Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Matthew Haworth | -- | 2317 | 2023-12-20 15:44:09 | | | |
| 2 | Fanny Huang | Meta information modification | 2317 | 2023-12-22 06:40:50 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Haworth, M.; Marino, G.; Atzori, G.; Fabbri, A.; Daccache, A.; Killi, D.; Carli, A.; Montesano, V.; Conte, A.; Balestrini, R.; et al. Plant Leaf Gas Exchange to Plant Phenotyping. Encyclopedia. Available online: https://encyclopedia.pub/entry/52984 (accessed on 07 February 2026).
Haworth M, Marino G, Atzori G, Fabbri A, Daccache A, Killi D, et al. Plant Leaf Gas Exchange to Plant Phenotyping. Encyclopedia. Available at: https://encyclopedia.pub/entry/52984. Accessed February 07, 2026.
Haworth, Matthew, Giovanni Marino, Giulia Atzori, Andre Fabbri, Andre Daccache, Dilek Killi, Andrea Carli, Vincenzo Montesano, Adriano Conte, Raffaella Balestrini, et al. "Plant Leaf Gas Exchange to Plant Phenotyping" Encyclopedia, https://encyclopedia.pub/entry/52984 (accessed February 07, 2026).
Haworth, M., Marino, G., Atzori, G., Fabbri, A., Daccache, A., Killi, D., Carli, A., Montesano, V., Conte, A., Balestrini, R., & Centritto, M. (2023, December 20). Plant Leaf Gas Exchange to Plant Phenotyping. In Encyclopedia. https://encyclopedia.pub/entry/52984
Haworth, Matthew, et al. "Plant Leaf Gas Exchange to Plant Phenotyping." Encyclopedia. Web. 20 December, 2023.
Copy Citation
Plant physiological status reflects the interaction between the plant genome and the prevailing growth conditions. Accurate characterization of plant physiology is, therefore, fundamental to effective plant phenotyping studies; particularly those focused on identifying traits associated with improved yield, lower input requirements, and climate resilience. Leaf gas exchange is a core component of plant physiological research. The exchange of gases between a part of the leaf, the whole leaf, or the entire plant with the atmosphere underpins photosynthetic CO2 uptake and transpiration.
plant phenotyping
photosynthesis
gas exchange
1. Introduction
Plant physiology is central to photosynthetic performance, growth, and resilience to abiotic stress [1][2]. Physiology represents the functional link between the environment and plant genome [3][4]. Characterization of plant physiology is fundamental to the utility of genome-wide association studies [5][6][7][8]. Analysis of plant physiological status is, therefore, an essential component of plant phenotyping, where the characteristics of a specific genotype are quantified under designated environmental conditions [9][10]. Traditional approaches to plant physiological analyses are often time-consuming and orientated towards a leaf- or organ-level measurement, precluding rapid screening and measurements over a wider canopy level suited to field-based phenotyping. However, given the importance of plant phenomics and precision digital agriculture to future food and bioenergy security [11][12], a number of advances in phenotyping approaches and technologies may reduce this ‘physiological bottleneck’ and facilitate wide-scale rapid analysis of plant physiological status [13][14]. The capability to infer physiological status over wide spatial scales at high temporal resolution is crucial for digital agriculture applications to optimize yield outputs and minimize resource inputs [15].
2. Leaf Gas Exchange
Leaf gas exchange is a core component of plant physiological research [16][17]. The exchange of gases between a part of the leaf, the whole leaf, or the entire plant with the atmosphere underpins photosynthetic CO2 uptake and transpiration [18][19]. The expansion in the availability of commercial plant photosynthetic gas exchange systems has led to their increased use in quantifying rates of photosynthesis, biochemical/diffusive limits to CO2 uptake, and stomatal physiological behaviour in regulating photosynthesis [20][21]. However, these plant photosynthesis systems are relatively complex and prone to mechanical or user error, e.g., [22]. This has impaired their utilization in wide-scale phenotyping studies.
Plant photosynthetic leaf gas exchange systems utilize infra-red gas analyzers to quantify fluxes of CO2 and water vapour. A leaf, or a portion of a leaf, is placed in an enclosed leaf cuvette where the concentrations of [CO2] and [H2O] in the air entering and then exiting the cuvette after passing over the leaf are measured. The delta values of [CO2] and [H2O] are then used to calculate parameters such as photosynthesis (PN), stomatal conductance (Gs), and the internal sub-stomatal concentration of CO2 within the leaf (Ci), as shown in [23] and the citations contained within. The external concentration of [CO2] outside the leaf (Ca), photosynthetic photon flux density (PPFD), temperature, relative humidity, leaf-to-air vapour pressure deficit, and velocity of air flow can be controlled within the leaf cuvette. This enables rapid manipulation of cuvette conditions to quantify responses to specific factors influencing photosynthesis and the exchange of gases between the leaf and the external atmosphere. Many plant photosynthesis systems also include the capacity to simultaneously measure chlorophyll fluorescence (ChlF) parameters alongside leaf gas exchange.
2.1. Instantaneous Point Measurements
Instantaneous point measurements give a ‘snap shot’ of leaf photosynthetic status. Point measurements are the simplest and most rapid type of leaf gas exchange measurement to perform, and are, therefore, the most widely used in plant phenotyping studies. Despite the relative simplicity of instantaneous point measurements, these measurements can be time-consuming. If set controlled conditions of PPFD, temperature, Ca, and leaf-to-air vapour pressure deficit are utilized within the leaf cuvette, each leaf requires a period of time to adjust to those cuvette conditions, precluding the ability to take large numbers of measurements at the same time/conditions in phenotyping trials of multiple genotypes. Less time-consuming instantaneous point measurements of leaf gas exchange parameters can be performed without using controlled cuvette conditions. Such measurements where PPFD and temperature track ‘ambient’ conditions do not require the same adjustment period for the leaf, but variations in ambient conditions, diminishes comparability between measurements, and may render any phenotypic or treatment effect indiscernible [24][25]. A significant disadvantage of these instantaneous point measurements of leaf gas exchange is that they only reflect photosynthetic status at a single point in time over a comparatively small area of leaf (~1.75 to 6 cm2). Moreover, leaf gas exchange measurements tend to be comparatively variable between individual plants (and sometimes between leaves of the same plant). A more comprehensive insight into leaf physiological processes that are relevant to phenotyping can be observed in the more detailed leaf gas exchange measurements outlined below; however, these measurements are more complex and time-consuming than instantaneous measurements, further reducing their applicability for high-throughput phenotyping.
2.2. Biochemical Efficiency of Photosynthesis
The rate of photosynthesis is determined by biochemical and diffusive constraints on the uptake and assimilation of CO2 [23][26]. The biochemical efficiency of photosynthesis is a key parameter in determining plant growth rate and crop yield [27][28][29][30][31][32][33], and, therefore, of primary importance to plant phenotyping applications. Leaf level rates of photosynthesis in many staple crops such as rice are relatively low [34]. Identification of genotypes with higher leaf level photosynthetic capacities has the potential to positively affect future crop yields [30][35][36], and identification of genotypes that retain biochemical assimilation during abiotic stress such as water deficit or drought can contribute to more climate-resilient agriculture [27][37]. However, analysis of the biochemical efficiency of leaf level photosynthesis is highly time-consuming, and it is not possible to rapidly and accurately assess large volumes of genotypes at sufficient levels of replication [38][39].
The most commonly used method to gauge plant photosynthetic capacity in vivo involves the use of photosynthetic leaf gas exchange systems to perform photosynthetic response curves, where the PN is measured relative to increasing steps in the concentration of [CO2] (commonly known as A–Ci curves where, instead of PN, A stands for the assimilation rate of CO2) (Figure 1).
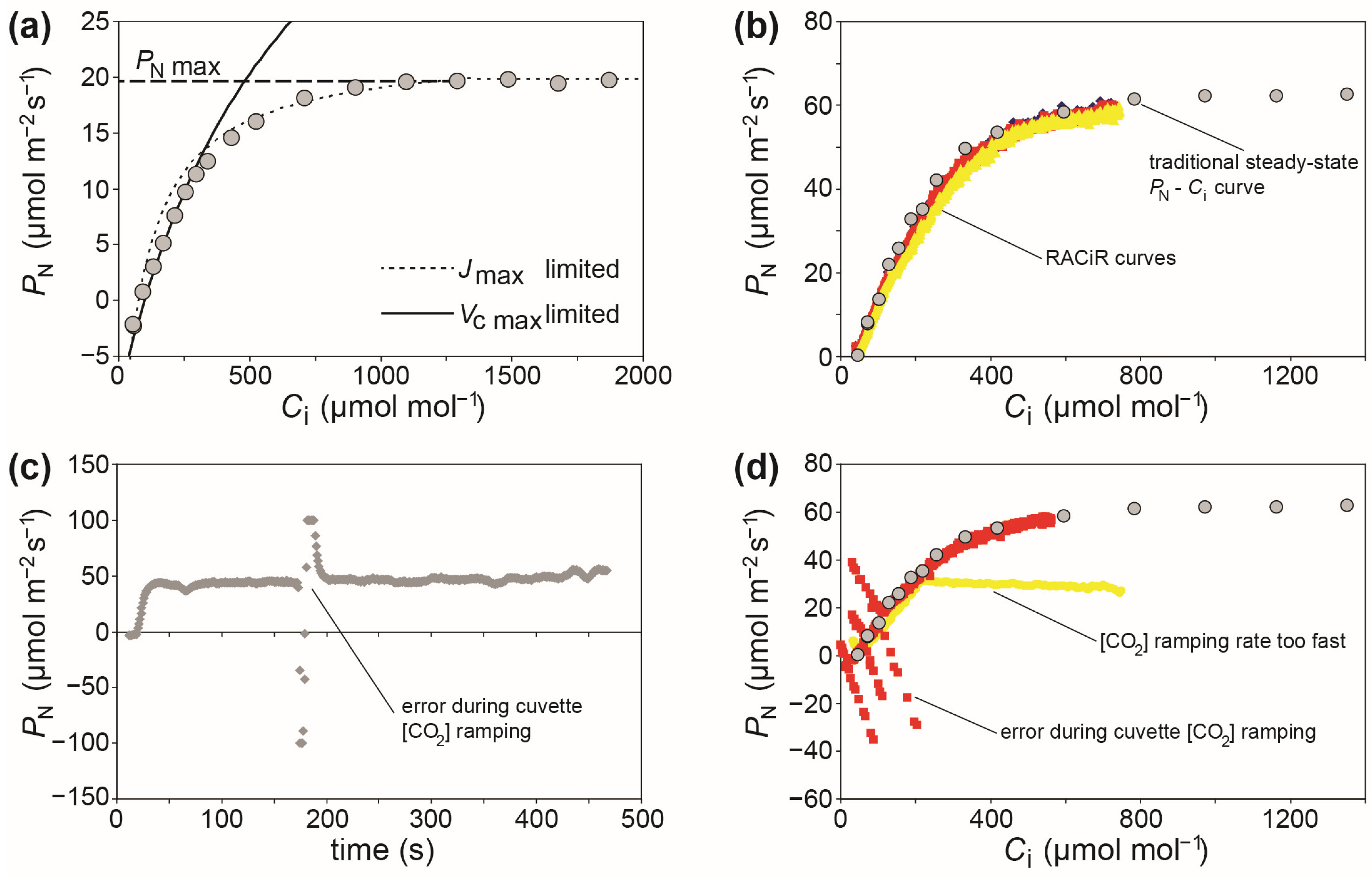
Figure 1. (a) PN–Ci response curve (Olea europaea L.) showing the stage of the curve limited by the rate of electron transport required for ribulose-1,5-bisphosphate (RuBP) regeneration (Jmax), the part of the curve limited by the carboxylation capacity of ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) (Vcmax), and the maximum rate of photosynthesis at PARsat and high [CO2] (PNmax), grey circles indicate steady state measurements of photosynthetic gas exchange taken at each [CO2] level; (b) RACiR curves (red, yellow, and blue symbols) overlain with a traditional PN–Ci steady state response curve (Phragmites australis (Cav.) Trin. ex Steud.); (c) example of an error during [CO2] ramping that can affect post-processing of a RACiR curve, and; (d) the results of the [CO2] ramping error outlined in (c) on the corrected RACiR curve (red symbols) of a P. australis leaf, alongside a RACiR error caused by the use of an excessively fast [CO2] ramping rate (yellow symbols).
Figure 1a shows a typical PN–Ci curve characteristic of a C3 plant Michaelis–Menten enzymatic reaction. Photosynthesis is limited by the availability of CO2 at the initial part of the curve, and this represents the maximum rate of carboxylation of ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) (Vcmax). At higher levels of [CO2], PN is no longer limited by substrate availability, but the regeneration of ribulose-1,5-bisphosphate (RuBP) (Jmax). The parameters Vcmax and Jmax are critical components of crop and climate models [19][40][41], and would likely be integrated into digital agriculture applications [42][43]; thus, demonstrating their importance to phenotyping of plant physiology [30]. More detailed reviews of PN–Ci curves can be found in Sharkey et al. [21], Centritto et al. [44], Ethier and Livingston [45], and Duursma [20]. The parameters derived from these PN–Ci curves are fundamental to the characterization of the biochemical efficiency of PN, but these measurements are time-consuming (each curve taking ~40–120 min), prone to error, and require expensive equipment operated by staff trained in leaf gas exchange. This makes traditional steady state photosynthetic response curves unsuited to high-throughput phenotyping systems [38][39][46].
Given the importance of the biochemical efficiency of photosynthetic CO2 assimilation to crop productivity [24][30], attempts have been made to adapt these measures for use in phenomic applications. Newer plant photosynthetic gas exchange systems are able to continuously ‘ramp’ [CO2] in the leaf cuvette and dynamically measure the concurrent effect on PN. These response curves are known as RACiR curves, standing for “Rapid A-Ci Response” [46]. The RACiR curves can be performed in ~5–15 min, significantly increasing the capability to perform detailed physiological analysis of photosynthetic capacity [31][46][47][48][49]. However, a number of limitations constrain the potential widespread application of RACiR curves: (1) RACiR curve measurements require set-up time to perform empty cuvette measurements to determine the lag in the system between the reference and sample IRGAs [46]; (2) RACiR curves require a degree of prior knowledge of the plant species under analysis (for example, if there is a wide range of Gs values of plant varieties in a phenotyping study, this may require adjustment of the [CO2] ramping speed between varieties) [50][51]; (3) preparation of the RACiR curves involves more extensive processing of data after measurements than traditional PN–Ci curves, and this reduces the opportunity to identify and correct any errors during measurements (Figure 1b), and; (4) variations associated with photosystem I electron transport dynamics [52]. To minimize some of these limitations by eliminating data post-processing, a recent modification of the method known as the single-step CO2 response (SSCO2R) has been proposed. This involves equalizing the pathways (or air volume) travelled by the reference and analysis sample air flows to the respective IRGAs during the CO2 ramping process [53].
The parameters Vcmax and Jmax are highly significant to models of photosynthesis [26][54], stomatal behaviour [55][56][57], crop yield [58][59], and climate modelling [19], but the application of Vcmax and Jmax is constrained by the length of time and the complexities outlined above in performing sufficient numbers of reliable PN–Ci curves. To speed up data collection, Vcmax has also been calculated from single-point measurements of light-saturated photosynthesis (PN sat) [60][61]. This ‘one-point method’ assumes that in the light-saturated state, the leaves are limited by CO2 availability, allowing calculation of Vcmax from the C3 photosynthesis model [26][62]. Accurate prediction of Vcmax from a single-point measurement of leaf gas exchange requires the PN of the leaf to be limited by CO2 availability, knowledge/estimation of respiration in the light, and for the leaf to be fully acclimated to saturating PPFD. In cases where these conditions are not met, estimates of Vcmax may be rendered inaccurate [63][64]. This uncertainty leaves the more time-consuming PN–Ci curves as the benchmark methodology for the determination of Vcmax and Jmax.
2.3. Light-Use Efficiency and Photoprotection
The conversion of light energy to sugars underpins plant growth, and is central to crop yield [65]. However, excess light can induce photo-oxidative stress through the production of reactive oxygen species [66]. During episodes of abiotic stress, such as drought, when the capacity to utilize energy for photochemistry declines, a greater proportion of the absorbed light energy must be dissipated as heat or re-emitted at a longer, less energetic wavelength as ChlF [67][68]. It is, therefore, important for crop phenotyping efforts to enhance productivity and climate resilience to characterize the physiology of light harvesting and photoprotection.
Photosynthetic light capture and use can be analyzed a number of ways using leaf gas exchange, often in combination with ChlF [69]. The most common approach is to measure PN at decreasing/increasing steps of PPFD using the LED lights within the plant photosynthesis system leaf cuvette. This enables calculation of the maximum quantum efficiency (ΦCO2), the light saturation point (PPFD above which PN no longer increases: PN sat), identification of levels of PPFD where photoinhibition may occur [38][70], and respiration in both the light (Rlight) and the dark (Rdark) [71]. These physiological parameters are useful in identifying crop varieties with greater quantum efficiencies that are more likely to correlate to yield [72].
Light response curves incur many of the same impediments for high-throughput phenotyping as PN–Ci curves. Despite their utility in the accurate characterization of light harvesting and protective physiologies, light response curves using leaf gas exchange are likely to be unsuitable for phenotyping in comparison to the leaf-level sensor and remote-sensing-based approaches outlined below. Nevertheless, light response curves derived from leaf gas exchange may still be required to ‘support’ data and observations derived from more rapid approaches suited to high-throughput phenotyping.
Photosynthetic Response to Variable Growth Conditions
Plant responses to variations in radiation are becoming increasingly important to efforts to improve crop productivity [65][73][74]. Under normal growing conditions in the field, the light environment experienced by leaves can be highly variable (passing clouds, temporary shading from other leaves in the canopy, changes in leaf orientation as the canopy moves due to wind, etc.) Small increases in the efficiency of plants to use this heterogeneous light would translate into improved yields when scaled over whole canopies and an entire growing season [74][75]. The mechanisms involved in the transitions between photochemical and protective energy dissipation may be evident in canopy-level measurement of sun-induced fluorescence [76]. This improved efficiency in photosynthetic light harvesting is related to the physiological processes involved in the transitions between light capture and energy dissipation, and also physiological stomatal behaviour through stomatal control of PN [77][78].
Stomatal physiological regulation plays a central role in plant carbon and water efficiencies [79][80]. Analysis of Gs values over time to changes in factors such as PPFD, [CO2], chemical signals of drought, or leaf-to air vapour pressure deficit enables quantification of stomatal physiological responsiveness [81][82][83][84]. These ‘stomatal kinetics’ are performed by placing a leaf within a cuvette and monitoring Gs over time while cuvette conditions are adjusted [38]. Stomatal kinetic responses can be used to show physiological differences between varieties that may translate into enhanced productivity [85][86], resilience to stresses such as drought [83][87], or fumigation with atmospheric pollutants [88]. However, measurement of stomatal kinetics using leaf gas exchange is particularly time-consuming given the requirement for the leaf to completely adjust to cuvette conditions prior to recording the full extent of any response to a change in cuvette conditions and the inherent variability in many Gs measurements requiring high numbers of replicates for statistical robustness [38].
References
- Xue, Y.; Bai, X.; Zhao, C.; Tan, Q.; Li, Y.; Luo, G.; Wu, L.; Chen, F.; Li, C.; Ran, C.; et al. Spring photosynthetic phenology of Chinese vegetation in response to climate change and its impact on net primary productivity. Agric. For. Meteorol. 2023, 342, 109734.
- Pinheiro, C.; Chaves, M.M. Photosynthesis and drought: Can we make metabolic connections from available data? J. Exp. Bot. 2011, 62, 869–882.
- Kumar, A.; Pathak, R.K.; Gupta, S.M.; Gaur, V.S.; Pandey, D. Systems biology for smart crops and agricultural innovation: Filling the gaps between genotype and phenotype for complex traits linked with robust agricultural productivity and sustainability. OMICS J. Integr. Biol. 2015, 19, 581–601.
- York, L.M. Functional phenomics: An emerging field integrating high-throughput phenotyping, physiology, and bioinformatics. J. Exp. Bot. 2018, 70, 379–386.
- Chen, W.; Wang, W.; Peng, M.; Gong, L.; Gao, Y.; Wan, J.; Wang, S.; Shi, L.; Zhou, B.; Li, Z. Comparative and parallel genome-wide association studies for metabolic and agronomic traits in cereals. Nat. Commun. 2016, 7, 12767.
- Zhu, F.; Ahchige, M.W.; Brotman, Y.; Alseekh, S.; Zsögön, A.; Fernie, A.R. Bringing more players into play: Leveraging stress in genome wide association studies. J. Plant Physiol. 2022, 271, 153657.
- Ro, N.; Haile, M.; Ko, H.-C.; Cho, G.-T.; Lee, J.; Kim, B.; Lee, S.; Kim, S.-H. Genome-wide association study of phenolic content and antioxidant properties in eggplant germplasm. Genes 2023, 14, 1315.
- Alvarez-Morezuelas, A.; Barandalla, L.; Ritter, E.; Ruiz de Galarreta, J.I. Genome-wide association study of agronomic and physiological traits related to drought tolerance in potato. Plants 2023, 12, 734.
- Baslam, M.; Mitsui, T.; Hodges, M.; Priesack, E.; Herritt, M.T.; Aranjuelo, I.; Sanz-Sáez, Á. Photosynthesis in a changing global climate: Scaling up and scaling down in crops. Front. Plant Sci. 2020, 11, 882.
- Prado, S.A.; Cabrera-Bosquet, L.; Grau, A.; Coupel-Ledru, A.; Millet, E.J.; Welcker, C.; Tardieu, F. Phenomics allows identification of genomic regions affecting maize stomatal conductance with conditional effects of water deficit and evaporative demand. Plant Cell Environ. 2018, 41, 314–326.
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428.
- Gebbers, R.; Adamchuk, V.I. Precision agriculture and food security. Science 2010, 327, 828–831.
- Tardieu, F.; Cabrera-Bosquet, L.; Pridmore, T.; Bennett, M. Plant phenomics, from sensors to knowledge. Curr. Biol. 2017, 27, R770–R783.
- Furbank, R.T.; Tester, M. Phenomics–technologies to relieve the phenotyping bottleneck. Trends Plant Sci. 2011, 16, 635–644.
- Sun, P.; Wahbi, S.; Tsonev, T.; Haworth, M.; Liu, S.; Centritto, M. On the use of leaf spectral indices to assess water status and photosynthetic limitations in Olea europaea L. during water-stress and recovery. PLoS ONE 2014, 9, e105165.
- Long, S.P. Leaf Gas Exchange. In Photosynthetic Mechanisms and the Environment; Barber, J., Baker, N.R., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1985; pp. 453–499.
- Heath, O.V.S. Studies in stomatal behaviour. V. The role of carbon dioxide in the light response of stomata. J. Exp. Bot. 1950, 1, 29–62.
- Lammertsma, E.I.; Boer, H.J.D.; Dekker, S.C.; Dilcher, D.L.; Lotter, A.F.; Wagner-Cremer, F. Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc. Natl. Acad. Sci. USA 2011, 108, 4035–4040.
- Rogers, A. The use and misuse of Vcmax in Earth System Models. Photosynth. Res. 2014, 119, 15–29.
- Duursma, R.A. Plantecophys—An R Package for Analysing and Modelling Leaf Gas Exchange Data. PLoS ONE 2015, 10, e0143346.
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C-3 leaves. Plant Cell Environ. 2007, 30, 1035–1040.
- Rodeghiero, M.; Niinemets, Ü.; Cescatti, A. Major diffusion leaks of clamp-on leaf cuvettes still unaccounted: How erroneous are the estimates of Farquhar et al. model parameters? Plant Cell Environ. 2007, 30, 1006–1022.
- Von Caemmerer, S. Biochemical Models of Leaf Photosynthesis; Csiro Publishing: Collingwood, Australia, 2000; p. 152.
- Haworth, M.; Moser, G.; Raschi, A.; Kammann, C.; Grünhage, L.; Müller, C. Carbon dioxide fertilisation and supressed respiration induce enhanced spring biomass production in a mixed species temperate meadow exposed to moderate carbon dioxide enrichment. Funct. Plant Biol. 2016, 43, 26–39.
- Haworth, M.; Gallagher, A.; Elliott-Kingston, C.; Raschi, A.; Marandola, D.; McElwain, J.C. Stomatal index responses of Agrostis canina to carbon dioxide and sulphur dioxide: Implications for palaeo- using the stomatal proxy. New Phytol. 2010, 188, 845–855.
- Farquhar, G.D.; Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90.
- Killi, D.; Bussotti, F.; Raschi, A.; Haworth, M. Adaptation to high temperature mitigates the impact of water deficit during combined heat and drought stress in C3 sunflower and C4 maize varieties with contrasting drought tolerance. Physiol. Plant. 2017, 159, 130–147.
- Sun, D.; Robbins, K.; Morales, N.; Shu, Q.; Cen, H. Advances in optical phenotyping of cereal crops. Trends Plant Sci. 2022, 27, 191–208.
- McAusland, L.; Atkinson, J.A.; Lawson, T.; Murchie, E.H. High throughput procedure utilising chlorophyll fluorescence imaging to phenotype dynamic photosynthesis and photoprotection in leaves under controlled gaseous conditions. Plant Methods 2019, 15, 109.
- Silva-Pérez, V.; De Faveri, J.; Molero, G.; Deery, D.M.; Condon, A.G.; Reynolds, M.P.; Evans, J.R.; Furbank, R.T. Genetic variation for photosynthetic capacity and efficiency in spring wheat. J. Exp. Bot. 2020, 71, 2299–2311.
- Pilon, C.; Snider, J.L.; Sobolev, V.; Chastain, D.R.; Sorensen, R.B.; Meeks, C.D.; Massa, A.N.; Walk, T.; Singh, B.; Earl, H.J. Assessing stomatal and non-stomatal limitations to carbon assimilation under progressive drought in peanut (Arachis hypogaea L.). J. Plant Physiol. 2018, 231, 124–134.
- De Souza, A.P.; Long, S.P. Toward improving photosynthesis in cassava: Characterizing photosynthetic limitations in four current African cultivars. Food Energy Secur. 2018, 7, e00130.
- Pinheiro, C.; Emiliani, G.; Marino, G.; Fortunato, A.S.; Haworth, M.; De Carlo, A.; Chaves, M.M.; Loreto, F.; Centritto, M. Metabolic background, not photosynthetic physiology, determines drought and drought recovery responses in C3 and C2 Moricandias. Int. J. Mol. Sci. 2023, 24, 4094.
- Lauteri, M.; Haworth, M.; Serraj, R.; Monteverdi, M.C.; Centritto, M. Photosynthetic diffusional constraints affect yield in drought stressed rice cultivars during flowering. PLoS ONE 2014, 9, e109054.
- Sudhakar, P.; Latha, P.; Reddy, P. Phenotyping Crop Plants for Physiological and Biochemical Traits; Academic Press: Cambridge, MA, USA, 2016.
- Costa, J.M.; Marques da Silva, J.; Pinheiro, C.; Barón, M.; Mylona, P.; Centritto, M.; Haworth, M.; Loreto, F.; Uzilday, B.; Turkan, I.; et al. Opportunities and limitations of crop phenotyping in southern European countries. Front. Plant Sci. 2019, 10, 1125.
- Killi, D.; Haworth, M. Diffusive and metabolic constraints to photosynthesis in quinoa during drought and salt stress. Plants 2017, 6, 49–64.
- Haworth, M.; Marino, G.; Centritto, M. An introductory guide to gas exchange analysis of photosynthesis and its application to plant phenotyping and precision irrigation to enhance water use efficiency. J. Water Clim. Chang. 2018, 9, 786–808.
- van Bezouw, R.F.; Keurentjes, J.J.; Harbinson, J.; Aarts, M.G. Converging phenomics and genomics to study natural variation in plant photosynthetic efficiency. Plant J. 2019, 97, 112–133.
- Thompson, A.L.; Thorp, K.R.; Conley, M.; Andrade-Sanchez, P.; Heun, J.T.; Dyer, J.M.; White, J.W. Deploying a proximal sensing cart to identify drought-adaptive traits in upland cotton for high-throughput phenotyping. Front. Plant Sci. 2018, 9, 507.
- Williams, K.; Gornall, J.; Harper, A.; Wiltshire, A.; Hemming, D.; Quaife, T.; Arkebauer, T.; Scoby, D. Evaluation of JULES-crop performance against site observations of irrigated maize from Mead, Nebraska. Geosci. Model Dev. 2017, 10, 1291–1320.
- Barnes, M.L.; Breshears, D.D.; Law, D.J.; Van Leeuwen, W.J.; Monson, R.K.; Fojtik, A.C.; Barron-Gafford, G.A.; Moore, D.J. Beyond greenness: Detecting temporal changes in photosynthetic capacity with hyperspectral reflectance data. PLoS ONE 2017, 12, e0189539.
- Yu, Y.; Yang, X.; Fan, W. Remote sensing inversion of leaf maximum carboxylation rate based on a mechanistic photosynthetic model. IEEE Trans. Geosci. Remote Sens. 2021, 60, 1–12.
- Centritto, M.; Loreto, F.; Chartzoulakis, K. The use of low to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant Cell Environ. 2003, 26, 585–594.
- Ethier, G.J.; Livingston, N.J. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant Cell Environ. 2004, 27, 137–153.
- Stinziano, J.R.; Morgan, P.B.; Lynch, D.J.; Saathoff, A.J.; McDermitt, D.K.; Hanson, D.T. The rapid A–Ci response: Photosynthesis in the phenomic era. Plant Cell Environ. 2017, 40, 1256–1262.
- Coursolle, C.; Prud’homme, G.O.; Lamothe, M.; Isabel, N. Measuring rapid A–Ci curves in boreal conifers: Black spruce and balsam fir. Front. Plant Sci. 2019, 10, 1276.
- Lawrence, E.H.; Stinziano, J.R.; Hanson, D.T. Using the rapid A-Ci response (RACiR) in the Li-Cor 6400 to measure developmental gradients of photosynthetic capacity in poplar. Plant Cell Environ. 2019, 42, 740–750.
- Bunce, J. Three methods of estimating mesophyll conductance agree regarding its CO2 sensitivity in the RubisCO-limited Ci range. Plants 2018, 7, 62.
- Stinziano, J.R.; Adamson, R.K.; Hanson, D.T. Using multirate rapid A/Ci curves as a tool to explore new questions in the photosynthetic physiology of plants. New Phytol. 2019, 222, 785–792.
- Taylor, S.H.; Long, S.P. Phenotyping photosynthesis on the limit–a critical examination of RACiR. New Phytol. 2019, 221, 621–624.
- McClain, A.M.; Sharkey, T.D. Rapid CO2 changes cause oscillations in photosynthesis that implicate PSI acceptor-side limitations. J. Exp. Bot. 2023, 74, erad084.
- PP-Systems. The Single-Step CO2 Response (SSCO2R™) Method—Rapid A/Ci Curves in Real Time Without Post Processing. Available online: https://ppsystems.com/wp-content/uploads/AN_CIRAS-4_SSCO2R-Method.pdf (accessed on 25 November 2023).
- Walker, A.P.; Beckerman, A.P.; Gu, L.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The relationship of leaf photosynthetic traits–Vcmax and Jmax–to leaf nitrogen, leaf phosphorus, and specific leaf area: A meta-analysis and modeling study. Ecol. Evol. 2014, 4, 3218–3235.
- Konrad, W.; Roth-Nebelsick, A.; Grein, M. Modelling of stomatal density response to atmospheric CO2. J. Theor. Biol. 2008, 253, 638–658.
- Roth-Nebelsick, A.; Oehm, C.; Grein, M.; Utescher, T.; Kunzmann, L.; Friedrich, J.-P.; Konrad, W. Stomatal density and index data of Platanus neptuni leaf fossils and their evaluation as a CO2 proxy for the Oligocene. Rev. Palaeobot. Palynol. 2014, 206, 1–9.
- Haworth, M.; Killi, D.; Materassi, A.; Raschi, A.; Centritto, M. Impaired stomatal control is associated with reduced photosynthetic physiology in crop species grown at elevated . Front. Plant Sci. 2016, 7, 1568.
- Evers, J.B.; Vos, J.; Yin, X.; Romero, P.; van der Putten, P.E.L.; Struik, P.C. Simulation of wheat growth and development based on organ-level photosynthesis and assimilate allocation. J. Exp. Bot. 2010, 61, 2203–2216.
- Peng, B.; Guan, K.; Chen, M.; Lawrence, D.M.; Pokhrel, Y.; Suyker, A.; Arkebauer, T.; Lu, Y. Improving maize growth processes in the community land model: Implementation and evaluation. Agric. For. Meteorol. 2018, 250–251, 64–89.
- Kattge, J.; Knorr, W.; Raddatz, T.; Wirth, C. Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob. Chang. Biol. 2009, 15, 976–991.
- Niinemets, Ü. Research review. Components of leaf dry mass per area–thickness and density–alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol. 1999, 144, 35–47.
- Laisk, A. Kinetics of Photosynthesis and Photorespiration in C3 Plants; Science Research: Moscow, Russia, 1977. (In Russian)
- Burnett, A.C.; Davidson, K.; Serbin, S.P.; Rogers, A. The ‘one-point method’for estimating maximum carboxylation capacity of photosynthesis: A cautionary tale. Plant Cell Environ. 2019, 42, 2472–2481.
- De Kauwe, M.G.; Lin, Y.S.; Wright, I.J.; Medlyn, B.E.; Crous, K.Y.; Ellsworth, D.S.; Maire, V.; Prentice, I.C.; Atkin, O.K.; Rogers, A. A test of the ‘one-point method’for estimating maximum carboxylation capacity from field-measured, light-saturated photosynthesis. New Phytol. 2016, 210, 1130–1144.
- Slattery, R.A.; Ort, D.R. Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiol. 2021, 185, 34–48.
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative stress in plants. Physiol. Plant. 1994, 92, 696–717.
- Killi, D.; Raschi, A.; Bussotti, F. Lipid peroxidation and chlorophyll fluorescence of Photosystem II performance during drought and heat stress is associated with the antioxidant capacities of C3 sunflower and C4 maize varieties. Int. J. Mol. Sci. 2020, 21, 4846.
- Dias, M.C.; Correia, S.; Serôdio, J.; Silva, A.M.S.; Freitas, H.; Santos, C. Chlorophyll fluorescence and oxidative stress endpoints to discriminate olive cultivars tolerance to drought and heat episodes. Sci. Hortic. 2018, 231, 31–35.
- Yin, X.; Sun, Z.; Struik, P.C.; Gu, J. Evaluating a new method to estimate the rate of leaf respiration in the light by analysis of combined gas exchange and chlorophyll fluorescence measurements. J. Exp. Bot. 2011, 62, 3489–3499.
- Lobo, F.D.A.; De Barros, M.; Dalmagro, H.; Dalmolin, Â.; Pereira, W.; de Souza, É.; Vourlitis, G.; Ortíz, C.R. Fitting net photosynthetic light-response curves with Microsoft Excel—A critical look at the models. Photosynthetica 2013, 51, 445–456.
- Kok, B. A critical consideration of the quantum yield of Chlorella photosynthesis. Enzymologia 1948, 13, 1–56.
- Riggi, E.; Avola, G.; Marino, G.; Haworth, M.; Cosentino, S.L.; Centritto, M. Open field experiment for the evaluation of Arundo donax ecotypes ecophysiology and yield as affected by soil water content. Ind. Crops Prod. 2019, 140, 111630.
- De Souza, A.P.; Burgess, S.J.; Doran, L.; Hansen, J.; Manukyan, L.; Maryn, N.; Gotarkar, D.; Leonelli, L.; Niyogi, K.K.; Long, S.P. Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 2022, 377, 851–854.
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861.
- Sakowska, K.; Alberti, G.; Genesio, L.; Peressotti, A.; Vedove, G.D.; Gianelle, D.; Colombo, R.; Rodeghiero, M.; Panigada, C.; Juszczak, R.; et al. Leaf and canopy photosynthesis of a chlorophyll deficient soybean mutant. Plant Cell Environ. 2018, 41, 1427–1437.
- Acebron, K.; Matsubara, S.; Jedmowski, C.; Emin, D.; Muller, O.; Rascher, U. Diurnal dynamics of nonphotochemical quenching in Arabidopsis npq mutants assessed by solar-induced fluorescence and reflectance measurements in the field. New Phytol. 2021, 229, 2104–2119.
- Haworth, M.; Cosentino, S.L.; Marino, G.; Brunetti, C.; Riggi, E.; Avola, G.; Loreto, F.; Centritto, M. Increased free abscisic acid during drought enhances stomatal sensitivity and modifies stomatal behaviour in fast growing giant reed (Arundo donax L.). Environ. Exp. Bot. 2018, 147, 116–124.
- Gerardin, T.; Douthe, C.; Flexas, J.; Brendel, O. Shade and drought growth conditions strongly impact dynamic responses of stomata to variations in irradiance in Nicotiana tabacum. Environ. Exp. Bot. 2018, 153, 188–197.
- Cowan, I.R. Stomatal behaviour and environment. Adv. Bot. Res. 1978, 4, 117–228.
- Raschke, K. How stomata resolve the dilemma of opposing priorities. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1976, 273, 551–560.
- Haworth, M.; Killi, D.; Materassi, A.; Raschi, A. Co-ordination of stomatal physiological behavior and morphology with carbon dioxide determines stomatal control. Am. J. Bot. 2015, 102, 677–688.
- Elliott-Kingston, C.; Haworth, M.; Yearsley, J.M.; Batke, S.P.; Lawson, T.; McElwain, J.C. Does size matter? Atmospheric CO2 may be a stronger driver of stomatal closing rate than stomatal size in taxa that diversified under low CO2. Front. Plant Sci. 2016, 7, 1253.
- Durand, M.; Brendel, O.; Buré, C.; Le Thiec, D. Altered stomatal dynamics induced by changes in irradiance and vapour-pressure deficit under drought: Impacts on the whole-plant transpiration efficiency of poplar genotypes. New Phytol. 2019, 222, 1789–1802.
- Doi, M.; Kitagawa, Y.; Shimazaki, K.-i. Stomatal blue light response is present in early vascular plants. Plant Physiol. 2015, 169, 1205–1213.
- Haworth, M.; Marino, G.; Loreto, F.; Centritto, M. Integrating stomatal physiology and morphology: Evolution of stomatal control and development of future crops. Oecologia 2021, 197, 867–883.
- Sillo, F.; Marino, G.; Franchi, E.; Haworth, M.; Zampieri, E.; Pietrini, I.; Fusini, D.; Mennone, C.; Centritto, M.; Balestrini, R. Impact of irrigation water deficit on two tomato genotypes grown under open field conditions: From the root-associated microbiota to the stress responses. Ital. J. Agron. 2022, 17, 3.
- McAusland, L.; Vialet-Chabrand, S.; Davey, P.; Baker, N.R.; Brendel, O.; Lawson, T. Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytol. 2016, 211, 1209–1220.
- Agathokleous, E.; Kitao, M.; Hoshika, Y.; Haworth, M.; Tang, Y.; Koike, T. Ethylenediurea protects against ozone phytotoxicity not by adding nitrogen or controlling stomata in a stomata-unresponsive hybrid poplar. Sci. Total Environ. 2023, 875, 162672.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
571
Revisions:
2 times
(View History)
Update Date:
22 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




