Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hong Jin Kim | -- | 1145 | 2023-12-19 09:20:10 | | | |
| 2 | Catherine Yang | -13 word(s) | 1132 | 2023-12-19 09:38:59 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Keum, B.; Kim, H.J.; Kim, G.; Chang, D. The Mechanisms for Bone Regeneration. Encyclopedia. Available online: https://encyclopedia.pub/entry/52911 (accessed on 13 January 2026).
Keum B, Kim HJ, Kim G, Chang D. The Mechanisms for Bone Regeneration. Encyclopedia. Available at: https://encyclopedia.pub/entry/52911. Accessed January 13, 2026.
Keum, Byeong-Rak, Hong Jin Kim, Gun-Hwa Kim, Dong-Gune Chang. "The Mechanisms for Bone Regeneration" Encyclopedia, https://encyclopedia.pub/entry/52911 (accessed January 13, 2026).
Keum, B., Kim, H.J., Kim, G., & Chang, D. (2023, December 19). The Mechanisms for Bone Regeneration. In Encyclopedia. https://encyclopedia.pub/entry/52911
Keum, Byeong-Rak, et al. "The Mechanisms for Bone Regeneration." Encyclopedia. Web. 19 December, 2023.
Copy Citation
The bone regeneration process has historically been studied with the repair of fracture as a unique ability of our body by restoring it to its pre-injured functions. In bone biology, homeostasis is regulated by two main cellular components: osteoblasts (bone-forming cells) and osteoclasts (bone-resorbing cells). Furthermore, various inflammatory cells and cytokines dynamically interact with these cells in bone environments, which are responsible for their repair capacity. For the bone regeneration process, previous studies have emphasized the role of osteoblasts with morphogen gradients such as bone morphogenetic proteins (BMPs).
bone morphogenetic proteins
osteoblast
mesenchymal stem cells
1. Bone Homeostasis
Normal bone homeostasis is balanced by osteoblasts and osteoclasts (Figure 1A). Importantly, these two cells have different lineages between mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs), respectively. MSCs, when induced by transcriptional factors, such as SRY-box transcriptional factor 9 (SOX9), Runt-related transcriptional factor 2 (RUNX2), fibroblast growth factor (FGF), and BMP, differentiate into osteoprogenitor and pre-osteoblast cells [1][2]. These cells, in turn, have the potential to differentiate further into osteoblasts, osteocytes, and chondrocytes, which are primarily involved in bone and cartilage formation. Of the many transcriptional factors, SOX9 and RUNX2 are the most important factors, and dominance between SOX9 and RUNX2 in MSC-derived progenitors determines their fates between chondrogenesis (SOX9 dominance) and osteogenesis (RUNX2 dominance) [3]. Osteoblast lineage cells not only differentiate into osteocytes but also promote bone mineralization by secreting hydroxyapatite and calcium (Figure 1B).
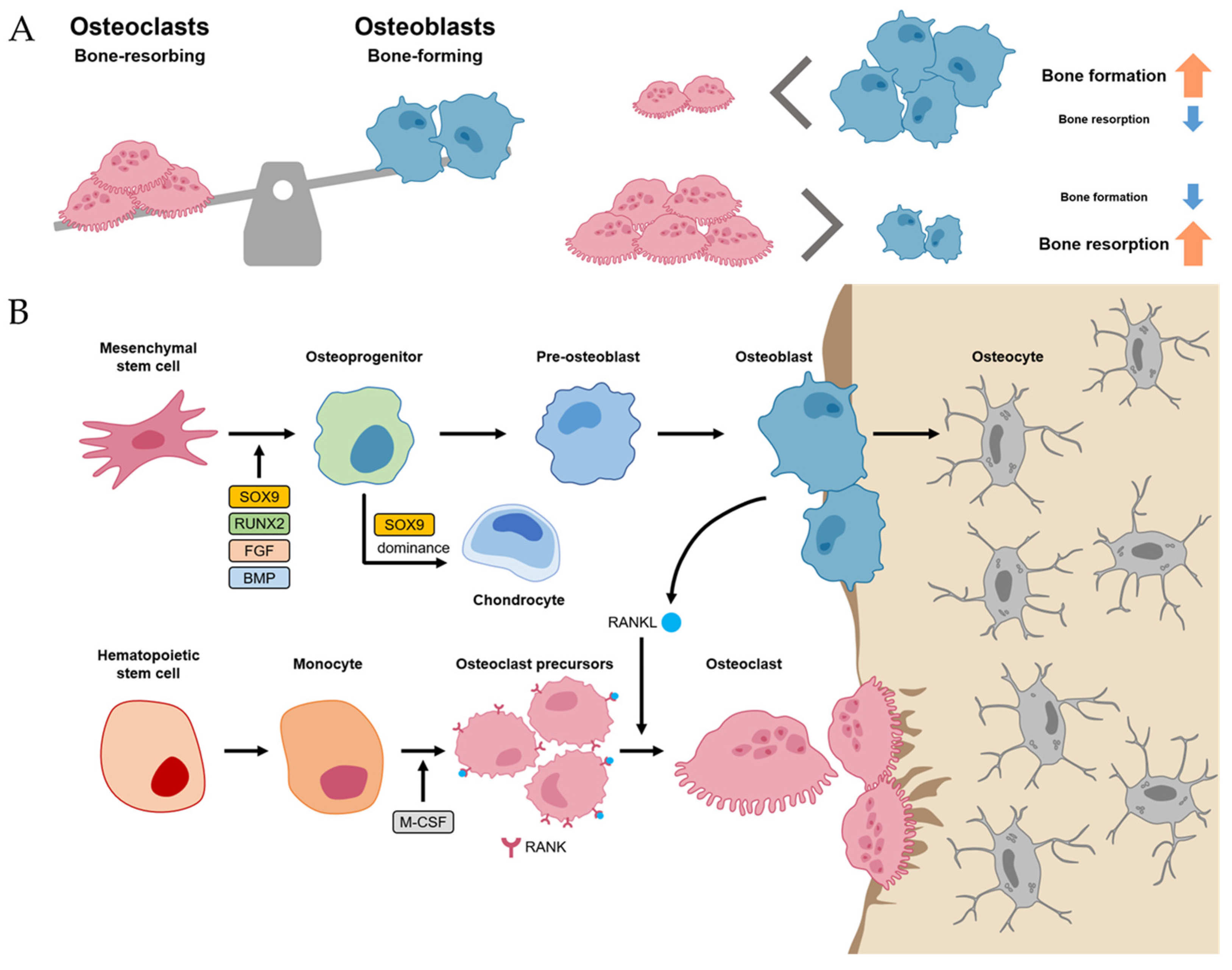
Figure 1. Bone homeostasis. (A) Normal bone homeostasis is balanced by osteoblasts and osteoclasts. (B) The detailed differentiation pathways of osteoblasts and osteoclasts in bone homeostasis.
Meanwhile, osteoclasts, which have the function of bone resorption, are large multinucleated cells derived from HSC lineage [1]. Hematopoietic monocytes and macrophages were differentiated into osteoclast precursors mediated by macrophage colony-stimulating factor (M-CSF) [4]. M-CSF also proliferated the osteoclast precursors. Then, the osteoclast precursors differentiated into mature osteoclasts mediated by the receptor activator of nuclear factor kappa-B ligand (RANKL). Thus, M-CSF and RANK-RANKL signaling within bone environments is essential for osteoclastogenesis, which is initiated by the recruitment of osteoclast precursors by osteoblasts that express RANKL (Figure 1B) [1][5].
The homeostasis between osteoblasts and osteoclasts is very important in physiological bone environments [6]. If an imbalance between osteoclasts exists, it can develop into a pathological process such as osteoporosis (the environment for osteoclast activity surpasses osteoblast activity) or osteopetrosis (the environment for osteoblast activity surpasses osteoclast activity) [6][7].
2. Bone Regeneration Process
The regeneration process of bone has been studied with the fracture healing phase [8]. It involves a series of coordinated events, including hematoma-forming inflammation, soft callus formation, the hard callus stage, and bone remodeling (Figure 2) [9][10].
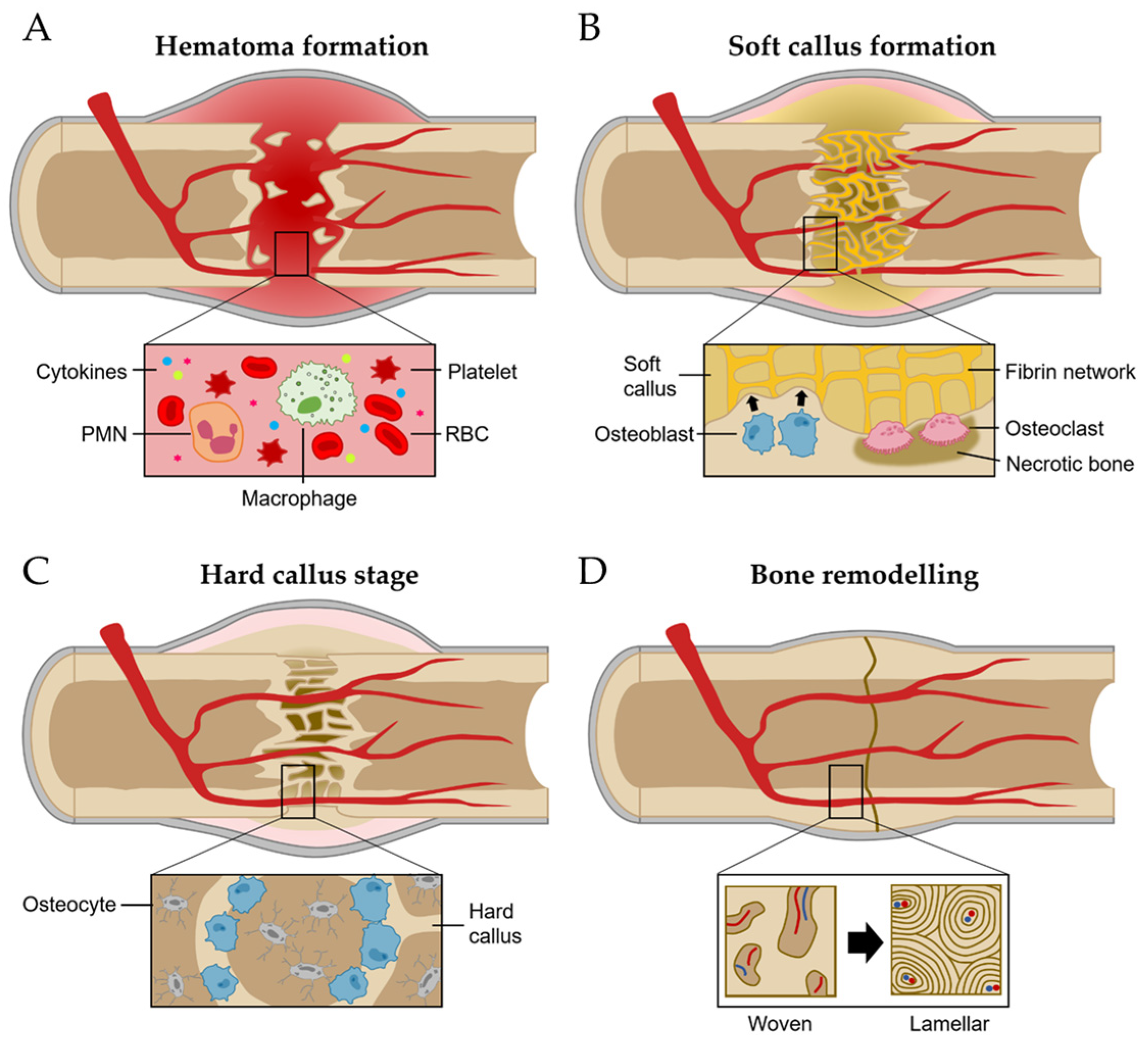
Figure 2. Bone regeneration process. (A) Hematoma formation. (B) Soft callus formation. (C) Hard callus formation. (D) Bone remodeling stage.
Inflammation stages occur for more than a few days after fracture [11]. The most important initial phase of the inflammation stages for bone regeneration is hematoma formation and inflammatory exudation from the injured blood vessels at the breakage location [11][12]. Injured soft tissues and degranulation of platelets release various cytokines, which results in typical inflammation responses such as vasodilation, hyperemia, polymorphonuclear neutrophils (PMNs), and macrophage migration and proliferation (Figure 2A) [8].
The network of fibrin and reticulin fibrils in hematoma is gradually substituted with granulation tissues (called soft calluses) and osteoclasts act to remove the necrotic bone at the fragment’s ends [8]. For soft callus formation, the osteoprogenitor cells in the cambium layer and endosteum are differentiated into osteoblasts, starting intramembranous appositional bone growth (Figure 2B) [1][8]. When the gaps between bones are linked with the soft callus, it starts to change the hard callus, called the hard callus stage [1]. For intramembranous bone formation, the soft callus within the gap is developed into the hard callus that is constituted with rigid calcified tissue by endochondral ossification [1]. Hard callus formation starts peripherally and progressively moves toward the center of the fracture and the fracture gap (Figure 2C) [1].
Following the solid union of gaps with woven bones, the remodeling stage begins [8]. The woven bone undergoes gradual replacement with lamellar bone, facilitated by surface erosion, condensation, and osteonal remodeling [8]. It persists from a few months to several years until the bone’s morphology is fully restored to its original state (Figure 2D) [8].
3. Signaling Pathways for Bone Formation
Since the osteoblasts have a critical function in bone formation, many studies have been focused on various osteoblast differentiation-related signaling pathways, which act in a coordinated manner to bone development and fracture repair [5][9][10][13]. The researchers introduce the five representative signaling pathways, including Hedgehog, Notch, Wnt/β-catenin, FGF, and BMP signaling (Figure 3).
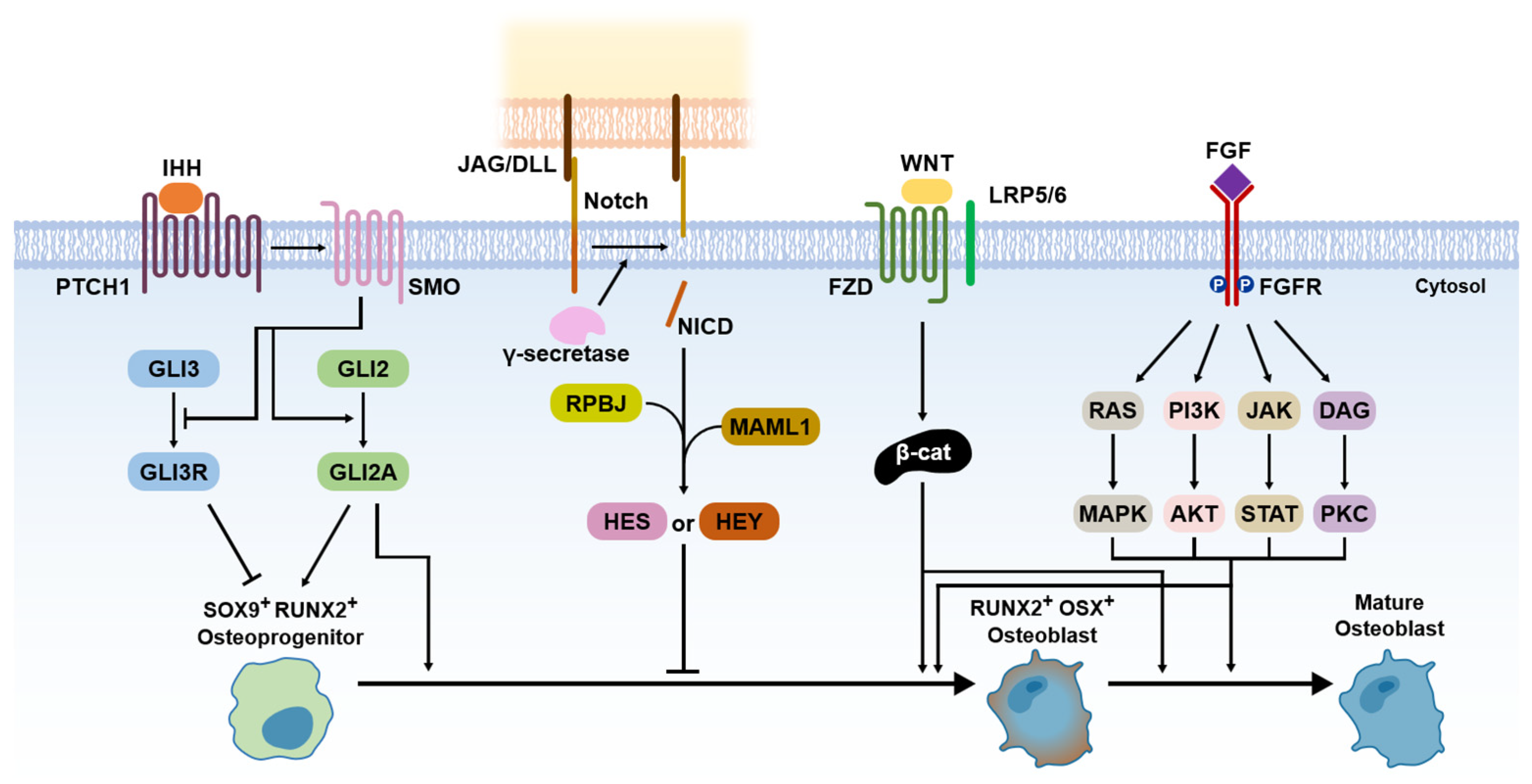
Figure 3. Signaling pathways for bone formation.
Hedgehog has been known to be an important morphogen in limb development for the anteroposterior body axis [14]. The Hedgehog pathway also has a crucial role in endochondral ossification [1]. Indian hedgehog (IHH) acts with parathyroid hormone-related peptides in a negative feedback loop [15]. IHH binds smoothened homologue (SMO), which blocks the cleavage of GLI3 to the GLI3 repressor (GLI3R) and activates GLI2 to the GLI2 activator (GLI2A) [1]. It leads to the expression of SOX9 and RUNX2 in osteoprogenitor cells, which are master regulators for osteoblast differentiation [16].
Compared to other signaling pathways, Notch signaling is a negative regulator of osteoblast differentiation [1]. Notch receptors initially interact with Jagged (JAG) or Delta-like protein (DLL) families by direct cell-to-cell contact [1]. This leads to proteolytic cleavage of the γ-secretase complex, which releases Notch intracellular domain (NCID) [1]. The NCID interaction with RPBJ and Mastermind-like protein 1 (MAML1) induces the expression of Notch target genes, including Hairy and Enhancer of Split (HES) and HES-related with YRPW motif (HEY), which inhibits osteoblast differentiation [1].
Wnt/β-catenin signaling acts as a positive regulator of osteoblast differentiation [1]. The Wnt ligand binds Frizzled (FZD; a cell surface receptor) and low-density lipoprotein receptor-related protein 5 (LRP 5) or LRP6, which leads to β-catenin accumulation in the cytoplasm [17]. It allows for translocation to the nucleus expressing RUNX2 and osterix (OSX) [17]. The expression of RUNX2 and OSX enables the differentiation of osteoblasts [17]. The role of β-catenin is important for various signaling pathways for osteoblast differentiation because it stimulates transcriptional activity by interacting with the T cell factor (TCF)/lymphoid enhancing factor (LEF) [18]. Thus, the presence of β-catenin is also essential for osteoblast differentiation in the Hedgehog pathway [1].
FGFs are known as a family of signaling proteins that are crucial for normal development [9]. For osteoblast differentiation, FGF initially binds with the extracellular ligand-binding domain of the fibroblast growth factor receptor (FGFR), which leads to the phosphorylation of tyrosine kinase in the FGFR intracellular domain [1][19]. It activates the intracellular signaling cascade, including Ras/MAPK, PI3K/AKT, JAK/STAT, and protein kinase (PKC) pathways [1]. For fusion as a bone regeneration process, FGF2, among the more than 20 types of FGFs, has been shown to have superior osteogenic effects with a combination of BMP2 [1][9]. Thus, FGF2 is currently recognized as enhancing the BMP-induced bone regeneration process in the inflammatory and endochondral bone formation stages.
References
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711.
- Wang, X.; Wang, Y.; Gou, W.; Lu, Q.; Peng, J.; Lu, S. Role of mesenchymal stem cells in bone regeneration and fracture repair: A review. Int. Orthop. 2013, 37, 2491–2498.
- Zhou, G.; Zheng, Q.; Engin, F.; Munivez, E.; Chen, Y.; Sebald, E.; Krakow, D.; Lee, B. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19004–19009.
- Sun, Y.; Li, J.; Xie, X.; Gu, F.; Sui, Z.; Zhang, K.; Yu, T. Macrophage-Osteoclast Associations: Origin, Polarization, and Subgroups. Front. Immunol. 2021, 12, 778078.
- Lademann, F.; Hofbauer, L.C.; Rauner, M. The Bone Morphogenetic Protein Pathway: The Osteoclastic Perspective. Front. Cell Dev. Biol. 2020, 8, 586031.
- Eastell, R.; O’Neill, T.W.; Hofbauer, L.C.; Langdahl, B.; Reid, I.R.; Gold, D.T.; Cummings, S.R. Postmenopausal osteoporosis. Nat. Rev. Dis. Primers 2016, 2, 16069.
- Wu, C.C.; Econs, M.J.; DiMeglio, L.A.; Insogna, K.L.; Levine, M.A.; Orchard, P.J.; Miller, W.P.; Petryk, A.; Rush, E.T.; Shoback, D.M.; et al. Diagnosis and Management of Osteopetrosis: Consensus Guidelines From the Osteopetrosis Working Group. J. Clin. Endocrinol. Metab. 2017, 102, 3111–3123.
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54.
- Tateiwa, D.; Kaito, T. Advances in bone regeneration with growth factors for spinal fusion: A literature review. N. Am. Spine Soc. J. 2023, 13, 100193.
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237.
- Perren, S.M.; Rickman, M.; Varghese, V.D.; Koh, A.; Guerado, E.; Giannoudis, P.V.; Rajfer, R.A.; Kilic, A.; Neviaser, A.S.; Schulte, L.M.; et al. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: Choosing a new balance between stability and biology. J. Bone Jt. Surg. Br. 2002, 84, 1093–1110.
- Kelly, P.J.; Montgomery, R.J.; Bronk, J.T. Reaction of the circulatory system to injury and regeneration. Clin. Orthop. Relat. Res. 1990, 254, 275–288.
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221.
- Tickle, C.; Towers, M. Sonic Hedgehog Signaling in Limb Development. Front. Cell Dev. Biol. 2017, 5, 14.
- Van der Eerden, B.C.; Karperien, M.; Gevers, E.F.; Löwik, C.W.; Wit, J.M. Expression of Indian hedgehog, parathyroid hormone-related protein, and their receptors in the postnatal growth plate of the rat: Evidence for a locally acting growth restraining feedback loop after birth. J. Bone Miner. Res. 2000, 15, 1045–1055.
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445.
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3.
- Doumpas, N.; Lampart, F.; Robinson, M.D.; Lentini, A.; Nestor, C.E.; Cantù, C.; Basler, K. TCF/LEF dependent and independent transcriptional regulation of Wnt/β-catenin target genes. EMBO J. 2019, 38, e98873.
- Marie, P.J. Fibroblast growth factor signaling controlling osteoblast differentiation. Gene 2003, 316, 23–32.
More
Information
Subjects:
Orthopedics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
19 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




