
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio Stecco | -- | 2152 | 2023-12-20 10:57:47 | | | |
| 2 | Lindsay Dong | + 1 word(s) | 2153 | 2023-12-22 02:37:49 | | |
Video Upload Options
The mechanical stress can depolymerize into small pieces at low molecular weight and have a high inflammatory capacity. Many of these pieces are then further degraded into small oligosaccharides. Recently, it has been demonstrated that oligosaccharides are able to stop this inflammatory process. These data support that deep friction could metabolize self-aggregated hyaluronan (HA) chains responsible for increasing loose connective tissue viscosity, catalyzing a local HA fragment cascade that will generate soreness but, at the same time, facilitate the reconstitution of the physiological loose connective tissue properties. This information can help to explain the meaning of the inflammatory process as well as the requirement for it for the long-lasting resolution of these alterations.
1. Introduction
2. Mechanical Stress on Hyaluronan Fragments’ Inflammatory Cascade
2.1. The Role of HA Weight: A Decremental Cascade during Inflammation
2.2. The Inflammation Cascade: Influencing Factors
2.3. HA Polymer Fragments: Diverse Biological Activities
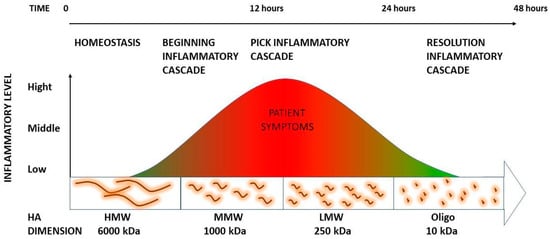
2.4. Summary
References
- Paanalahti, K.; Holm, L.W.; Nordin, M.; Asker, M.; Lyander, J.; Skillgate, E. Adverse events after manual therapy among patients seeking care for neck and/or back pain: A randomized controlled trial. BMC Musculoskelet. Disord. 2014, 15, 77.
- Fidut-Wrońska, J.; Chołuj, K.; Chmiel, J.; Pikto-Pitkiewicz, K.; Majcher, P. Observation using thermography of post-operative reaction after fascial manipulation®. Ann. Agric. Environ. Med. 2019, 26, 468–471.
- Stecco, C.; Day, J.A. The fascial manipulation technique and its biomechanical model: A guide to the human fascial system. Int. J. Ther. Massage Bodyw. 2010, 3, 38–40.
- Day, J.A.; Copetti, L.; Rucli, G. From clinical experience to a model for the human fascial system. J. Bodyw. Mov. Ther. 2012, 16, 372–380.
- Pintucci, M.; Simis, M.; Imamura, M.; Pratelli, E.; Stecco, A.; Ozcakar, L.; Battistella, L.R. Successful treatment of rotator cuff tear using Fascial Manipulation® in a stroke patient. J. Bodyw. Mov. Ther. 2017, 21, 653–657.
- Branchini, M.; Lopopolo, F.; Andreoli, E.; Loreti, I.; Marchand, A.M.; Stecco, A. Fascial Manipulation® for chronic aspecific low back pain: A single blinded randomized controlled trial. F1000Res 2015, 4, 1208.
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Res 2015, 4, 622.
- Lee, J.Y.; Spicer, A.P. Hyaluronan: A multifunctional, megaDalton, stealth molecule. Curr. Opin. Cell Biol. 2000, 12, 581–586.
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic Acid: Redefining Its Role. Cells 2020, 9, 1743.
- Anderegg, U.; Simon, J.C.; Averbeck, M. More than just a filler—The role of hyaluronan for skin homeostasis. Exp. Dermatol. 2014, 23, 295–303.
- Lee, D.H.; Oh, J.H.; Chung, J.H. Glycosaminoglycan and proteoglycan in skin aging. J. Dermatol. Sci. 2016, 83, 174–181.
- Tømmeraas, K.; Melander, C. Kinetics of hyaluronan hydrolysis in acidic solution at various pH values. Biomacromolecules 2008, 9, 1535–1540.
- Menon, R.G.; Oswald, S.F.; Raghavan, P.; Regatte, R.R.; Stecco, A. T1ρ-Mapping for Musculoskeletal Pain Diagnosis: Case Series of Variation of Water Bound Glycosaminoglycans Quantification before and after Fascial Manipulation® in Subjects with Elbow Pain. Int. J. Environ. Res. Public Health 2020, 17, 708.
- Menon, R.G.; Raghavan, P.; Regatte, R.R. Quantifying muscle glycosaminoglycan levels in patients with post-stroke muscle stiffness using T1ρ MRI. Sci. Rep. 2019, 9, 14513.
- Han, W.; Lv, Y.; Sun, Y.; Wang, Y.; Zhao, Z.; Shi, C.; Chen, X.; Wang, L.; Zhang, M.; Wei, B.; et al. The anti-inflammatory activity of specific-sized hyaluronic acid oligosaccharides. Carbohydr. Polym. 2022, 276, 118699, Erratum in Carbohydr. Polym. 2022, 282, 119101.
- Bohaumilitzky, L.; Huber, A.K.; Stork, E.M.; Wengert, S.; Woelfl, F.; Boehm, H. A Trickster in Disguise: Hyaluronan’s Ambivalent Roles in the Matrix. Front. Oncol. 2017, 7, 242.
- Laurent, T.C.; Fraser, J.R. Hyaluronan. FASEB J. 1992, 6, 2397–2404.
- Cowman, M.K. Hyaluronan and Hyaluronan Fragments. Adv. Carbohydr. Chem. Biochem. 2017, 74, 1–59.
- Balazs, E.A. Viscoelastic properties of hyaluronic acid and biological lubrication. Univ. Mich. Med. Cent. J. 1968, 1, 255–259.
- Anderegg, U.; Halfter, N.; Schnabelrauch, M.; Hintze, V. Collagen/glycosaminoglycan-based matrices for controlling skin cell responses. Biol. Chem. 2021, 402, 1325–1335.
- Spicer, A.P.; Tien, J.Y. Hyaluronan and morphogenesis. Birth Defects Res. C Embryo Today 2004, 72, 89–108.
- Garg, H.; Hales, C. Chemistry and Biology of Hyaluronan, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004.
- Yu, L.; Quinn, D.A.; Garg, H.G.; Hales, C.A. Cyclin-dependent kinase inhibitor p27Kip1, but not p21WAF1/Cip1, is required for inhibition of hypoxia-induced pulmonary hypertension and remodeling by heparin in mice. Circ. Res. 2005, 97, 937–945.
- Heldin, P. Chemistry and Biology of Hyaluronan. Edited by Hari G. Garg and Charles A. Hales. ChemBioChem 2005, 6, 1288–1289.
- Tian, X.; Azpurua, J.; Hine, C.; Vaidya, A.; Myakishev-Rempel, M.; Ablaeva, J.; Mao, Z.; Nevo, E.; Gorbunova, V.; Seluanov, A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 2013, 499, 346–349.
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092.
- Parsons, B.J.; Spickett, C.M. Special issue on “Analytical methods for the detection of oxidized biomolecules and antioxidants”. Free Radic. Res. 2015, 49, 473–476.
- Kasai, S.; Furuichi, Y.; Ando, N.; Kagami, K.; Abe, M.; Nakane, T.; Goi, K.; Inukai, T.; Saitoh, S.; Ohno, S.; et al. Inflammatory mediator ultra-low-molecular-weight hyaluronan triggers necrosis of B-precursor leukemia cells with high surface CD44 expression. Cell Death Dis. 2017, 8, e2857.
- Stern, R. Hyaluronan catabolism: A new metabolic pathway. Eur. J. Cell Biol. 2004, 83, 317–325.
- Avenoso, A.; Bruschetta, G.; D’Ascola, A.; Scuruchi, M.; Mandraffino, G.; Saitta, A.; Campo, S.; Campo, G.M. Hyaluronan Fragmentation During Inflammatory Pathologies: A Signal that Empowers Tissue Damage. Mini Rev. Med. Chem. 2020, 20, 54–65.
- Horton, M.R.; Shapiro, S.; Bao, C.; Lowenstein, C.J.; Noble, P.W. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J. Immunol. 1999, 162, 4171–4176.
- Yamasaki, K.; Muto, J.; Taylor, K.R.; Cogen, A.L.; Audish, D.; Bertin, J.; Grant, E.P.; Coyle, A.J.; Misaghi, A.; Hoffman, H.M.; et al. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J. Biol. Chem. 2009, 284, 12762–12771.
- Harada, H.; Takahashi, M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 2007, 282, 5597–5607.
- Powell, J.D.; Horton, M.R. Threat matrix: Low-molecular-weight hyaluronan (HA) as a danger signal. Immunol. Res. 2005, 31, 207–218.
- Tolg, C.; McCarthy, J.B.; Yazdani, A.; Turley, E.A. Hyaluronan and RHAMM in wound repair and the “cancerization” of stromal tissues. Biomed. Res. Int. 2014, 2014, 103923.
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019, 286, 2883–2908.
- Tammi, M.I.; Oikari, S.; Pasonen-Seppänen, S.; Rilla, K.; Auvinen, P.; Tammi, R.H. Activated hyaluronan metabolism in the tumor matrix—Causes and consequences. Matrix Biol. 2019, 78–79, 147–164.
- McDevitt, A.W.; Cooper, C.G.; Friedrich, J.M.; Anderson, D.J.M.; Arnold, E.A.; Clewley, D.J. Effect of Physical Therapy Timing on Patient Reported Outcomes for Individuals with Acute Low Back Pain: A Systematic Review with Meta Analysis of Randomized Controlled Trials. PM&R 2023, 15, 1466–1477.
- Daecke, W.; Kusnierczak, D.; Loew, M. Long-term effects of extracorporeal shockwave therapy in chronic calcific tendinitis of the shoulder. J. Shoulder Elbow Surg. 2002, 11, 476–480.
- Matteini, P.; Dei, L.; Carretti, E.; Volpi, N.; Goti, A.; Pini, R. Structural behavior of highly concentrated hyaluronan. Biomacromolecules 2009, 10, 1516–1522.
- Nagy, N.; Kuipers, H.F.; Marshall, P.L.; Wang, E.; Kaber, G.; Bollyky, P.L. Hyaluronan in immune dysregulation and autoimmune diseases. Matrix Biol. 2019, 78–79, 292–313.




