpur, Jamalganj, Sadar, Salla, and Tahirpur | 0.37 | 133 | 0.27 |
|
Balagonj, Beanibazar, Biswanath, Fenchuganj, and Jaintiapur | 0.35 | 43 | 0.19 |
| Total | 2.00 | 423 | 0.86 | |
Boro rice, cultivated under irrigated conditions from mid-November to mid-May (Rabi season), is the primary source of income for Haor families. Most land remains submerged throughout the monsoon season, fostering a productive fishery. According to Alam et al., a total of 0.41 M ha of cultivable land is available in the seven Haor districts (Table 2), which is about 48% of the Haor areas [1][4]. The Boro rice–Fallow–Fallow is the primary cropping pattern of the Haor areas. However, some Aus (summer) and T. Aman (monsoon) rice is also grown very small in the Haors of Habiganj, Netrakona, Moulvibazar, and Sylhet [1]. The average cropping intensity of Haor areas is 176% (Table 3) as reported by the DAE [3]. Haor regions play a crucial role by (i) contributing around 18% of the overall Boro rice production [5] and (ii) generating job opportunities for non-farm wage employees by harvesting Boro rice about one month before other locations [6]. Data presented in the Table 4 shows that Haor districts and regions account for around 19% and 9% of the nation’s total Boro rice acreage, respectively [7]. Kabir et al. [6] also reported that about 36% of the land is under Boro rice cultivation in Sunamganj compared to 23% in Kishoreganj and 11% in Habiganj (Figure 4). Around 22 to 73% of the total land area is under cultivation (Figure 5).
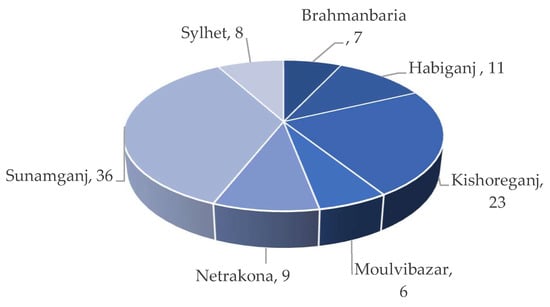
Figure 4. Percentage of Boro areas in the Haor districts.
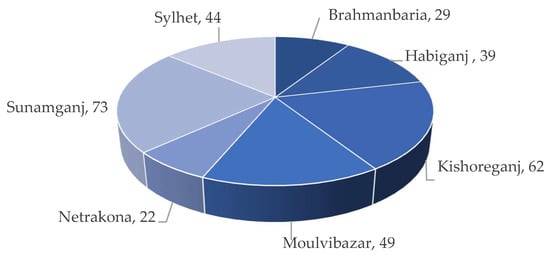
Figure 5. Percentage of arable land area in the Haor districts.
Table 2. Cultivable land areas of Hoar districts.
| Districts | Total Cultivated Land (M ha) |
Cultivated Land under Haor | |
|---|---|---|---|
| M ha | % Area | ||
|
0.15 | 0.01 | 33.77 |
|
0.19 | 0.04 | 36.53 |
|
0.17 | 0.12 | 89.59 |
|
0.13 | 0.02 | 42.02 |
|
0.21 | 0.05 | 63.02 |
|
0.20 | 0.14 | 52.14 |
|
0.21 | 0.03 | 15.80 |
| Total | 1.26 | 0.41 | 47.76 |
Table 3. Seasonal coverage (M ha) of diverse crops in the Haor regions.
| District | Aus (Summer) Rice | T. Aman (Monsoon) Rice | Boro (Winter) Rice | Other Rabi (Winter) Crops | Cropping Intensity (%) |
|---|---|---|---|---|---|
|
- | - | 0.014 | 0.003 | 189 |
|
- | 0.010 | 0.033 | 0.001 | 171 |
|
- | - | 0.117 | 0.007 | 215 |
|
- | 0.007 | 0.010 | 0.001 | 171 |
|
0.005 | 0.004 | 0.046 | 0.004 | 186 |
|
- | - | 0.136 | 0.002 | 143 |
|
- | 0.004 | 0.020 | 0.002 | 160 |
| Total | 0.005 | 0.025 | 0.376 | 0.02 |
Table 4. Boro acreages in the Haor districts and Haor regions.
| Districts | Boro Area (M ha) in the | |
|---|---|---|
| District | Haor | |
|
0.111 | 0.032 |
|
0.120 | 0.046 |
|
0.167 | 0.103 |
|
0.055 | 0.027 |
|
0.185 | 0.041 |
|
0.219 | 0.161 |
|
0.081 | 0.035 |
| Total | 0.937 | 0.445 |
Generally, farmers widely cultivate BRRI dhan28 (20% area), BRRI dhan29 (39% area), hybrid varieties (22% area), and other HYVs and local varieties (19% area) of Boro rice (Table 5) as reported by Kamruzzaman and Shaw [4]. It occupies 0.37 M ha, which is >90% of the cultivated land [1], and it accounts for around 16% of the total Boro rice production in the country [5]. It provides a living for 20 million people [8]. In 2022, the area under Boro rice production expanded to 0.45 M ha (Table 4) from 0.37 M ha in 2010 (Table 3). In the Haor areas, the mean yield of HYV Boro rice is about 4.5 t ha−1 compared to 5.8 t ha−1 of hybrid rice (Table 5). The average growth duration of BRRI dhan28 and other hybrid varieties; and BRRI dhan29 are 140 and 160 days, respectively. The typical planting time is 15–20 November, and harvest is on 15 April–15 May. Unfortunately, in the first week of April, just before the harvest, they had severe flash flooding when the water covered all of the immature or almost matured rice [9]. This has resulted in enormous losses for farmers and affects the food security and livelihoods of the Haor families. In April 2017, sudden floods submerged more than 0.20 million hectares of mature, blooming, or dough-stage Boro rice, resulting in 0.90 million ton loss in rice production [10]. Again, in March 2022, about 7,083 hectares of Boro rice went underwater in these regions, causing a loss of around USD 14 million [11].
Table 5. Area coverage and yield (t ha−1) of HYV and Hybrid Boro rice in the Haor areas.
| Districts | Percent (%) Area | Yield (t ha−1) | ||||
|---|---|---|---|---|---|---|
| BRRI dhan28 | BRRI dhan29 | Hybrid Rice | Other HYV + Local | HYV | Hybrid | |
|
27 | 40 | 15 | 18 | 4.85 | 5.92 |
|
14 | 52 | 24 | 10 | 4.93 | 5.45 |
|
12 | 49 | 20 | 18 | 4.37 | 5.82 |
|
28 | 27 | 20 | 25 | 4.10 | 5.78 |
|
20 | 40 | 18 | 20 | 5.07 | 6.72 |
|
15 | 43 | 22 | 20 | 4.30 | 5.50 |
|
21 | 22 | 31 | 24 | 4.33 | 5.65 |
| Average | 19.6 | 39.0 | 21.4 | 19.3 | 4.56 | 5.83 |
Rice breeders, agronomists, and researchers have been suggesting farmers seed Boro rice earlier than usual during the last week of October that could be harvested in March to reduce the risks of flash flooding. However, this runs the risk of rice plants experiencing cold shock between mid-February and mid-March at rice’s reproductive stages (panicle initiation through blooming). The grains of early-planted Boro rice become sterile when the mean temperature remains under 20 °C for one week [12]. Hence, cultivating short-duration Boro rice varieties of 135–140 days and medium duration of 141–150 days with modest adaptation to cold during the reproduction phase is a significant thrust to avoid flash floods while being planted in the last week of October to mid-November. Therefore, for the Haor regions, rice varieties that are cold tolerant to the reproductive phase should be developed and have a short to medium lifespan that could be harvested before the arrival of flash floods.
2. Flash Floods and Cold Risks of Damage to Boro Rice in the Haor Regions
Three different types of flooding impact the Haor regions: (i) early flash floods, (ii) rainfed floods, and (iii) river floods. The flash/early flash flood had a devastating impact on Haor [3]. According to BBS [13], from 1955 to 2022, this form of flood occurred 70% of the time, which was followed by rainfed floods (19%) and river floods (11%) (Figure 6). Overall, 90% to 100% of low and medium-low land regions are inundated by flash/early flash floods, while only 6 to 13% of medium-high and high-land areas are affected (Figure 7). That damaged 97% of Boro rice and 3% of other Rabi (winter) crops like mustard, ground nut, winter vegetables, etc. The cultivation of Aus (summer) and T. Aman (monsoon) rice is unaffected (if grown) [1][2][4].
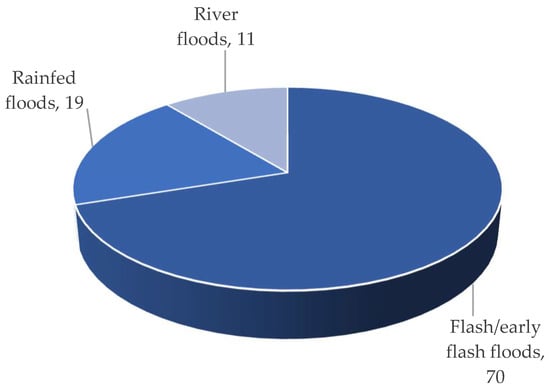
Figure 6. Types of floods that cause crop damage.
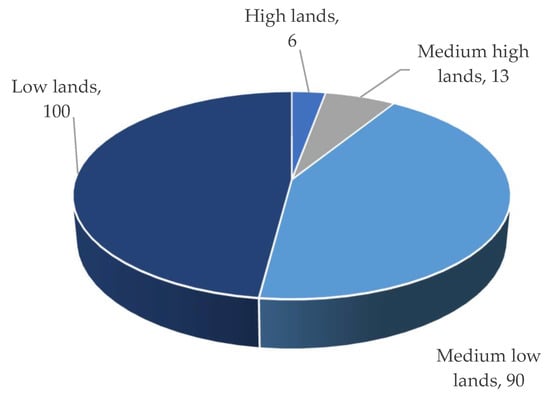
Figure 7. The type of land most affected by flash/early flash flooding.
In the Haor areas, Boro rice (cv. BRRI dhan28, BRRI dhan29, and hybrid varieties) seeds are typically sown on 15 November and transplanted at the end of December, which matured and were harvested between mid-April and early May [14]. During the first to the second week of April, flash floods often strike these regions, called the Boishakhi Dhall [9], causing an enormous loss of Boro rice (Figure 8) in the Hoar regions [15]. Flash flooding events are the most devastating, causing almost 80% or even total rice production loss, specifically in the Haor wetlands of the country [16]. For instance, the flash flood in 2017 caused a loss of 0.88 million metric tons of Boro rice in the Haor districts [17]. The monetary value of rice production loss was estimated at USD 450 million. Assuming a 33% probability of flash floods occurring in the Haor areas (once in three years), the economic value of rice production loss is estimated at USD 150 million per year. As a result, sustaining a safe production system in rice-based Haor agricultural regions is becoming difficult due to flash floods, and it is also a formative example of the problems associated with achieving sustainability in agriculture [18].


Figure 8. Flash flooding caused damage to Boro rice in the Haor areas.
Flash flood hits by the 3rd or 4th week of March, known as Chaitali Dhall, induce irreversible destruction to the immature/almost matured Boro rice [15]. An early flash flood or flash flood does not occur every year. In March 2017 and 2022, a devastating flash flood occurred unexpectedly, and the flooding of immature Boro rice produced a devastating catastrophe [10][11]. According to meteorological data analysis, from 1955 to 2022, early flash floods have occurred in the Haor area in 1964, 1982, 1996, 2003, 2010, 2017, and 2022 (Figure 9). The Haor regions of Bangladesh, located downstream of India’s Cherapunji area, are prone to annual flash flooding. Five or six days of precipitation over 150 mm can cause floods in the Haor basin and its tributaries [9]. Since 1955, the rainfall in the Haor areas from 20 March to 15 April has exceeded 150–200 mm in 1964, 1982, 1996, 2003, 2010, 2017 and 2022 (Figure 9). In 1964, 1982, 2003, and 2010, it was 186, 216, 184 and 296 mm, respectively. In comparison, rainfall in 2017 and 2022 was 627 and 356 mm, respectively (Figure 9). The precipitation in 2010 and 2022 may be characterized as more intense, but in 2017, it was overwhelming [19]. Observations indicate that these exceptional rainfalls occurred every seven years up to 2017. Still, in 2022, it was two years earlier, even though the Meteorological Department of Bangladesh projected an increase in the incidence of early flash floods. In the years to come, the frequency of early flash floods may increase due to global climate change.
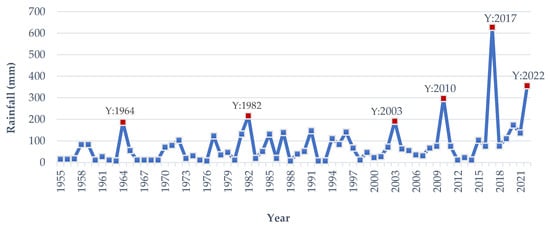
Figure 9. The average rainfall in Haor regions was from 20 March to 15 April from 1955 to 2022. Y: Year.
Boro rice must be harvested before the end of March to prevent early flash floods. The crops will then be protected from early flash floods. This variety’s growth period must be 135–140 days while planted at the standard time. A variety with such accommodating growth time is currently unavailable. The BRRI dhan28 is the most popular mega rice variety in the Haor region due to its high yield and shorter growth duration. The average growth duration of this variety is about 140 days under the recommended cultivation procedure. Farmers cultivate this variety at the medium–low land to low land areas of the Haor. BRRI released rice varieties viz., BRRI dhan35, 36, 45, 67, 84, 88, BRRI hybrid dhan3, and 5 are the coeval of BRRI dhan28 [14]. These short-duration varieties would not survive the early flash floods because they can be harvested by 10 April if 30-day-old seedlings are transplanted on 15 November at the regular time (Figure 10). Suppose these varieties are sown earlier, for example, on 15 October and even 1 November. In that case, they could be harvested by 28 March and 4 April, respectively, but will be in cold injury at the reproductive phase (Table 6).
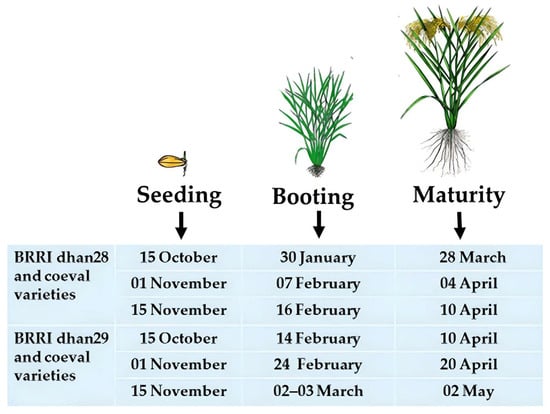
Figure 10. Effect of seeding dates on the maturity time of Boro rice in the Haor areas.
Table 6. Different planting dates of different Boro rice varieties of Bangladesh and associated risks in the Haor regions.
| Rice Varieties | Seeding by | Transplanting by | Panicle Initiation by | Heading by | Harvesting by | Risks |
|---|---|---|---|---|---|---|
|
15 October | 15 November | 15 January | 15 February | 21 March | Spikelet sterility |
| 1 November | 7 December | 1st week of February | 7 March | 7 April | Spikelet sterility Early flash floods |
|
| 15 November | 15 December | 21 February | 15 March | 15 April | Flash floods | |
|
15 October | 30 November | 15 February | 7 March | 7 April | Early/flash floods |
| 1 November | 15 December | 28 February | 22 March | 22 April | Early/flash floods | |
| 15 November | 30 December | 7 March | 7 April | 30 April–7 May | Early/flash floods |
Farmers favor cultivating the highest producing, long duration (160 days) varieties viz., BRRI dhan29, BRRI dhan69, BRRI dhan89, and BRRI dhan92 at the medium–high to high land areas of the Haor. Because flood water first submerges the low-lying areas, followed by the medium–high to high land areas, they have some extra time to harvest these long-duration varieties. They typically plant seeds in seedbeds in the first to second week of November. Due to its extended growth period, it is generally not susceptible to cold damage during the reproductive stage, even if seeded in the second week of October. Yet, flash flood is a significant danger as it matures by 7–8 May (Figure 10). Due to its extended growth period, it is generally not susceptible to cold harm during the reproductive stage, even if seeded in the second week of October. Still, flash floods at maturity are a significant danger (Table 6). Some local varieties, such as Habiganj Boro IV (KhoiaBoro) and Habiganj Boro VI (Pushusail), with a 150-day lifespan, are not suitable to cultivate here due to their poor yield of about 3.0 t ha−1 [15].
3. Temperature Requirements for Rice Plant
Regular (15 November) planting of Boro rice in Haor locations does not result in cold harm during the reproductive stage, but flash flood damage is probable at harvest [9]. To avoid this disaster, early transplanted Boro rice has been suggested. However, there is a possibility that low temperatures will be encountered during the particular phases of rice establishment (Figure 11). Since rice is a thermophilic plant native to tropical and subtropical environments [20], it has a definite temperature requirement. The ideal range of temperatures for cultivating the Indica variant is 25–35 °C, and for the Japonica variant, it is 20–33 °C [21]. The critical low temperature is often believed to be below 20 °C. It varies from one phase to the next in rice development (Table 7) as stated by Yoshida [21]. However, it varies by variety, length of critical temperature, growth circumstances, and daily fluctuations [22]. Yoshida [21] demonstrated that 10–13 °C is the lower temperature limit for rice plants that induce cold damage during the seed germination and vegetative phases. The threshold temperature falls between 18 and 20 °C at the reproductive stage and below 20 °C during the development of pollen mother cells, frequently producing a significant proportion of sterile spikelets. The germination and seedling development occur best at 25–35 °C [23]. At the booting stage, the threshold temperatures for cold-sensitive and cold-tolerant rice are 20 and 15 °C, respectively [24]. Spikelet sterility occurs when the temperature goes below this critical limit for three consecutive days. However, the harm is catastrophic if the condition lingers for five to six days [14]. The critical night-time temperature ranges from 13 to 15 °C during the reproductive period and is capable of causing cold damage [22]. Reports show that 12–13 °C night and 28–29 °C day temperatures are essential during the reproductive stage since these temperatures reduce rice output by 50% compared to its initial level [9].
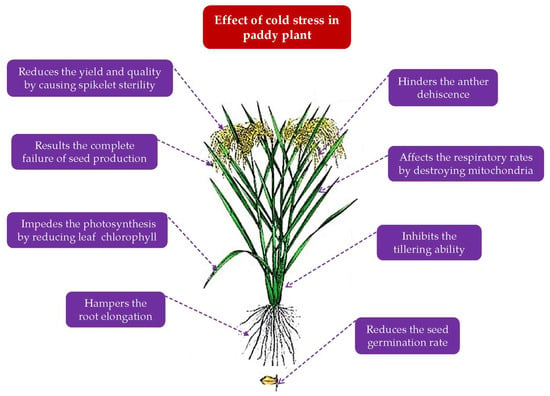
Figure 11. Effect of cold temperature on different growth phases of Boro rice.
Table 7. Temperature (°C) requirements at different growth phases of rice plants.
| Rice Growth Phases | Optimum | Minimum | Maximum |
|---|---|---|---|
|
20–35 | 10 | 45 |
|
25–30 | 12–13 | 35 |
|
25–28 | 16 | 35 |
|
31 | 7–12 | 45 |
|
25–31 | 9–16 | 33 |
|
30–33 | 22 | 35 |
|
20–25 | 12–18 | 30 |
3.1. Influence of Cold Temperature on the Vegetative Growth of Rice
Every developmental phase of rice, from vegetative to reproductive (Table 7), including germination, seedling, tillering, blooming, grain filling, and maturity, is influenced by cold temperature [25][26][27]. Common signs of cold temperature damage during the germination stage include a terrible delay in germination and a lower germination rate [28][29][30]. Low temperature affects seedling vigor and development [31][32], decreases the seedling quantity and tillering [33], increases plant mortality [23][34], and lengthens the growth time [35]. At the vegetative stage, cold injury in rice emerges: (i) leaves turn yellow, (ii) tiller number is reduced, (iii) plant height is impeded, (iv) biomass synthesis decreases, and (v) growth duration lengthens [36].
3.2. Influence of Cold Temperature on the Reproductive Growth of Rice
3.2.1. Floral Organs Development
During the reproductive phase, cold temperature inhibits anthers development, viz., the disappearance of anthers, little or no bursting of the anther, immature pollens, and few or no pollen shedding. Thus, pollen cannot germinate [21][37][38]. Panicle initiation is heavily controlled by temperature since rice must accrue a particular number of degrees days before the commencement of the reproductive stage [39]. Research suggests that 1–2 weeks before heading, the booting time, is especially vulnerable to cold. Flowering and heading are also susceptible to low temperatures [21]. Cold stress also causes late flowering, partial panicle exertion, and the deformation of spikelets [27][40]. However, spikelet sterility is the most prevalent damage caused by pollen aborting [37][41][42]. Young microspores are particularly vulnerable to the chilling injury that causes male sterility [43]. Temperatures as low as 12 °C for six days create almost complete sterility [21]. Under cold stress, inferior spikelets and a delay in blooming result in poor grain growth and reduced grain production associated with a deficiency of carbohydrates [36][44].
3.2.2. Grain Formation and Development
During the growth of rice grains, low temperature causes incomplete and delayed maturation, and grain development is regulated by a source–sink relationship that is affected by low temperature [45]. Grain filling duration and rate are reduced under cold stress, resulting in smaller grain sizes [46]. Moreover, hormones in plants, viz., abscisic acid (ABA) and cytokinin (CKT), also impact rice grain development [45][47]. Under cold stress, ABA and CKT concentrations increase and function as stress regulators in rice. Due to a lack of carbon dioxide (CO2) within cells, stomatal closure occurs at elevated ABA levels in leaves, hence restricting photosynthesis. Cold stress decreases the grain filling duration and rate, reducing rice grain yield. Somersalo and Krause [48] also showed that cold temperatures restrict photosynthesis, negatively affecting grain production and quality since there is less accessible glucose for grain production. Other studies have shown that low temperatures result in grain’s partial and late maturity [27][49].
4. Global Scenario of Cold Damage in Rice
In many Asian countries, rice is especially susceptible to cold stress and has been extensively reported in Bhutan, China, India, Japan, Korea, and Nepal [40][49]. In Bhutan’s upper altitudes, throughout the seedling development in March–April and maturation between October and November, air temperatures persist below 15 °C, rendering rice cultivars extremely susceptible to cold harm [50]. In case of delayed transplanting, rice suffers cold damage during the blooming and ripening periods in October and November, resulting in the sterility of the spikelets [48]. In southern China, early-planted rice suffers severely from frost damage, mainly between February and March, during the seedling stage, while late-planted rice suffers from cold injury in the reproductive phase (October–November) in northeast, east, and southwest China [51][52].
The average temperature in the Telangana state, India, ranges between 8 and 13 °C from December to 15 February, representing a substantial abiotic constraint for rice cultivation [53][54]. The cold damage is the greatest constraint for rice in the temperate zone of Nepal (1000–2000 m above sea level). Reports found that yield loss is moderately severe due to high levels of sterility and panicle degeneration at 1300 m above sea level when the temperature drops below 20 °C [55][56]. Low temperatures caused severe damage to Korean rice at every stage, between seed sprouting and maturity, in all rice-growing regions [57]. In Japan, the air temperature drops below the critical point during September and October, when rice is in the flowering or maturation phases [58]. The winter season in Australia (late January–early February) impedes the development of pollen grains and results in the sterility of spikelets [59][60]. Rice must also be cold-tolerant, since the cold stress frequently threatens the yield in Africa, Europe, South America, and the United States [36].
5. Rice Cold-Stress Problem in Bangladesh
Cold damage during the dry season of Boro rice is a countrywide issue in many locations, including the Haor districts of Bangladesh [14]. The meteorological data of the World Bank [19] reported that, during the Bangladesh’s Boro season, the air temperature drops below the essential temperature for rice, particularly in the country’s north (Figure 12 and Figure 13). Boro rice seedlings faced exceptionally low temperatures in December. Seed germination rates became poor in several circumstances. With insufficient development, the seedling became yellowish. Farmers were unhappy with their rice seedlings since they did not receive excellent seedlings from their seed beds. Following transplantation, seedling mortality occurred. Transplantation was occasionally delayed by two weeks. Some farmers must purchase seedlings from the market at a significant cost [14]. The BRRI suggests sowing short and long-duration rice during 15–30 and 5–25 November, respectively. Due to the early receding of floodwaters, farmers in some Haor regions may be unable to adhere to the approved planting timetable, since they must use the residual floodwater for seedling growing and crop establishment. To prevent the flash flood, short-duration cultivars are typically advised for early sowing (from late October to early November) in a Haor region, which may expose the rice crop to low temperatures during the transition from the vegetative to reproductive development stages. Even early planting of a long-duration cultivar may be susceptible to cold damage during its reproductive phase [9]. As an example, for short-duration rice (135–140 days) sown on 15 October, the most sensitive stage, panicle initiation (PI), and flowering/anthesis occur during the second week of January and the second week of February, respectively (Table 6). These dates are shifted to the 1st week of February and 1st week of March when seeded on 1 November. Seedings seeded on 15 October have a 100% probability of suffering from cold injury, while the probability is 60% for 1 November seedings [12] because the mean air temperature from January to mid-February remains 15–20 °C in the Haor areas (Figure 12). However, January is the coldest month in Bangladesh followed by December and February (Figure 13).
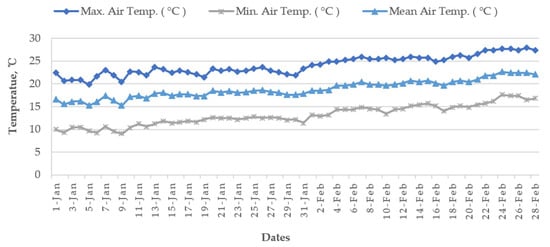
Figure 12. Average air temperature of January–February from 1955 to 2022 at the seven Haor districts of Bangladesh.
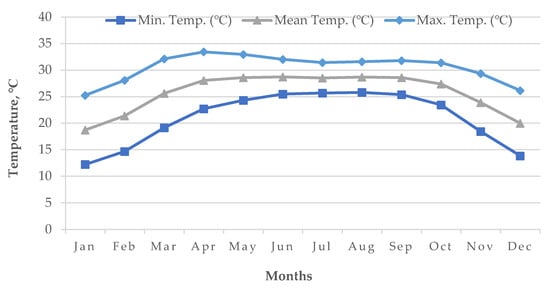
Figure 13. Average air temperature (1955–2022) of Bangladesh.
On the other hand, for the long-duration (151–160 days) rice, dates of PI and flowering for rice seeded on 15 October are the 2nd week of February and 1st week of March, respectively. Kabir et al. [12] reported a 17% chance of critical low temperatures for a rice crop seeded on 1 November, estimating dates of PI and flowering as the 4th week of February and the 3rd week of March, respectively. The rice crop can escape cold due to its long-duration nature [9]. In a field study, Polash et al. [61] observed that BRRI dhan28 had the highest percentages (40%) of sterile grains when seeded on 15 October. In January, vegetative and reproductive phases were observed at cold temperatures when the monthly mean air temperature at the PI stage was 13.1 °C. The number of grains was naturally degenerated, and only 0.71-ton yield per ha was produced. This date generated the shortest plant and panicle because of the low temperature. When the sowing dates were 30 October, 15 November, and 30 November, the duration of PI, growth, and maturity phases was reduced. In Bangladesh, yield variation of Boro rice is often linked to low air temperature at the reproductive stage because of (i) incomplete panicle exertion, (ii) delayed blooming time with uneven heading, (iii) degeneration of spikelet, (iv) asymmetrical maturity, and (v) sterile and deformed grains [62][63][64][65][66].
6. Alleviation of Flash Flood and Cold Injury Damage of Boro Rice in the Haor Areas
6.1. Cultivation of Appropriate Boro Rice Varieties
Cultivating short-duration and cold-tolerant cultivars is the ultimate response to rice flash flooding and cold damage in the Haor regions of Bangladesh. However, Bangladesh currently lacks such suitable cultivars. New cold-tolerant short-duration (135–140 days) and medium-duration (141–150 days) cultivars should be developed. Farmers must sow seeds between 25 October and 1 November and transplant seedlings when they are 30–35 days old so they can be harvested by the end of March (Figure 14). If seeds are seeded during this time, the booting and heading period will inevitably fall into the cold current (Figure 12). Hence, cultivating cold-tolerant rice that can withstand <20 °C temperature during the reproductive phase could be the best alternative Boro rice for the Haor areas of Bangladesh.
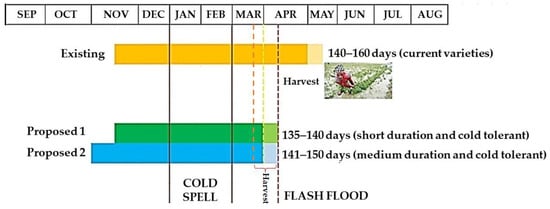
Figure 14. Existing and proposed planting schedule of Boro rice for the Haor areas.
BRRI developed a variety, BRRI dhan36, which exhibited some cold tolerance but was insufficient to satisfy the farmers’ needs. Sadly, farmers did not embrace these varieties. BRRI tried to introduce BRRI dhan45, another short-duration type, but it was not accepted by the Haor farmers due to a lack of cold tolerance. Recently, BRRI observed that the seedling and reproductive stages of BRRI dhan67 and BRRI dhan69 showed a moderate cold tolerance. The adoption of these cultivars in the Haor environment might reduce unanticipated production losses. These varieties could be popularized in the Hoar areas through large-scale demonstrations, adaptive trials, etc. Farmers must cultivate short-duration variety varieties (Table 6) in the low-lying part of the Haor. Long-duration varieties (Table 6) are suggested for cultivation in medium–high to high-land areas. These varieties will avoid cold because of their longer growing period but run the danger of being inundated because of early and frequent flash floods. The cold tolerance of distinct rice germplasm accessions (Table 8) is available in various countries [15][67][68].
Table 8. Available cold-tolerant rice genotypes in various countries.
| Country | Rice Varieties/Genotypes | References |
|---|---|---|
| Australia | Sherpa, Quest | Basuchaudhuri [69] Reinke et al. [59] |
| Bhutan | Barkat, Chhomrong dhan, Jakar Rey Naab, Khangma Maap, Kuchum, Yusi Rey Kaap1, Yusi Rey Kaap2, Yusi Rey Maap1, Yusi Rey Maap2 | Ghimiray and Gurung [70], Endo et al. [71] |
| China | B55, Banjiemang, Daohuaxiang2, KY131, Lijiangheigu, Longgeng31,TR22183, Yunlu 29 | Basuchaudhuri [69], Jiang et al. [72], and Li et al. [73] |
| Hungary | HSC55 | Basuchaudhuri [69] |
| India | Akshaydhan, Bhadrakali, Himalaya1, Himali, Himdhan, HPU1, JGL 3844, K332, Kalimpong1, Kanchan, Khonorullo, Meghalaya, RNR 17813, RNR 18805, Sheetal, Taramati, Tellahamsa, WGL 44 | Neelima et al. [54] Basuchaudhuri [69] |
| Japan | Hitomebore, Iwate 100, Jyoudeki, Ouu 415, Ouu-PL5, Tohoku 207, Tohoku PL3 | Nakagomi [58], |
| Korea | IR83222-F8-156, IR83222-F8-14, Jinbubyeo, Junganbyeo, SR30084-F8-156, SR30084-F8-14 | Wang et al. [74] |
| Nepal | Chainan-2, Chainung-242, Chandannath-1, Chandannath-3, Chhomrong dhan, Himali, Jumli Marsi, Kanchan, Khumal-2, Khumal-3, Khumal-4, Khumal-5, Khumal-6, Khumal-7, Khumal-8, Khumal-9 Machhapuchre-3, Machhapuchre-3, Manjushree 2, Palung-2, Taichung-176, Tainan-1 | Gautam and Shrestha [55] Karki et al. [75] |
| Russia | Kuban-3, Severny | Wang |
| USA | M103, M104 | Basuchaudhuri [69] |
In a series of cold-tolerance nurseries in Australia, the cold-tolerant rice variety Sherpa exhibited superior spikelet fertility compared to the standard checks. Furthermore, its spikelet fertility was comparable to that of the cold-tolerant standard Chinese variety Baijieming and Japanese variety Jyoudeki [59]. The Quest has also exhibited moderate cold tolerance in Australia [69]. According to Ghimiray and Gurung, Barkat, a cold-tolerant variety cultivated in the mid-altitude valleys of Bhutan attains maturity early [70]. The feasibility of utilizing the Bhutanese rice variety Kuchum to develop a cold-tolerant variety was documented by Endo et al. [71]. Additionally, Yusi Rey Maap1, Yusi Rey Kaap2, and Jakar Rey Naab are cold-tolerant varieties indigenous to Bhutan [50].
The lofty mountains of Nepal are crucial for the development of a cold-tolerant rice genome. In Chhumchaur, Jumla, Nepal, the renowned Jumli Marsi variety is cultivated at an astounding elevation of 3000 m above sea level (ASL). According to Gautam and Shrestha [55], Chhomrong dhan, Machhapuchre-3, and Palung-2 have been introduced for the mild-temperate high hill regions of Nepal (1300–2000 m ASL), whereas Chandannath-1 and Chandannath-3 have been designated for the Jumla valley (2300 m ASL) [15]. However, several cold-tolerant rice varieties viz., Manjushree 2, Tainan-1, Chainan-2, Chainung-242, Taichung-176, Himali, Kanchan, Khumal-2, Khumal-3, Khumal-4, Khumal-5, Khumal-6, Khumal-7, Khumal-8, Khumal-9, and Khumal-9 have been released for mild temperate regions of Nepal. According to a study by Karki et al., farmers in Nepal have thus far embraced Chhomrong, Machhapuchre-3, Chandannath-1, Chandannath-3, and Palung-2 as cultivars that tolerate cold conditions. The Chhomrong variety has become the most significant variety in Madagascar and Bhutan [75].
The study conducted by Neelima et al. in Telangana, India, observed that the cold-tolerant varieties Akshaydhan, Taramati, Bhadrakali, RNR 18805, RNR 17813, and WGL 44 performed better over the other cold tolerant varieties viz., Sheetal, Tellahamsa, and JGL 3844 [54]. Moreover, HPU1, Kanchan, Himali, Himdhan, Himalaya1, K332, Kalimpong1, Khonorullo, and Meghalaya1 are typical cold-tolerant rice varieties cultivated at high altitudes in India and Nepal [69].
Again, Basuchaudhuri reported that certain cultivars originating from China (B55, Banjiemang, Lijiangheigu, and Yunlu 29), Hungary (HSC55), Japan (Jyoudeki), and the United States (M103 and M104) exhibited low-temperature tolerance or a moderate degree of tolerance during the booting and flowering phases [69]. At all growth stages, three Chinese varieties and one Hungarian variety exhibited consistent resistance to cold temperatures. The TR22183, a temperate japonica variety originating in northern China, exhibits exceptional resistance to low temperatures [72]. In the Tohoku region of Japan, there are notable cultivars of cold-tolerant rice—namely, Hitomebore, Ouu 415, Tohoku PL 3, Iwate 100, and Tohoku 207 [58]. Regarding grain fertility, Wang et al. documented that Jinbubyeo, Junganbyeo, SR30084-F8-156, IR83222-F8-156, and IR83222-F8-14 from Korea, and Kuban-3 and Severny from Russia exhibited cold tolerance against cold susceptible commercial rice cultivars [74]. After careful evaluation, these genotypes might be collected and included in the Bangladesh rice breeding package to make cold-tolerant cultivars. The development of cold-tolerant Boro rice varieties utilizing cutting-edge breeding approaches is the most appropriate solution to overcome flash food and cold injury in the Haor region of the country.
6.2. Breeding of Cold-Tolerant Rice
6.2.1. Physiological Basis of Cold Tolerance
Cold temperatures cause physiological fluctuations that appear as physical damage in the field or laboratory. Physiological fluctuations have been categorized into six categories related to (i) amino acids, (ii) antioxidants, (iii) chlorophyll concentration and fluorescence ratio, (iv) electrolyte leakage, (v) ROS and MDA, and (v). soluble sugars. Changes in concentrations of these metabolites may indicate cold-stress reactions in rice [20].
The accumulation of amino acids, e.g., proline, is accelerated by cold stress. Proline serves as a cellular enzyme denaturation inhibitory agent and a C and N reservoir [76]. Protein synthesis is sustained due to the stabilizing effect of this amino acid [77]. It is also involved in the removal of excess H+ caused by stress and in maintaining the optimal cytosolic pH for oxidative respiration. Furthermore, it enhances the water-binding capacity of proteins [78]. Kim and Tai have confirmed the correlations between proline accumulation and cold tolerance in rice [67].
Antioxidants namely, ascorbic acid (AsA) and glutathione (GSH), scavenge ROS as an additional metabolic adaptation to safeguard rice plants from oxidative harm caused by cold stress [67][79]. They are present in chloroplasts and other cellular compartments in high concentrations; they are essential for plant defense against oxidative stress [80]. According to Sato et al., cold tolerance can be enhanced through the upregulation of OsAPXa, an ascorbate peroxidase gene, which elevates ascorbate peroxidase activity and decreases lipid peroxidation and H2O2 levels in response to cold stress [81].
The chlorophyll concentration and fluorescence ratio are important photosynthetic parameters affecting plant cold responses. According to Haboudane et al., chlorophyll levels may indicate nutritional stress, particularly N or S stress [82]. Cold stress may hinder rice leaf chlorophyll production and chloroplast development. Reduced chlorophyll concentration in rice plants may reflect the impact of low temperatures [83]. Cold stress causes a minor drop in fluorescence ratio values in tolerant plants but a large decrease in sensitive plants [84][85]. This metric helps assess plants’ cold tolerance and sensitivity, which may vary due to genetics or acclimatization.
Alterations in membrane rigidity, the physical state of membrane proteins, and osmotic pressure enable rice cells to detect cold stress [86]. Elevated membrane rigidity is initiated by low temperatures, which may result in elevated electrolyte leakage, and indicates the activity of genes associated with cold tolerance, such as TERF2, OVP1, and OsNAC5 [87][88]. An early occurrence cold stress, the influx of Ca2+ into the cytoplasm, may be facilitated by Ca2+ channels stimulated by ligands, membrane rigidification, or mechanical stimuli [89]. The calcium signaling cascade interprets and amplifies this cold-sensing signal, which then activates the dehydration-responsive element pathway in rice. This pathway is crucial for rice cold sensing and response [20][90].
Reactive oxygen species (ROS) are oxygen-containing molecules that are generated in trace amounts as typical by-products of cellular metabolism within chloroplasts, mitochondria, and peroxisomes [20]. Cellular oxidative injury can occur when an inordinate amount of ROS is produced [91][92]. The ROS may impede CO2 fixation, restrict electron transport chain mitochondria, and disrupt the photosynthetic process in chloroplasts [93]. It also degrades polyunsaturated lipids to produce malondialdehyde (MDA), which induces cellular toxic stress, tissue injury, and cellular dysfunction [94]. Additional research has demonstrated that the accumulation of ROS within the cells of rice that has been cold-treated induces the activation of cold-responsive genes and controls the cold-responsive signaling network [95].
Soluble sugars, viz., glucose, fructose, hexose, raffinose, sucrose, and trehalose function as compatible solutes and osmoprotectants against chilling-dehydration injury under cold stress [96][97]. Again, stachyose, raffinose, sucrose, and trehalose can increase in concentration when exposed to low temperatures [98]; thus, they can serve as indicators for assessing the potential cold tolerance of rice.
6.2.2. Assessment of Cold Tolerance in Rice
In all stages, cold temperatures have a negative impact, but the booting stage is more detrimental. There are distinct criteria for evaluating cold tolerance in rice [20]. The seedling to reproductive phases is the primary time when rice cultivars with increased cold tolerance are evaluated. Germination phase cold tolerance is essential for rapid seedling establishment and uniform crop stand [99]. The evaluation of cold tolerance in seedlings is vital for adaptation to situations with chilly temperatures that may occur at night-time, the beginning or end of the growing season. To produce the most grain, the survival of pollen, seed germination, and seedling establishment all require cold tolerance [100]. Table 9 summarizes the evaluation criteria for cold tolerance in rice at various phases of development [20].
Table 9. Criteria for evaluating the cold tolerance of rice at various development stages.
| Criteria in Different Stages | Indicators | Temperature and Duration | References | |
|---|---|---|---|---|
| Treatment | Recovery | |||
|
||||
|
The ratio of sprouted seeds to the total number of seeds used | 14 °C for 7–17 days | - | Han et al. [101] |
|
The ratio of survived seedlings over the total number of sprouted seeds | 2 °C for 72 h | 20 °C for 7 days | Zhou et al. [102] |
|
||||
|
Changes in fresh weight of plants after cold treatment | 10 °C for 1–48 h | - | Bonnecarrère [85] |
|
The ratio of live plants to the plants treated | 4 °C for 6 days | 26 °C for 6 days | Zhang et al. [88] |
|
The emergence of new leaves | 4 °C for 7 days | 25 °C for 10 days | Xie et al. [79] |
|
Visual scoring of 1: dark green, 3: light green, 5: yellow, 7: brown, 9: dead | 9 °C for 8–14 days | - | Kim and Tai [67], IRRI [103], Andaya and Tai [104] |
|
Visual scoring of 1–3: normal leaf without any injury tolerant, all leaves (tolerant), 4–9: wilted leaf and dead seedling (susceptible) | 10 °C for 7 days | 25 °C for 7 days | Suh et al. [105] |
|
||||
|
The ratio of filled grains to the total number of grains | 12 °C for 6 days | Until maturity | Sato et al. [81] |
|
The ratio of sterile grains to the total number of grains | 18–19 °C for ~60 days | Until maturity | Shirasawa et al. [106] |
-
Assessment of Cold Tolerance at the Germination Stage
Two primary parameters—the sprouting rate of seeds and the survival rate of seedlings—are employed to assess the cold tolerance of rice during this phase. The seed sprouting rate (%) is the ratio of sprouted seeds to the total number of seeds used and is measured at 7 days, 11 days, 14 days, and 17 days after germination at 14 °C in the dark [101]. Assuming the length of the root is equivalent to the length of the seed and the bud is equal to half the length of the seed, the germination of a rice grain is conventionally determined at that juncture. The survival rate of seedlings (%) is the ratio of survived seedlings over the total number of sprouted seeds. When the shoots reach a length of approximately 5 mm, the assessment of cold tolerance in seedlings involves the following procedure: after germinated seedlings have been deposited in soil and exposed to a cold treatment at 2 °C for three days, they are transferred to a sunny indoor environment maintaining a temperature above 20 °C to facilitate regular growth [102]. Survival rates of seedlings are evaluated seven days after recovery growth. Therefore, cold stress is more likely to affect rice seedlings than during seed sprouting. Hence, when assessing cold tolerance, the seedling survival rate emerges as a more pragmatic metric in comparison to characteristics evaluated during the germination phase.
-
Assessment of the Cold Tolerance at Seedlings Phase
Physiological and visual indicators are employed to assess the frigid tolerance of rice seedlings. Visual assessment of cold tolerance generally involves the consideration of five criteria: fresh weight, survival rate, emergence of new leaves, growth of seedlings, and growth of leaves (Table 9). Changes in fresh weight can serve as an indicator of water loss and growth retardation in rice plants subjected to cold stress given that water loss frequently transpires in conjunction with plant injury [85]. Nevertheless, water loss may not consistently serve as a reliable indicator of cold stress due to the influence of additional stressors and characteristics such as leaf size and plant variety. Another indicator, the seedling survival rate (ratio of live plants to the plants treated), is calculated following a 4 °C treatment in the lab. This extreme temperature condition is detrimental to rice growth and differentiates the cold tolerance of cultivars within 6 to 7 days [88]. Furthermore, an additional application of new leaf emergence is an indicator of cold tolerance in wild types of plants and their transgenic because cold treatment at 4 °C merely inhibits development without inducing fatal consequences [79]. The seedling development scale is a metric that is sourced from the International Rice Research Institute [103]. The seedlings are scored by the information provided in Table 9 following a 14-day cold treatment at a temperature of 9 °C [67][104]. Suh et al. [105] established a leaf growth scale that utilizes a comparable visual scaling approach. This scale is determined by the extent of leaf wilting and is quantified on the scale provided in Table 9. In contrast to the seedling growth scale, which involves the immediate observation of seedlings after low-temperature treatment, the leaf growth assay detects symptoms beginning on the seventh day of the recovery period [105]. As a result of the moderate treatment (9–10 °C), the leaf growth assay and seedling growth scale are frequently used to distinguish minute variations in cold tolerance among cultivars. Furthermore, due to the 7-day recuperation period at 25 °C, the leaf growth assay is more capable of differentiating cultivars that have comparable cold tolerance levels than the seedling growth scale.
-
Assessment of the Cold Tolerance at Reproductive Phase
In rice, spikelet sterility and consequently significant yield reduction can result from reproductive stage exposure to low temperatures. During this phase, cold tolerance can be assessed through the fertility of spikelets using cold greenhouse cultivation (CGC) or cold deep-water irrigation (CDWI) methods [20]. Using the CGC method, leaving the mother tiller, additional tillers are excised at the tillering stage from each greenhouse-grown plant. Subsequently, each plant is transferred to a greenhouse that is maintained at a temperature of 12 °C and reintroduced into the milder greenhouse after 5–6 days, and it is left there until maturity. Subsequently, the mean spikelet fertility is computed to assess cold tolerance. Using CDWI, rice plants are transplanted into containers filled to a depth of 20–25 cm with water maintained at 18–19 °C. The plants remain submerged in cold deep-water irrigation containers for the duration of the booting phase. The determination of spikelet fertility/sterility involves the ratio of the number of fertile/sterile seeds to the number of florets [81][105][106].
The evaluation of antioxidant enzyme activities could both identify cold-tolerant plants and elucidate the mechanisms underlying cold tolerance as opposed to relying solely on physical assessment of whole-plant cold tolerance. As illustrated by Kim and Tai, temperate varieties of O. japonica demonstrate reduced levels of electrolyte leakage (EL), whereas O. indica varieties accumulate greater quantities of proline, malondialdehyde (MDA), ascorbic acid (AsA), and glutathione (GSH) [67]. Cold tolerance in rice can also be determined by analyzing the activities of antioxidant enzymes, including superoxide dismutase, catalase, and ascorbate peroxidase [85][107]. Enhanced levels of OsPOX1, APXa, and the associated kinase OsTrx23 have been observed to potentially regulate the expression of numerous genes in response to cold stress [79][81][108].
6.2.3. Molecular Basis of Cold Tolerance
Several genes control the quantitative property of rice cold tolerance. Due to the difficulties in directly linking plant phenotypes to the genes that govern cold tolerance, marker-assisted selection is a helpful technique for creating cold-tolerant cultivars [106][109]. Rice cold tolerance has been linked to specific QTL using molecular markers and linkage maps. Studies of the QTLs associated with cold tolerance from germination to reproductive phases [20] could be helpful to speed up rice breeding for cold tolerance (Table 10), which could be utilized for developing cold-tolerant rice varieties for the Haor areas of Bangladesh.
Table 10. QTLs linked to rice’s cold tolerance.
| Name of QTL | Trait for Mapping | Chromosome No. | Reference |
|---|---|---|---|
| Ctb1, Ctb2 | Seed fertility | 4 | Saito et al. [46] |
| Dth, cl, fer, pe, dc | Days to flowering, culm length, seed fertility, and panicle exertion | 1, 3, 5, 6, 7, 8, 9, 11 | Oh et al. [110] |
| Os01g0357800, Os05g0171300, Os05g0400200 | Seedling vigor | 1, 4, 5 | Ham et al. [111] |
| qCTB-1-1, −4-1/2, −5-1/2, −10-1/2, −11-1 | Seed fertility | 1, 4, 5, 10, 11 | Xu et al. [26] |
| qCTB2a, qCTB3 | Seed fertility | 2, 3 | Andaya and Mackill [25] |
| qCTB-5-1/2/3, −7 | Seed germination | 5, 7 | Lin et al. [112] |
| qCTP11, qCTP12 | Seed germination | 11, 12 | Baruah et al. [34] |
| qCTS12, qCTS12a | Seedling growth | 12 | Andaya and Tai [104], Andaya and Mackill [113] |
| qCTS-2 | Seedling growth | 2 | Lou et al. [30] |
| qCTS4, qCTS4a, qCTS4b | Seedling growth | 4 | Andaya and Tai [114], Suh et al. [105] |
| qCtss11 | Seedling growth | 11 | Koseki et al. [115] |
| qLTB3 | Seed fertility | 3 | Shirasawa et al. [106] |
| qLTG3-1 | Seed germination | 3 | Fujino et al. [116] |
| qLVG2, qLVG7-2, qCIVG7-2 | Seed germination | 2, 7 | Han et al. [101] |
| qPSST-3, −7, −9 | Seed fertility | 3, 7, 8, 9, 11 | Suh et al. [100] |
| qSV-3-1/2, −5, −8-1/2 | Seedling growth | 3, 5, 8 | Zhang et al. [117] |
6.3. Emphasis on Cultural Techniques to Reduce the Risk of Flash Flooding and Cold Injury
6.3.1. Early Planting and Seedbed Covering
Planting seeds earlier than normal time might be advantageous. Transparent polyethylene may cover the seedbed between 10 a.m. and dusk to shield for early-planted seedlings from cold injury and promote good seedling growth during a cold period [118]. It boosts the temperature inside the polythene cover, resulting in enhanced seedling development. Higher temperatures at the seedling stage in the shade may compensate for certain degree days to lessen the plant’s growth time. However, this approach may not work well for the whole Haor region.
6.3.2. Direct Seeding
The direct sowing of sprouted seeds in puddle soils might minimize the plant growth time by about one week, but this method is highly dependent on the farmer’s preferences [15].
6.3.3. Double Transplanting
This cultural practice may be preferable to an earlier harvest by one week [9]. In this technique, seeding is completed on 1 November. Then, 30–35 days aged seedlings are transplanted in the highlands with a very densely spacing of 10 cm × 10 cm by the 1st week of December. After 40–45 days of the first transplantation, the second transplantation are completed by 15 January with regular spacing of 20 cm × 20 cm in the low-lying fields.
6.3.4. Transplanting of Young Seedlings
According to Biswas et al. [9], manipulation of seedling age may be another technique for shortening growth length. Growth duration could be shortened by 7–8 days by transplanting seedlings of 30 days age instead of 45 days.
6.3.5. Water Management
Water management is essential for reducing rice cold damage. Around a field, deep irrigation, circular channels, and polyethylene tubes may enhance the water temperature. Deep irrigation (20–25 cm) throughout the reproductive phase may assist panicle development, improve the filled-grain ratio, and protect the crop against cold temperatures [15].
6.3.6. Judicious Nutrient Management
Minimizing cold damage to rice may also be achieved by prudent nutrient management because the danger of yield loss in chilly weather is considerably raised by a crop’s higher nitrogen (N) status [119]. Adding nitrogen at colder temperatures during the flag leaf stage increases the risk of crop loss. The application of adequate phosphorus (P) prevents cold damage in rice [120][121]. Organic matter may enhance soil’s physical and chemical qualities, reducing cold damage to rice fields by improving their capacity to retain water and fertilizer. Zhu et al. [122] demonstrated that compost treatments improved root growth, increased fertile grain proportion, and decreased cold damage. However, all these agronomic techniques are highly environment sensitive and not sustainable. Developing and deploying cold-tolerant short-duration varieties would be the best solution for improving the poor livelihood of the Haor people in Bangladesh.
6.3.7. Applications of Plant Growth Regulators and Stimulants
Rice plants may be able to withstand cold stress through the exogenous application of growth regulators. According to a study by Wang et al., cold stress on rice can be effectively mitigated through the administration of brassinolide (BR), which is a plant hormone. The application of 2 mg L–1 BR at the seedling stage effectively preserved the levels of N, P, and K while also postponing the depletion of chlorophyll during the booting phase of rice. Additionally, it bolstered the functionality of antioxidant enzymes found in rice leaves, namely superoxide dismutase and peroxidase, and prevented the buildup of malondialdehyde, which is a lipid peroxidation product that is responsible for sustaining rice grain yield in cold-stress conditions [123]. Abscisic acid (ABA), a phytohormone, was found to play a crucial role in beginning the ABA signaling cascade, which in turn influences the expression of ABA-responsive genes via the activation of cis-acting ABA-response elements (ABRE). The OsNAC gene functions as a transducer of the abscisic acid (ABA) signal using an ABA-responsive element (ABRE) located in its promoter region. This gene plays a crucial role in regulating the expression of NACRS-containing genes that can exert control over cold tolerance in rice [87][124][125].
A promising strategy for enhancing cold stress tolerance has been identified in seed priming and seedling treatments utilizing exogenous stimulants. Previous research has documented that increasing α-amylase activity through hormonal priming with salicylic acid (SA), redox priming with hydrogen peroxide (H2O2), and chemical priming with selenium (Se), nitric oxide (NO), spermidine (Spd), and trehalose (TH) can substantially enhance the ability of seeds and seedlings to degrade starch under chilling stress [126]. The H2O2 and NO seed priming exhibited notable safeguarding impacts on germination and growth parameters in addition to bestowing substantial resistance to cold stress by increasing the activity of antioxidant enzymes and protecting chlorophyll from chilling-induced degradation [127]. KCl, NaCl, polyethylene glycol (PEG), and CaCl2 seed priming were also associated with an increased rate of rice seedling survival under chilling stress [128][129].
Several chemical and organic stimulants, including zinc oxide nanoparticles (ZnO NP), nitric oxide (NO), hydrogen (H2), orysastrobin, glycine, and biochar, have been observed to enhance the cold stress tolerance of rice seedlings when applied to foliar or in the root medium during the seedling stage. The ZnO nanoparticles mitigated cold injury in rice seedlings subjected to cold stress by restoring chlorophyll accumulation and decreasing the severity of oxidative damage induced by cooling [130]. The application of 0.39 mM H2 externally to seedlings was observed to mitigate the detrimental effects of cold on growth and photosynthesis [131]. Rice seedlings cultivated in frigid conditions exhibited enhanced mineral homeostasis, antioxidant capacity, N-uptake growth attributes, photosynthetic pigments, and water balance when treated with NO and glycine [127][132]. Orysastrobin enhanced the resistance of rice seedlings to chilling stress by inhibiting transpiration and stimulating water-retaining activity [133]. The cold tolerance of rice seedlings was enhanced through the administration of biochar. Research has indicated that rice seedlings treated with biochar exhibited notably elevated concentrations of putative cold-inducible proteins, including OsCOIN, OsiSAP8, OsWRKY76, OsMYB2, OsDREB1A, and OsDREB1B. These proteins were found to improve the cold tolerance of the rice [134].
References
- Alam, M.; Quayum, M.; Islam, M. Crop Production in the Haor Areas of Bangladesh: Insights from Farm Level Survey. Agriculturists 2010, 8, 88–97.
- Khan, M.N.H.; Mia, M.Y.; Hossain, M.R. Impacts of Flood on Crop Production in Haor Areas of Two Upazillas in Kishoregonj. J. Environ. Sci. Nat. Resour. 2012, 5, 193–198.
- Department of Agricultural Extension (DAE). Annual Report-2016; Field Crops Wing, Ministry of Agriculture, Government of The People’s Republic of Bangladesh; Department of Agricultural Extension: Khamar Bari, Farmgate, Dhaka, Bangladesh, 2017; p. 197.
- Kamruzzaman, M.; Shaw, R. Flood and Sustainable Agriculture in the Haor Basin of Bangladesh: A Review Paper. Univers. J. Agric. Res. 2018, 6, 40–49.
- Bangladesh Bureau of Statistics (BBS). YAS—Yearbook of Agricultural Statistics-2021, 33rd ed.; Bangladesh Bureau of Statistics, Statistics & Informatics Division, Ministry of Planning, Government of The People’s Republic of Bangladesh: Dhaka, Bangladesh, 2022.
- Kabir, M.J.; Kabir, M.S.; Salam, M.A.; Islam, M.A.; Omar, M.I.; Sarkar, M.A.R.; Rahman, M.C.; Chowdhury, A.; Rahaman, M.S.; Deb, L.; et al. Harvesting of Boro Paddy in Haor Areas of Bangladesh: Interplay of Local and Migrant Labour, Mechanized Harvesters and Covid-19 Vigilance in 2020; ZBW—Leibniz Information Centre for Economics, Bangladesh Rice Research Institute (BRRI): Gazipur, Bangladesh, 2020; p. 40.
- CEGIS. Master Plan of Haor Area; Center for Environmental and Geographic Information Services, Bangladesh Haor and Wetlands Development Board, Ministry of Water Resources, The People’s Republic of Bangladesh: Dhaka, Bangladesh, 2012; p. 82.
- Rabby, T.G.; Alam, G.M.; Mishra, P.K.; Hoque, K.E.; Nair, S. Different Economic and Policy Perspectives in Micro Population for Sustainable Development: A Study of the Haor Livelihood in Bangladesh. Afr. J. Bus. Manag. 2011, 5, 2475–2492.
- Biswas, J.; Hossain, M.; Mamin, M.; Muttaleb, M. Manipulation of Seeding Date and Seedling Age to Avoid Flash Flood Damage of Boro Rice at the Northeastern Haor Areas in Bangladesh. Bangladesh Rice J. 2008, 13, 57–61.
- ReliefWeb. Bangladesh: Floods in Northeast (Haor) Areas (April–May 2017); Government of Bangladesh: Dhaka, Bangladesh, 2017; p. 48.
- NIRAPAD. Flash Flood Situation, March, 2022; Network for Information, Response and Preparedness Activities on Disaster: Dhaka, Bangladesh, 2022.
- Kabir, M.S.; Howlader, M.; Biswas, J.K.; Mahbub, M.A.; Elahi, M.N.E. Probability of Low Temperature Stress at Different Growth Stages of Boro Rice. Bangladesh Rice J. 2015, 19, 19–27.
- Bangladesh Bureau of Statistics (BBS). Statistical Year Book of Bangladesh, 41st ed.; Statistics & Informatics Division, Ministry of Planning, Government of The People’s Republic of Bangladesh: Dhaka, Bangladesh, 2021.
- BRRI. Annual Research Review Workshop 2018, Plant Breeding Division 2016–2017; Bangladesh Rice Research Institute (BRRI): Gazipur, Bangladesh, 2018.
- Rashid, M.M.; Yasmeen, R. Cold Injury and Flash Flood Damage in Boro Rice Cultivation in Bangladesh: A Review. Bangladesh Rice J. 2017, 21, 13–25.
- Rahman, H.M.T.; Mia, M.E.; Ford, J.D.; Robinson, B.E.; Hickey, G.M. Livelihood Exposure to Climatic Stresses in the North-Eastern Floodplains of Bangladesh. Land Use Policy 2018, 79, 199–214.
- Kamruzzaman, M.; Anne Daniell, K.; Chowdhury, A.; Crimp, S. Facilitating Learning for Innovation in a Climate-Stressed Context: Insights from Flash Flood-Affected Rice Farming in Bangladesh. J. Agric. Educ. Ext. 2023, 29, 463–487.
- Tran, D.D.; Huu, L.H.; Hoang, L.P.; Pham, T.D.; Nguyen, A.H. Sustainability of Rice-Based Livelihoods in the Upper Floodplains of Vietnamese Mekong Delta: Prospects and Challenges. Agric. Water Manag. 2021, 243, 106495.
- World Bank Climate Change Knowledge Portal. Available online: https://climateknowledgeportal.worldbank.org/country/bangladesh (accessed on 23 December 2022).
- Zhang, Q.; Chen, Q.; Wang, S.; Hong, Y.; Wang, Z. Rice and Cold Stress: Methods for Its Evaluation and Summary of Cold Tolerance-Related Quantitative Trait Loci. Rice 2014, 7, 24.
- Yoshida, S. Fundamentals of Rice Crop Science; International Rice Research Institute: Los Baños, Philippines, 1981; ISBN 971-10-4052-2.
- Farrell, T.C.; Fox, K.M.; Williams, R.L.; Fukai, S. Genotypic Variation for Cold Tolerance during Reproductive Development in Rice: Screening with Cold Air and Cold Water. Field Crops Res. 2006, 98, 178–194.
- Fujino, K.; Sekiguchi, H.; Sato, T.; Kiuchi, H.; Nonoue, Y.; Takeuchi, Y.; Ando, T.; Lin, S.Y.; Yano, M. Mapping of Quantitative Trait Loci Controlling Low-Temperature Germinability in Rice (Oryza sativa L.). Theor. Appl. Genet. 2004, 108, 794–799.
- Satake, T.; Hayase, H. Male Sterility Caused by Cooling Treatment at the Young Microspore Stage in Rice Plants: V. Estimations of Pollen Developmental Stage and the Most Sensitive Stage to Coolness. Jpn. J. Crop Sci. 1970, 39, 468–473.
- Andaya, V.; Mackill, D. QTLs Conferring Cold Tolerance at the Booting Stage of Rice Using Recombinant Inbred Lines from a Japonica × Indica Cross. Theor. Appl. Genet. 2003, 106, 1084–1090.
- Xu, L.-M.; Zhou, L.; Zeng, Y.-W.; Wang, F.-M.; Zhang, H.-L.; Shen, S.-Q.; Li, Z.-C. Identification and Mapping of Quantitative Trait Loci for Cold Tolerance at the Booting Stage in a Japonica Rice Near-Isogenic Line. Plant Sci. 2008, 174, 340–347.
- Ye, C.; Fukai, S.; Godwin, I.; Reinke, R.; Snell, P.; Schiller, J.; Basnayake, J. Cold Tolerance in Rice Varieties at Different Growth Stages. Pasture Sci. 2009, 60, 328–338.
- Cruz, R.; Milach, S. Cold Tolerance at the Germination Stage of Rice: Methods of Evaluation and Characterization of Genotypes. Sci. Agric. 2004, 61, 1–8.
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393.
- Lou, Q.; Chen, L.; Sun, Z.; Xing, Y.; Li, J.; Xu, X.; Mei, H.; Luo, L. A Major QTL Associated with Cold Tolerance at Seedling Stage in Rice (Oryza sativa L.). Euphytica 2007, 158, 87–94.
- Ali, M.G.; Naylor, R.E.L.; Matthews, S. Distinguishing the Effects of Genotype and Seed Physiological Age on Low Temperature Tolerance of Rice (Oryza sativa L.). Exp. Agric. 2006, 42, 337–349.
- Reyes, B.G.; Myers, S.J.; McGrath, J.M. Differential Induction of Glyoxylate Cycle Enzymes by Stress as a Marker for Seedling Vigor in Sugar Beet (Beta vulgaris). Mol. Genet. Genom. 2003, 269, 692–698.
- Shimono, H.; Hasegawa, T.; Iwama, K. Response of Growth and Grain Yield in Paddy Rice to Cool Water at Different Growth Stages. Field Crops Res. 2002, 73, 67–79.
- Baruah, A.R.; Ishigo-Oka, N.; Adachi, M.; Oguma, Y.; Tokizono, Y.; Onishi, K.; Sano, Y. Cold Tolerance at the Early Growth Stage in Wild and Cultivated Rice. Euphytica 2009, 165, 459–470.
- Ghadirnezhad, R.; Fallah, A. Temperature Effect on Yield and Yield Components of Different Rice Cultivars in Flowering Stage. Int. J. Agron. 2014, 2014, e846707.
- Hussain, S.; Khaliq, A.; Ali, B.; Hussain, H.A.; Qadir, T.; Hussain, S. Temperature Extremes: Impact on Rice Growth and Development. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 153–171. ISBN 978-3-030-06118-0.
- Da Cruz, R.P.; Milach, S.C.K.; Federizzi, L.C. Rice cold tolerance at the reproductive stage in a controlled environment. Sci. Agric. 2006, 63, 255–261.
- Da Cruz, R.P.; Sperotto, R.A.; Cargnelutti, D.; Adamski, J.M.; de FreitasTerra, T.; Fett, J.P. Avoiding Damage and Achieving Cold Tolerance in Rice Plants. Food Energy Secur. 2013, 2, 96–119.
- Shrestha, S.; Asch, F.; Brueck, H.; Giese, M.; Dusserre, J.; Ramanantsoanirina, A. Phenological Responses of Upland Rice Grown along an Altitudinal Gradient. Environ. Exp. Bot. 2013, 89, 1–10.
- Shimono, H.; Okada, M.; Kanda, E.; Arakawa, I. Low Temperature-Induced Sterility in Rice: Evidence for the Effects of Temperature before Panicle Initiation. Field Crops Res. 2007, 101, 221–231.
- Jacobs, B.C.; Pearson, C.J. Cold Damage and Development of Rice: A Conceptual Model. Aust. J. Exp. Agric. 1994, 34, 917–919.
- Mackill, D.J.; Coffman, W.R.; Garrity, D.P. Rainfed Lowland Rice Improvement.; International Rice Research Institute (IRRI): Los Baños, Manila, Philippines, 1996.
- Heenan, D.P. Low-Temperature Induced Floret Sterility in the Rice Cultivars Calrose and Inga as Influenced by Nitrogen Supply. Aust. J. Exp. Agric. 1984, 24, 255–259.
- Ahmed, N.; Maekawa, M.; Tetlow, I.J.; Ahmed, N.; Maekawa, M.; Tetlow, I.J. Effects of Low Temperature on Grain Filling, Amylose Content, and Activity of Starch Biosynthesis Enzymes in Endosperm of Basmati Rice. Aust. J. Agric. Res. 2008, 59, 599–604.
- Oliver, S.N.; Dennis, E.S.; Dolferus, R. ABA Regulates Apoplastic Sugar Transport and Is a Potential Signal for Cold-Induced Pollen Sterility in Rice. Plant Cell Physiol. 2007, 48, 1319–1330.
- Saito, K.; Miura, K.; Nagano, K.; Hayano-Saito, Y.; Araki, H.; Kato, A. Identification of Two Closely Linked Quantitative Trait Loci for Cold Tolerance on Chromosome 4 of Rice and Their Association with Anther Length. Theor. Appl. Genet. 2001, 103, 862–868.
- Oliver, S.N.; Van Dongen, J.T.; Alfred, S.C.; Mamun, E.A.; Zhao, X.; Saini, H.S.; Fernandes, S.F.; Blanchard, C.L.; Sutton, B.G.; Geigenberger, P.; et al. Cold-Induced Repression of the Rice Anther-Specific Cell Wall Invertase Gene OSINV4 Is Correlated with Sucrose Accumulation and Pollen Sterility. Plant Cell Environ. 2005, 28, 1534–1551.
- Somersalo, S.; Krause, G.H. Photoinhibition at Chilling Temperature. Planta 1989, 177, 409–416.
- Jena, K.K.; Kim, S.M.; Suh, K.J.P.; Yang, C.I.; Kim, Y.G. Identification of Cold-Tolerant Breeding Lines by Quantitative Trait Loci Associated with Cold Tolerance in Rice. Crop Sci. 2012, 52, 517–523.
- Ghimiray, M. Temperate Japonica Rice in Bhutan. In Advances in Temperate Rice Research; Jena, K., Hardy, M., Eds.; International Rice Research Institute (IRRI): Los Baños, Philippines, 2012; pp. 15–25.
- Feng, D.; Fu, L. Main Meteorological Problems of Rice Production and Protective Measures in China. Int. J. Biometeorol. 1989, 33, 1–6.
- Tao, F.; Zhang, S.; Zhang, Z. Changes in Rice Disasters across China in Recent Decades and the Meteorological and Agronomic Causes. Reg. Environ. Change 2013, 13, 743–759.
- Singh, B.; Sutradhar, M.; Singh, A.; Mandal, M. Cold Stress in Rice at Early Growth Stage—An Overview. Int. J. Pure Appl. Biosci. 2017, 5, 407–419.
- Neelima, P.; Rani, K.; Raju, C.; Keshavulu, K. Evaluation of Rice Genotypes for Cold Tolerance. Agric. Sci. Res. J. 2015, 5, 124–133.
- Gautam, A.; Shrestha, N. Temperate Japonica Rice in Nepal. In Advances in Temperate Rice Research; Jena, K., Hardy, M., Eds.; International Rice Research Institute (IRRI): Los Baños, Philippines, 2012; pp. 49–57.
- Sthapit, B. Chilling Injury of Rice Crop in Nepal: A Review. J. Inst. Agric. Anim. Sci. 1992, 13, 1–32.
- Lee, M.H. Low Temperature Tolerance in Rice: The Korean Experience. In Proceedings of the Increased Lowland Rice Production in the Mekong Region, Vientiane, Laos, 30 October–2 November 2000; pp. 109–117.
- Nakagomi, K. Selection of New Check Rice Varieties for Evaluating High Cold Tolerance and the Development of Breeding Lines with High Cold Tolerance in the Tohoku Region of Japan. Jpn. Agric. Res. Q. 2013, 47, 1–7.
- Reinke, R.; Beecher, G.; Dunn, B.; Snell, P. Temperate Rice in Australia. In Advances in Temperate Rice Research; Jena, K., Hardy, M., Eds.; International Rice Research Institute (IRRI): Los Baños, Philippines, 2012; pp. 1–14.
- Satake, T.; Lee, S.Y.; Koike, S.; Kariya, K. Male Sterility Caused by Cooling Treatment at the Young Microspore Stage in Rice Plants: XXVIII. Prevention of Cool Injury with the Newly Devised Water Management Practices: Effects of the Temperature and Depth of Water before the Critical Stage. Jpn. J. Crop Sci. 1988, 57, 234–241.
- Polash, M.M.H.; Biswas, J.K.; Mahmud, H.; Khatun, R. Effects of Low Temperature at Various Growth Stages and Yield of Different Rice (Oryza sativa L.) Genotypes. J. Stress Physiol. Biochem. 2020, 16, 57–66.
- Biswas, P.; Khatun, H.; Anisuzzaman, M. Molecular and Phenotypic Characterization for Cold Tolerance in Rice (Oryza sativa L.). Bangladesh Rice J. 2020, 23, 1–15.
- Islam, A.; Rasel, M.; Khanam, S.; Hassan, L. Screening of Rice (Oryza sativa L.) Genotypes for Cold Tolerance at the Seedling and Reproductive Stages Based on Morpho-Physiological Markers and Genetic Diversity Analysis *Address for Correspondence. Indian J. Nat. Prod. Resour. 2020, 10, 17964–17985.
- Islam, M.S.; Morison, J.I.L. Influence of Solar Radiation and Temperature on Irrigated Rice Grain Yield in Bangladesh. Field Crops Res. 1992, 30, 13–28.
- Nahar, K.; Biswas, J.; Shamsuzzaman, A.; Hasanuzzaman, M.; Barman, H. Screening of Indica Rice (Oryza sativa L.) Genotypes Against Low Temperature Stress. Bot. Res. Int. 2009, 2, 295–303.
- Nahar, K.; Hasanuzzaman, M.; Majumder, R. Effect of Low Temperature Stress in Transplanted Aman Rice Varieties Mediated by Different Transplanting Dates. Acad. J. Plant Sci. 2009, 2, 132–138.
- Kim, S.-I.; Tai, T.H. Evaluation of Seedling Cold Tolerance in Rice Cultivars: A Comparison of Visual Ratings and Quantitative Indicators of Physiological Changes. Euphytica 2011, 178, 437–447.
- Sharifi, P. Evaluation on Sixty-Eight Rice Germplasms in Cold Tolerance at Germination Stage. Rice Sci. 2010, 17, 77–81.
- Basuchaudhuri, P. Cold Tolerance in Rice Cultivation, 1st ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2014.
- Ghimiray, M.; Gurung, T.R. Barkat, a Cold-Tolerant Variety for the First Crop in Rice-Rice Rotations in Mid-Altitude Valleys of Bhutan. Int. Rice Res. Notes 1993, 18, 35.
- Endo, T.; Chiba, B.; Wagatsuma, K.; Saeki, K.; Ando, T.; Shomura, A.; Mizubayashi, T.; Ueda, T.; Yamamoto, T.; Nishio, T. Detection of QTLs for Cold Tolerance of Rice Cultivar ‘Kuchum’ and Effect of QTL Pyramiding. Theor. Appl. Genet. 2016, 129, 631–640.
- Jiang, W.; Jin, Y.-M.; Lee, J.; Lee, K.-I.; Piao, R.; Han, L.; Shin, J.-C.; Jin, R.-D.; Cao, T.; Pan, H.-Y.; et al. Quantitative Trait Loci for Cold Tolerance of Rice Recombinant Inbred Lines in Low Temperature Environments. Mol. Cells 2011, 32, 579–587.
- Li, X.; Chen, Z.; Zhang, G.; Lu, H.; Qin, P.; Qi, M.; Yu, Y.; Jiao, B.; Zhao, X.; Gao, Q.; et al. Analysis of Genetic Architecture and Favorable Allele Usage of Agronomic Traits in a Large Collection of Chinese Rice Accessions. Sci. China Life Sci. 2020, 63, 1688–1702.
- Wang, J.; Lin, X.; Sun, Q.; Jena, K.K. Evaluation of Cold Tolerance for Japonica Rice Varieties from Different Country. Adv. J. Food Sci. Technol. 2013, 5, 54–56.
- Karki, T.B.; Koirala, K.B.; Bk, S. Participatory Variety Slection of Cold Tolerant Rice in the Western Hills of Nepal. Agron. J. Nepal 2010, 1, 74–79.
- Shah, K.; Dubey, R.S. Effect of Cadmium on Proline Accumulation and Ribonuclease Activity in Rice Seedlings: Role of Proline as a Possible Enzyme Protectant. Biol. Plant. 1997, 40, 121–130.
- Kandpal, R.P.; Rao, N.A. Alterations in the Biosynthesis of Proteins and Nucleic Acids in Finger Millet (Eleucine coracana) Seedlings during Water Stress and the Effect of Proline on Protein Biosynthesis. Plant Sci. 1985, 40, 73–79.
- Schobert, B.; Tschesche, H. Unusual Solution Properties of Proline and Its Interaction with Proteins. Biochim. Biophys. Acta BBA Gen. Subj. 1978, 541, 270–277.
- Xie, G.; Kato, H.; Imai, R. Biochemical Identification of the OsMKK6-OsMPK3 Signalling Pathway for Chilling Stress Tolerance in Rice. Biochem. J. 2012, 443, 95–102.
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410.
- Sato, Y.; Masuta, Y.; Saito, K.; Murayama, S.; Ozawa, K. Enhanced Chilling Tolerance at the Booting Stage in Rice by Transgenic Overexpression of the Ascorbate Peroxidase Gene, OsAPXa. Plant Cell Rep. 2011, 30, 399–406.
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated Narrow-Band Vegetation Indices for Prediction of Crop Chlorophyll Content for Application to Precision Agriculture. Remote Sens. Environ. 2002, 81, 416–426.
- Sharma, P.; Sharma, N.; Deswal, R. The Molecular Biology of the Low-Temperature Response in Plants. BioEssays 2005, 27, 1048–1059.
- Farzad, P.; Nasri, M.; Tohidi Moghadam, H.R.; Zahedi, H.; Al-Alahmadi, M. Effects of Drought Stress on Chlorophyll Fluorescence Parameters, Chlorophyll Content and Grain Yield of Wheat Cultivars. J. Biol. Sci. 2007, 7, 841–847.
- Bonnecarrère, V.; Borsani, O.; Díaz, P.; Capdevielle, F.; Blanco, P.; Monza, J. Response to Photoxidative Stress Induced by Cold in Japonica Rice Is Genotype Dependent. Plant Sci. 2011, 180, 726–732.
- Los, D.A.; Murata, N. Membrane Fluidity and Its Roles in the Perception of Environmental Signals. Biochim. Biophys. Acta BBA Biomembr. 2004, 1666, 142–157.
- Song, S.-Y.; Chen, Y.; Chen, J.; Dai, X.-Y.; Zhang, W.-H. Physiological Mechanisms Underlying OsNAC5-Dependent Tolerance of Rice Plants to Abiotic Stress. Planta 2011, 234, 331–345.
- Zhang, X.; Guo, X.; Lei, C.; Cheng, Z.; Lin, Q.; Wang, J.; Wu, F.; Wang, J.; Wan, J. Overexpression of SlCZFP1, a Novel TFIIIA-Type Zinc Finger Protein from Tomato, Confers Enhanced Cold Tolerance in Transgenic Arabidopsis and Rice. Plant Mol. Biol. Report. 2011, 29, 185–196.
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Gene Regulation during Cold Acclimation in Plants. Physiol. Plant. 2006, 126, 52–61.
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Cold Stress Regulation of Gene Expression in Plants. Trends Plant Sci. 2007, 12, 444–451.
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399.
- Mittal, D.; Madhyastha, D.A.; Grover, A. Genome-Wide Transcriptional Profiles during Temperature and Oxidative Stress Reveal Coordinated Expression Patterns and Overlapping Regulons in Rice. PLoS ONE 2012, 7, e40899.
- Suzuki, N.; Mittler, R. Reactive Oxygen Species and Temperature Stresses: A Delicate Balance between Signaling and Destruction. Physiol. Plant. 2006, 126, 45–51.
- Pamplona, R. Advanced Lipoxidation End-Products. Chem. Biol. Interact. 2011, 192, 14–20.
- Xie, G.; Kato, H.; Sasaki, K.; Imai, R. A Cold-Induced Thioredoxin h of Rice, OsTrx23, Negatively Regulates Kinase Activities of OsMPK3 and OsMPK6 in Vitro. FEBS Lett. 2009, 583, 2734–2738.
- Shao, H.-B.; Guo, Q.-J.; Chu, L.-Y.; Zhao, X.-N.; Su, Z.-L.; Hu, Y.-C.; Cheng, J.-F. Understanding Molecular Mechanism of Higher Plant Plasticity under Abiotic Stress. Colloids Surf. B Biointerfaces 2007, 54, 37–45.
- Ma, Y.; Zhang, Y.; Lu, J.; Shao, H. Roles of Plant Soluble Sugars and Their Responses to Plant Cold Stress. Afr. J. Biotechnol. 2009, 8, 2004–2010.
- Morsy, M.R.; Jouve, L.; Hausman, J.-F.; Hoffmann, L.; Stewart, J. McD. Alteration of Oxidative and Carbohydrate Metabolism under Abiotic Stress in Two Rice (Oryza sativa L.) Genotypes Contrasting in Chilling Tolerance. J. Plant Physiol. 2007, 164, 157–167.
- Krishnasamy, V.; Seshu, D.V. Seed Germination Rate and Associated Characters in Rice. Crop Sci. 1989, 29, 904–908.
- Suh, J.P.; Jeung, J.U.; Lee, J.I.; Choi, Y.H.; Yea, J.D.; Virk, P.S.; Mackill, D.J.; Jena, K.K. Identification and Analysis of QTLs Controlling Cold Tolerance at the Reproductive Stage and Validation of Effective QTLs in Cold-Tolerant Genotypes of Rice (Oryza sativa L.). Theor. Appl. Genet. 2010, 120, 985–995.
- Han, L.-Z.; Zhang, Y.-Y.; Qiao, Y.-L.; Cao, G.-L.; Zhang, S.-Y.; Kim, J.-H.; Koh, H.-J. Genetic and QTL Analysis for Low-Temperature Vigor of Germination in Rice. Acta Genet. Sin. 2006, 33, 998–1006.
- Zhou, L.; Zeng, Y.; Hu, G.; Pan, Y.; Yang, S.; You, A.; Zhang, H.; Li, J.; Li, Z. Characterization and Identification of Cold Tolerant Near-Isogenic Lines in Rice. Breed. Sci. 2012, 62, 196–201.
- IRRI. Standard Evaluation System (SES) for Rice, 5th ed.; International Rice Research Institute: Los Baños, Philippines, 2013.
- Andaya, V.C.; Tai, T.H. Fine Mapping of the qCTS12 Locus, a Major QTL for Seedling Cold Tolerance in Rice. Theor. Appl. Genet. 2006, 113, 467–475.
- Suh, J.P.; Lee, C.K.; Lee, J.H.; Kim, J.J.; Kim, S.M.; Cho, Y.C.; Park, S.H.; Shin, J.C.; Kim, Y.G.; Jena, K.K. Identification of Quantitative Trait Loci for Seedling Cold Tolerance Using RILs Derived from a Cross between Japonica and Tropical Japonica Rice Cultivars. Euphytica 2012, 184, 101–108.
- Shirasawa, S.; Endo, T.; Nakagomi, K.; Yamaguchi, M.; Nishio, T. Delimitation of a QTL Region Controlling Cold Tolerance at Booting Stage of a Cultivar, “Lijiangxintuanheigu”, in Rice, Oryza sativa L. Theor. Appl. Genet. 2012, 124, 937–946.
- Huang, J.; Sun, S.-J.; Xu, D.-Q.; Yang, X.; Bao, Y.-M.; Wang, Z.-F.; Tang, H.-J.; Zhang, H. Increased Tolerance of Rice to Cold, Drought and Oxidative Stresses Mediated by the Overexpression of a Gene That Encodes the Zinc Finger Protein ZFP245. Biochem. Biophys. Res. Commun. 2009, 389, 556–561.
- Kim, S.-H.; Choi, H.-S.; Cho, Y.-C.; Kim, S.-R. Cold-Responsive Regulation of a Flower-Preferential Class III Peroxidase Gene, OsPOX1, in Rice (Oryza sativa L.). J. Plant Biol. 2012, 55, 123–131.
- Foolad, M.R.; Lin, G.Y.; Chen, F.Q. Comparison of QTLs for Seed Germination under Non-Stress, Cold Stress and Salt Stress in Tomato. Plant Breed. 1999, 118, 167–173.
- Oh, C.-S.; Choi, Y.-H.; Lee, S.-J.; Yoon, D.-B.; Moon, H.-P.; Ahn, S.-N. Mapping of Quantitative Trait Loci for Cold Tolerance in Weedy Rice. Breed. Sci. 2004, 54, 373–380.
- Ham, T.-H.; Kwon, Y.; Lee, Y.; Choi, J.; Lee, J. Genome-Wide Association Study Reveals the Genetic Basis of Cold Tolerance in Rice at the Seedling Stage. Agriculture 2021, 11, 318.
- Lin, J.; Zhu, W.; Zhang, Y.; Zhu, Z.; Zhao, L.; Chen, T.; Zhao, Q.; Zhou, L.; Fang, X.; Wang, Y.; et al. Detection of QTL for Cold Tolerance at Bud Bursting Stage Using Chromosome Segment Substitution Lines in Rice (Oryza sativa). Rice Sci. 2011, 18, 71–74.
- Andaya, V.C.; Mackill, D.J. Mapping of QTLs Associated with Cold Tolerance during the Vegetative Stage in Rice. J. Exp. Bot. 2003, 54, 2579–2585.
- Andaya, V.C.; Tai, T.H. Fine Mapping of the qCTS4 Locus Associated with Seedling Cold Tolerance in Rice (Oryza sativa L.). Mol. Breed. 2007, 20, 349–358.
- Koseki, M.; Kitazawa, N.; Yonebayashi, S.; Maehara, Y.; Wang, Z.-X.; Minobe, Y. Identification and Fine Mapping of a Major Quantitative Trait Locus Originating from Wild Rice, Controlling Cold Tolerance at the Seedling Stage. Mol. Genet. Genom. 2010, 284, 45–54.
- Fujino, K.; Sekiguchi, H.; Matsuda, Y.; Sugimoto, K.; Ono, K.; Yano, M. Molecular Identification of a Major Quantitative Trait Locus, qLTG3–1, Controlling Low-Temperature Germinability in Rice. Proc. Natl. Acad. Sci. USA 2008, 105, 12623–12628.
- Zhang, Z.-H.; Qu, X.-S.; Wan, S.; Chen, L.-H.; Zhu, Y.-G. Comparison of QTL Controlling Seedling Vigour under Different Temperature Conditions Using Recombinant Inbred Lines in Rice (Oryza sativa). Ann. Bot. 2005, 95, 423–429.
- Mahbub, M.; Biswas, J.; Yasmeen, R.; Haque, M. Effect of Polyethylene Cover on Quality Rice Seedling Production during Winter Season. Bangladesh Rice J. 2008, 14, 15–26.
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of Temperature Stress by Nutrient Management in Crop Plants: A Review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244.
- Hou, L.; Chen, W.; Zhao, G.; Ma, W.; Qi, C.; Liu, L.; Sun, H. Effects of Phosphate Fertilizer on Cold Tolerance and Its Related Physiological Parameters in Rice Under Low Temperature Stress. J. Northeast Agric. Univ. Engl. Ed. 2012, 19, 1–10.
- Andrianary, B.H.; Tsujimoto, Y.; Rakotonindrina, H.; Oo, A.Z.; Rabenarivo, M.; Ramifehiarivo, N.; Razakamanarivo, H. Phosphorus Application Affects Lowland Rice Yields by Changing Phenological Development and Cold Stress Degrees in the Central Highlands of Madagascar. Field Crops Res. 2021, 271, 108256.
- Zhu, X.; Chen, J.; Huang, S.; Li, W.; Penuelas, J.; Chen, J.; Zhou, F.; Zhang, W.; Li, G.; Liu, Z.; et al. Manure Amendment Can Reduce Rice Yield Loss under Extreme Temperatures. Commun. Earth Environ. 2022, 3, 147.
- Wang, S.; Zhao, H.; Zhao, L.; Gu, C.; Na, Y.; Xie, B.; Cheng, S.; Pan, G. Application of Brassinolide Alleviates Cold Stress at the Booting Stage of Rice. J. Integr. Agric. 2020, 19, 975–987.
- Nakashima, K.; Takasaki, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NAC Transcription Factors in Plant Abiotic Stress Responses. Biochim. Biophys. Acta 2012, 1819, 97–103.
- Hossain, M.A.; Cho, J.-I.; Han, M.; Ahn, C.-H.; Jeon, J.-S.; An, G.; Park, P.B. The ABRE-Binding bZIP Transcription Factor OsABF2 Is a Positive Regulator of Abiotic Stress and ABA Signaling in Rice. J. Plant Physiol. 2010, 167, 1512–1520.
- Wang, W.; Chen, Q.; Hussain, S.; Mei, J.; Dong, H.; Peng, S.; Huang, J.; Cui, K.; Nie, L. Pre-Sowing Seed Treatments in Direct-Seeded Early Rice: Consequences for Emergence, Seedling Growth and Associated Metabolic Events under Chilling Stress. Sci. Rep. 2016, 6, 19637.
- Sohag, A.A.M.; Tahjib-Ul-Arif, M.; Afrin, S.; Khan, M.K.; Hannan, M.A.; Skalicky, M.; Mortuza, M.G.; Brestic, M.; Hossain, M.A.; Murata, Y. Insights into Nitric Oxide-Mediated Water Balance, Antioxidant Defence and Mineral Homeostasis in Rice (Oryza sativa L.) under Chilling Stress. Nitric Oxide 2020, 100–101, 7–16.
- Hussain, S.; Khan, F.; Hussain, H.A.; Nie, L. Physiological and Biochemical Mechanisms of Seed Priming-Induced Chilling Tolerance in Rice Cultivars. Front. Plant Sci. 2016, 7, 116.
- Anwar, M.P.; Khalid, M.A.I.; Islam, A.K.M.M.; Yeasmin, S.; Ahmed, S.; Hadifa, A.; Ismail, I.; Hossain, A.; Sabagh, A. Potentiality of Different Seed Priming Agents to Mitigate Cold Stress of Winter Rice Seedling. Phyton 2021, 90, 1491–1506.
- Song, Y.; Jiang, M.; Zhang, H.; Li, R. Zinc Oxide Nanoparticles Alleviate Chilling Stress in Rice (Oryza sativa L.) by Regulating Antioxidative System and Chilling Response Transcription Factors. Molecules 2021, 26, 2196.
- Xu, S.; Jiang, Y.; Cui, W.; Jin, Q.; Zhang, Y.; Bu, D.; Fu, J.; Wang, R.; Zhou, F.; Shen, W. Hydrogen Enhances Adaptation of Rice Seedlings to Cold Stress via the Reestablishment of Redox Homeostasis Mediated by miRNA Expression. Plant Soil 2017, 414, 53–67.
- Cao, X.; Zhong, C.; Zhu, L.; Zhang, J.; Sajid, H.; Wu, L.; Jin, Q. Glycine Increases Cold Tolerance in Rice via the Regulation of N Uptake, Physiological Characteristics, and Photosynthesis. Plant Physiol. Biochem. 2017, 112, 251–260.
- Takahashi, N.; Sunohara, Y.; Fujiwara, M.; Matsumoto, H. Improved Tolerance to Transplanting Injury and Chilling Stress in Rice Seedlings Treated with Orysastrobin. Plant Physiol. Biochem. 2017, 113, 161–167.
- Yuan, J.; Meng, J.; Liang, X.; Yang, E.; Yang, X.; Chen, W. Organic Molecules from Biochar Leacheates Have a Positive Effect on Rice Seedling Cold Tolerance. Front. Plant Sci. 2017, 8, 1624.
More
Information
Subjects:
Agronomy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
21 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




