Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | George Louis Mendz | -- | 5708 | 2023-12-19 02:34:47 | | | |
| 2 | Lindsay Dong | + 2 word(s) | 5710 | 2023-12-22 01:20:05 | | | | |
| 3 | Lindsay Dong | -1 word(s) | 5709 | 2023-12-22 01:21:32 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mendz, G.L. Vaginal Microbiome during Pregnancy in Health and Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/52895 (accessed on 07 February 2026).
Mendz GL. Vaginal Microbiome during Pregnancy in Health and Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/52895. Accessed February 07, 2026.
Mendz, George L.. "Vaginal Microbiome during Pregnancy in Health and Disease" Encyclopedia, https://encyclopedia.pub/entry/52895 (accessed February 07, 2026).
Mendz, G.L. (2023, December 19). Vaginal Microbiome during Pregnancy in Health and Disease. In Encyclopedia. https://encyclopedia.pub/entry/52895
Mendz, George L.. "Vaginal Microbiome during Pregnancy in Health and Disease." Encyclopedia. Web. 19 December, 2023.
Copy Citation
Many aetiological agents pose a risk to pregnancy; in particular, infections of the genital tract by various pathogens that can cause serious health issues for both mothers and their fetuses have become a major public health issue all over the world for their high and growing prevalence. Genital infections can have a long duration, and women with these infections before pregnancy might also have increased risks of adverse outcomes including macrosomia, preterm birth (PTB), and spontaneous abortion. Multiple types of viral, bacterial, fungal, and parasitic infections have been identified that can affect the health of the pregnant woman, the pregnancy, and the infant after delivery.
vaginal microbiome
bacterial taxa
culture-independent techniques
new-generation sequencing
miscarriage
stillbirth
preterm birth
1. Introduction
In the 2021 Report of the UN Inter-agency Group for Child Mortality Estimation, the rates of miscarriage, defined as the loss of pregnancy before viability, was estimated at 11–22% of pregnancies [1]; an estimated 23 million miscarriages occur globally every year [2]. The rates of neonatal deaths and stillbirths, defined as a child born dead after 28 weeks of gestation, are estimated at 16.7/1000 and 13.9/1000 births, respectively [3]. The global burden of stillbirth is enormous: about 2 million infants die every year. Stillbirth rates vary significantly between countries from 1.4 to 32.2 per 1000 births; 34% of global stillbirths occur in Central and Southern Asia and 42% in Sub-Saharan Africa. In contrast, an average of about 1% of global stillbirths occur in North America, Europe, Australia, and Oceania [3].
Focussing on preterm birth (PTB, before 37 completed weeks of gestation), every year, over 15 million babies are born preterm, and this number is rising [1]. Across 184 countries, PTB rates range from 5% to 18% of babies born [1], and the average global PTB rate is about 11% [4]. It is the leading cause of death amongst children; one million children die due to PTB before the age of five years, and it accounts for 35% of all deaths amongst newborns (age < 28 days) and for 18% of all deaths amongst children aged under five years [4]. The rates of PTB and mortality vary significantly between countries and within countries, and, besides perinatal mortality, it is a major cause of serious neonatal morbidity and moderate to severe childhood disability in developed and underdeveloped countries. The burden of PTB is particularly high in low- and middle-income countries.
Ordinarily, pregnancy is a normal state of health, but changes in hormone levels and immune system function can make gravid women more susceptible to diseases that pose health risks to the mother, the child, or both. Complications during pregnancy have complex causes including alcohol or drug use, smoking, inadequate prenatal care, genetic background, maternal age, hypertension, nutritional status, diabetes, genitourinary tract infections, and several other risk factors [5].
The aetiology of pregnancy disorders is multifactorial, but the importance of the microbiota of the female genital tract (vagina and uterus) for maternal and infant health is shown by links between the dysbiosis of this tract and negative pregnancy outcomes. Some diseases that create problems primarily for the mother are urinary tract infections, vaginitis, postpartum infections, and pneumonia. For the child, adverse pregnancy outcomes related to maternal infections are miscarriage, stillbirth, PTB, low birth weight, and neonatal and perinatal mortality.
A little over a decade ago in 2011, volume 118 of the British Journal of Obstetrics and Gynaecology devoted a special issue to ‘Infections in Pregnancy’. The editorial stated “Infections of mothers and their babies (both in utero and ex utero) are a major global health challenge” [6]. The threat remains today.
2. Types of Infections during Pregnancy
2.1. Viral Infections
Different types of viruses have been identified as risk factors for maternal and infant heath. Arboviruses can cross the placenta and have been linked with fetal morbidity and mortality. Venezuelan equine encephalitis, West Nile, and Zika viruses have been associated with fetal malformations and demise, and neonatal death. Moreover, dengue and chikungunya viruses have been connected with adverse infant and mother outcomes [7].
The Hepatitis A virus is an important cause of materno-fetal infections in endemic regions owing to the spread of the disease. Most women infected with Hepatitis A during pregnancy and their infants do not experience serious complications. Nonetheless, this infection poses a higher risk of preterm labour, especially if it occurs during the second or third trimester. Other increased risks associated with Hepatitis A infection may include: premature uterine contractions. placental abruption, and the preterm prelabour rupture of membranes (PPROM). An increased chance for birth defects following exposure to Hepatitis A in pregnancy has not been shown [8]. Chronic Hepatitis B virus infection in pregnant women increases the risk of intrahepatic cholestasis, gestational diabetes mellitus, pre-eclampsia, and PTB rates [9]. In Hepatitis-E-endemic countries, there is a high burden of infection amongst pregnant women; this virus can cause fulminant hepatic failure, and, in third trimester infections, it has been associated with up to 30% mortality. Hepatitis E virus can be transmitted from infected mothers to their infants with significant perinatal morbidity and mortality [10].
The influenza virus has been related to various negative pregnancy outcomes; however, the results of a recent meta-analysis suggested a correlation with an increased risk of stillbirth, but no clear effects on small-for-gestational-age development, low birth weight, PTB, and fetal death [11]. Pregnant women with measles infection are at risk of severe pregnancy complications. The virus is not teratogenic but causes a dysregulation of the entire immune system that may interfere with the normal course of pregnancy. Measles virus infection alters the physiological mechanisms of immunotolerance present during pregnancy such that it can lead to a reaction similar to rejection, manifested by the spontaneous abortion or premature expulsion of the fetus. Measles in pregnancy can also lead to perinatal infections in the newborn, associated with neurological complications and a high mortality [12].
Pregnant women who contract rubella (German measles) are at risk of miscarriage or stillbirth, and their developing infants are at risk of severe birth defects. The timing of the infection is crucial: contracted by the fetus during the first 12 weeks of gestation, it is likely to result in many infant problems; in infections acquired between 12 and 20 weeks of pregnancy, usually, the problems will be milder; in infections acquired at later gestational ages, commonly, there will not be problems with the infant [13].
Herpes simplex (HSV) infection during pregnancy increases the risk of stillbirth, spontaneous abortion, and premature birth. These infections are well-known causes of childhood sequelae [14]. Human cytomegalovirus is a major cause of adverse nonhereditary birth outcomes, including hearing and visual loss, neurologic deficits, and intrauterine growth retardation; the virus may contribute to outcomes such as stillbirth and preterm delivery [15]. Human-papilloma-virus-positive women have an increased risk of pregnancy problems, such as miscarriage, pregnancy-induced hypertensive disorders, intrauterine growth restriction, low birth weight, the premature rupture of membranes, and PTB [16].
Untreated maternal human immunodeficiency (HIV) virus infection increases the risk of stillbirth, preterm delivery, low birth weight, and small-for-gestational-age infants. In 2013, the World Health Organization recommended the use of antiretroviral therapy for all pregnant and breastfeeding women for the prevention of mother-to-child HIV transmission [17].
2.2. Bacterial Infections
Bacterial colonisation of the female genital tract has been studied for a long time and extensively. These investigations have resulted in a considerable body of knowledge about the bacteria that have their habitats in the genital tract of healthy women, and those associated with disease; the latter represent a risk to the outcome of pregnancy.
Prominent amongst beneficial bacteria are four well-studied species of lactobacilli, L. crispatus, L. iners, L. gasseri, and L. jensenii [18]. A characteristic of female genital infections is that, usually, they are polymicrobial, of mixed aerobes and anaerobes that frequently outnumber aerobes. Common bacteria in genital tract infections are Chlamydia trachomatis, Clostridium spp., Gardnerella vaginalis, Mobiluncus mulieris, Nesseria gonorrhoeae, Peptostreptococcus spp., Porphyromonas spp., Prevotella bivia, Streptococcus agalactiae (Group B Streptococus, GBS), Ureaplasma urealyticum, and others [18].
Commonly, the presence of pathogens is tested by cultivation to identify the bacteria involved in genital infections and apply appropriate therapeutic measures, but, in many instances, no diagnosis had been possible owing to the absence of growth in cultures from genital tract swabs. Knowledge of this microflora has improved remarkably by employing culture-independent new sequencing methods that have yielded a vast increase in the number of taxa identified in the female genital tract.
2.3. Fungal Infections
The female genital mycobiota cincludes fungi residing in the reproductive tract: common taxa are Aspergillus, Candida, Sacharomyces, Sporobolomyces, and others. During pregnancy, increased levels of progesterone and oestrogen affect the vaginal microenvironment; these physiological changes are a risk factor for candidiasis (commonly known as thrush). Candida is the leading genus of fungal cervicovaginal infections present in approximately 20% of non-pregnant women; it increases to an average of 30% during pregnancy, with a risk that can be as high as 50% in the third trimester [19], and it can lead to intrauterine infection, endometritis, and choroamnionitis [20].
2.4. Parasitic Infections
Parasitic infections are common among pregnant women, owing to reduced body immunity, and they can affect the physiology of the mother’s body. Helminth infection may be particularly detrimental during pregnancy by increasing the risk of PTB, low birth weight, and maternal anaemia. Prenatal exposures to these infections are linked to infant impaired behavioral development [21]. In contrast, urogenital schistosomiasis is not significantly associated with adverse pregnancy outcomes [22].
Scabies mite infection accounts for 2% to 6% of skin conditions during pregnancy, but the infection is not known to cause directly negative pregnancy and fetal outcomes. Scratching the scabies rash may lead to secondary infections and even sepsis. Scabies-related infections may also increase the risk of having kidney diseases and rheumatic heart disease [23].
Pregnant women infected with malaria have adverse consequences on birth outcomes, including having small-for-gestational-age and preterm infants [24], as well as low birth weights, that predisposes them to infant mortality and lifelong morbidities [25]. An investigation involving 361 pregnant women with simple malaria and 85 women with severe malaria showed significant differences in the perinatal poor outcomes between simple and severe malaria and by the trimester of infection [26].
The global annual prevalence of congenital toxoplasmosis is estimated to be over 190,000 cases. A primary Toxoplasma gondii infection in pregnant women can cause the vertical transmission of the parasite and result in miscarriage, stillbirth, premature birth, abortions, malformations, neonatal mortality, and a variety of neurological sequelae [27]. Infants with congenital toxoplasmosis may exhibit clinical signs of hydrocephalus, mental retardation, eye disease, and other severe sequelae. The poor health condition of infants with congenital toxoplasmosis contributes to their heavy global health burden [28].
Trichomonas vaginalis infection is the most common non-viral sexually transmitted disease in the world. Often, it is asymptomatic and can be eliminated with proper treatment. Untreated trichomoniasis in pregnant women is associated with preterm delivery, the prelabour rupture of membranes, and low birth weight [29].
3. The Vaginal Microbiome during Pregnancy
During pregnancy, various physiological changes take place to adapt the mother’s body to the fetus and vice versa. This adaptation is governed by hormonal changes that lead to immune modulation, behavioural changes, physicochemical changes in the genital mucosa, and other adjustments in the genital tract. All these factors drive a modulation in the structure and function of the pregnant woman microbiome, making it different from that of non-pregnant females [30]. In addition to many other hormonal changes, pregnancy is characterised by high circulating oestrogen concentrations, mainly produced by the placenta, and increased glycogen deposition on the vaginal epithelium [31]. Such conditions favour the proliferation and dominance of Lactobacillus, which metabolises glycogen’s breakdown products to lactic acid [32]. As a result, during a healthy pregnancy, often, the vaginal microbiome has low bacterial species diversity.
Commonly, during the course of gestation, the vaginal microbiome of individual women with a healthy pregnancy undergoes a reduction in the diversity of microbial communities (alpha-diversity), but there are increases in microbial diversity between subjects (beta-diversity) and in the overall number of different taxa that result in a larger number of vagitypes being identified in pregnancies than in non-pregnancies. Also characteristic of pregnancy are shifts in vagitypes, discussed in more detail in the description of longitudinal studies. These changes are unrelated to those that arise from the pre-pregnancy body mass index, the development of gestational diabetes, or the number of previous births.
The prevalence of a specific vagitype stems from the stability of the microbial community, the lack of stability of other vagitypes, and the probabilities of switching among different dominant communities. Vagitypes dominated by lactobacilli, in particular, L. crispatus, are quite stable during pregnancy; others, for example, ones dominated by G. vaginalis, exhibit less stability [33]. Racial background plays an important role in the vaginal microbiota composition. For instance, the vaginal microbiota in non-pregnant or pregnant women of African ancestry includes commonly anaerobic bacteria such as G. vaginallis, A. vaginae, and S. amnii, and fewer Lactobacillus species. This occurrence increases the risk of bacterial vaginosis and PTB in women of African ancestry relative to women in other racial groups [32].
In addition to the known vaginal bacterial communities, there is a vaginal virome abundant in double-stranded DNA viruses, a lesser number of single-stranded DNA viruses, and a few unidentified viruses. Pathogenic viruses found in the vagina are taxa belonging to the order Herpesvirales and the family Papillomaviridae. Little information exists about the vaginal mycobiome [30].
Investigations of the vaginal microbiota in pregnant women can be classified as cross-sectional studies that provide snapshots at specific moments during pregnancy and longitudinal studies that address the temporal changes in this microbiome as pregnancy progresses. These studies have characterised normal pregnancies that finish at term, and/or abnormal pregnancies that end before a complete gestational period.
3.1. Cross-Sectional Studies
The vaginal microbiomes of pregnant women show similarities to those of non-pregnant women but with the following differences: (i) significant changes after delivery, and the inference that significant changes would have taken place too at the beginning of the pregnancy; (ii) stable populations throughout healthy pregnancies; and (iii) a varying number of vagitypes. Cross-sectional investigations highlighted the complexity and diversity of vaginal bacterial communities, indicating that there are racial differences in the vaginal microbiomes of women with healthy pregnancies. The findings of these studies also clarified that there are differences in the taxa abundance and diversity between healthy term pregnancies and those with adverse outcomes such as miscarriage, stillbirth, and preterm. However, many of these studies comprised a limited number of subjects, and yielded only an initial understanding of the vaginal microbiome during pregnancy.
3.1.1. Healthy Pregnancies
In pregnant women, the taxa more frequently observed and in greater abundance are Lactobacillus spp.; also present in the vagina with high frequency are the Actinomycetales, Clostridiales, and Bacteroidales taxa [34]. Lactobacillus spp. are abundant in the vaginal anaerobic habitat and create an environment hostile to many other bacteria. In particular, lactic acid keeps the vaginal pH below 4.5, thus establishing a defence against invading pathogens and contributing to a healthy vaginal environment. The most important role, as an antibacterial of the two L- and D-lactic acid forms, corresponds to the D-isomer from which hydrogen peroxide can be generated. Notably, the dominant Lactobacillus species play an important role in the protection of the vaginal ecosystem. The health and high stability of the vaginal community are enhanced by L. crispatus that produces D- and L-lactic acids [35]. L. iners does not generate D-lactic acid [36], and dysbiosis and lower stability are more commonly present in some racial backgrounds where the vaginal microbiota is dominated by this Lactobacillus.
To investigate the vaginal microbiome diversity that arises from racial background, the vaginal microflora of 300 Caucasian-, African-, or Hispanic-ancestry pregnant women were compared to those of 300 non-pregnant women case-matched for race, gestational age, and household income. The study found that pregnant women overall have a significantly higher prevalence of the four most common Lactobacillus vagitypes and a lower prevalence of vagitypes dominated by other taxa. Central to these differences was a higher prevalence of the L. iners vagitype during pregnancy at the expense of the G. vaginalis and other more complex vagitypes. Relative to non-pregnant women, a lower prevalence of G. vaginalis, A. vaginae, Prevotella cluster 2, P. bivia, and S. amnii was observed amongst African-American pregnant women, and a higher abundance of A. vaginae and P. bivia in Hispanic pregnant women. A lower prevalence of L. crispatus, accompanied by a higher prevalence of L. iners, was found in Caucasian pregnant women relative to non-pregnant women. In this group, the microbiome shifted towards a more stable, generally Lactobacillus-dominated profile; in women of African or Hispanic lineage, a major shift was observed in the early stages of pregnancy [33].
3.1.2. Pregnancies with Adverse Outcomes
Remarkable progress has been made in understanding the role of the vaginal microbiome in maintaining health and in preventing obstetric and gynaecological diseases, but further work is required to elucidate fully the contribution that a balanced vaginal microbiota makes to pregnancy. Cross-sectional investigations of the vaginal microbiome during pregnancy have served to discover relationships between taxa and the adverse pregnancy outcomes of miscarriage (a child born dead before 22 weeks of pregnancy), stillbirth (several criteria are employed—commonly, a child born dead at 22 weeks of pregnancy or later), and PTB (a live birth that occurs before 37 completed weeks of pregnancy).
Miscarriage
Miscarriage is an adverse obstetric outcome associated with a large number of pregnancy losses [37]. Statistics on the rate of miscarriage are difficult to obtain, but it is estimated that up to 10% of clinically recognised pregnancies end in miscarriage. A retrospective analysis was conducted on data from 24,835 pregnant women divided into two groups according to whether they had a first trimester threatened miscarriage or not. The women with the risk of miscarriage were older, and with higher rates of assisted reproduction and nulliparity. In pregnancies of this group of women, hyperemesis gravidarum, gestational diabetes mellitus, and placenta previa were more frequent than in the control group, and these women had lower gestational age and child birth weight [38]. Miscarriage is associated with a variety of conditions including embryo genetic and epigenetic disorders, immunological and endocrine factors, uterine malformations, maternal age, and lifestyle.
Recurrent miscarriage (RM) is defined as three or more consecutive pregnancy losses [39]. Vaginal infections have been associated with RM, but the pathophysiology is poorly understood. The innate immune system has various mechanisms for interacting with pathogens to protect vaginal tissues and to allow the survival of the commensal microbiota. In pregnancy, an organised immune response is essential for implantation, placentation, and blood vessel transformation, which an active infection may disturb, resulting in miscarriage. The mucosal epithelium and neutrophils in the vagina provide a first line of defence against micro-organisms, and, by secreting cytokines and chemokines, they attract innate immune cells such as macrophages, dendritic cells, and natural killer (NK) cells [40].
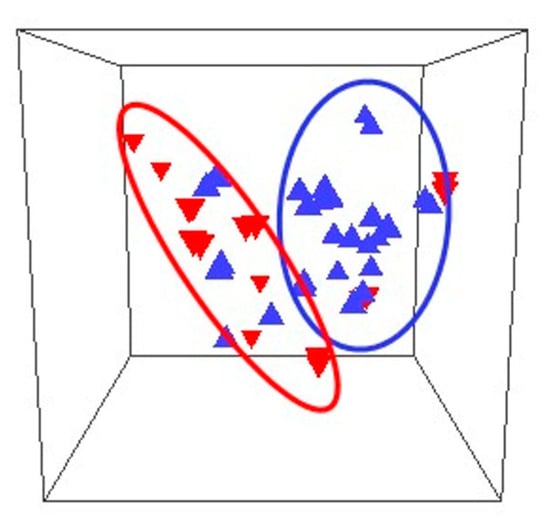
A prospective observational study characterised the vaginal microbiomes of 24 women in the first trimester of pregnancy. There were significant differences between the microbiomes of women who suffered miscarriage and those who continued to term delivery, both in the overall microbiome populations and in the abundances of individual taxa (Figure 1) [41].

Figure 1. Comparison of the composition of microbiomes of women who miscarried (inverted triangles) and controls (upright triangles) in 3 dimensions. The outlines refer to the miscarriage (red oval) and control (blue oval) microbiomes [41].
L. crispatus, L. gasseri, and Bifidobacerium were more abundant in women who delivered at term, whereas L. iners, L. jansenii, Gardnerella, Prevotella, and Escherichia/Shigella were found at higher relative abundances in women who suffered miscarriage [41]. Regarding pregnancy history, L. crispatus was the dominant bacterium in 50% of nulliparous women and L. iners was the dominant bacterium in 50% in women with a history of prior miscarriage and who had a miscarriage in the study, compared to 15% in women with no history of miscarriage, supporting the conclusion that the composition of the vaginal microbiome varies with pregnancy history. Lactobacillus spp. in the vaginal microbiome had a positive correlation with fertilisation success, implantation, and early embryonic development [42]. Notwithstanding the overall positive effects of Lactobacillus spp. on pregnancy and the known interactions between these bacteria and the components of the immune system located in the vagina, more detail remains to be known [43]. Lactic acid produced by lactobacilli shows immunomodulatory properties by inducing an anti-inflammatory response in vaginal and cervical epithelial cells [44] and, together with hydrogen peroxide, can lower the levels of pro-inflammatory cytokines, thus positively affecting fertility and pregnancy maintenance. In contrast, lipopolyssacharides from Gram-negative bacteria are potent immunostimulators that can activate peripheral natural killer cells with negative effects on reproduction [45].
In response to pathogens, neutrophil and macrophages produce extracellular traps composed of DNA strands, histones, elastase, and various peptides and enzymes that act as mechanisms to fight invading bacteria, but, if produced in excess, they can induce autoimmune and coagulation disorders and metastasis [46], and cause damage to the placenta and fetal membranes [47]. In women who had a miscarriage, elements of extracellular networks were found in the placenta, suggesting a relationship with this adverse outcome [48].
Stillbirth
In 2021, an estimated 2 million babies were stillborn, with a global rate of 13.9 stillbirths per 1000 total births [3], with large regional variations from 22.8 stillbirths per 1000 total births in West and Central Africa to 2.9 per 1000 in Western Europe [49]. Common causes of stillbirth include infections, birth defects, and pregnancy complications like pre-eclampsia. In more than 1 of every 10 stillbirths, the death was likely caused either by an infection in the fetus or in the placenta, or by a serious microbial disease in the mother. Pathogens were a more common cause of death in stillbirths before week 24 than later in the pregnancy. In the United States, stillbirths are more than twice as likely among African-American women than among Caucasian women, and it is an important factor in the former group [50].
The rate of pregnancy loss varies with the gestational age at the moment of infection and by the specific invading pathogen. For example, infection with Treponema pallidum causes pregnancy loss or fetal death in up to 50% of cases, whereas parvovirus B19 (PVB 19) infection causes pregnancy loss or stillbirth in less than 3% of cases. The mechanisms of pregnancy loss owing to pathogens that traverse the maternal–fetal barrier and cause congenital disease in the fetus are known. Infections by Toxoplasma gondii, rubella virus, cytomegalovirus, or HSV (TORCH) can be pathogen-mediated, placenta-mediated, and/or through inflammation-induced previable delivery [51]. Other bacteria linked to stillbirth include S. agalactiae, E. coli, Haemophilus influenza, and species of the genera Klebsiella, Enterococcus, Chlamydia, Mycoplasma, and Ureaplasma [49][52].
Group B Streptococcus infection is an important, potentially preventable, cause of worldwide burden of stillbirths, accounting for around 1% of cases in developed countries and 4% in Sub-Saharan Africa. Infection of the infant starting before delivery likely proceeds from bacteria ascending in utero from the maternal genitourinary tract. Evidence has been obtained from whole-genome sequencing studies that demonstrated GBS isolated at birth from the skin of newborns delivered by Caesarean section to be identical to those colonising the mother.
A secondary analysis of 512 stillbirths in a prospective, multisite, geographically, racially, and ethnically diverse population in the United States identified infection as a possible or probable cause of death in 66 cases. In the study, infection-related stillbirths occurred earlier than non-infection-related stillbirth. The most common bacterial pathogens identified were E. coli, S. agalactiae, and Enterococcus spp., and the most common viral pathogen was cytomegalovirus (CMV) [53].
Preterm Birth
The length of gestation is considered a key indicator of child health, and PTB is associated with poorer health outcomes in infants who have increased risks of short-term complications, mainly owing to the immaturity of multiple organ systems and neurodevelopmental disorders. Yearly, approximately 15 million infants are born preterm worldwide at a rate of 10.6%, with significant regional variations from 8.7% to 13.4% [54], with a higher prevalence in low- and middle-income countries [55]. Prematurity is the cause of about 35% of newborn deaths, and of 18% of children younger than 5 years old. Reducing PTB is part of the United Nations Sustainable Development Goal 3, target 3.2, which endeavours to end all preventable deaths of newborns and children aged under 5 years by 2030 [55].
Spontaneous PTB is associated with multiple, complex, and not completely understood causes of disease, but a leading cause of PTB is infection resulting from the microbial invasion of the amniotic cavity. A characteristic of acute chorioamnionitis is neutrophilic infiltration at the maternal–fetal interface, with ensuing inflammation. Commonly, acute chorioamnionitis is a result of ascending infection and recent studies suggest a link between vaginal dysbiosis, vaginal inflammation, and ascending infection. Less commonly, microbes can invade the maternal–fetal interface via the haematogenous route, e.g., Zika virus, CMV, and L. monocytogenes, where they can cause placental villitis and severe fetal inflammation and injury [56]. Although some studies found preterm labour to be associated with sterile intra-amniotic inflammation or infection [57], this does not preclude the involvement of the microbiota in the vagina modulating systemic host responses that could trigger an inflammatory response in the amniotic cavity leading to PTB. Studies linking the vaginal microbiota to PTB have yielded mixed results, exposing the need for further investigations [58].
A prospective study with 464 Caucasian-American women and 360 African-American women analysed the association between the vaginal microbiome at midpregnancy, race, and spontaneous PTB [59]. In the Caucasian-American cohort, 375 women delivered at term and 89 preterm, and, in the African-American cohort, 276 women delivered at term and 84 preterm. The vaginal microbiomes of both cohorts were significantly different; the African-American women had higher microbial diversity, a greater abundance of L. iners, and a lower abundance of L. crispatus. The vaginal microbiomes of both groups of women were significantly associated with race, PTB, and maternal factors such as poverty, education, marital status, age, and douching. A higher L. crispatus abundance in term controls was the main difference with the microbiota of women who delivered PTB. In L. crispatus-dominated microbiomes, diversity was significantly lower than in L. iners-dominated ones, suggesting that the former species is better at suppressing bacterial vaginosis-like microbiomes [59]. This result is consistent with the observation that L. iners-dominated vagitypes shift more often toward a diverse bacterial community structure than those where L. crispatus is predominant [60].
The diversity of taxa measured by the Shannon index or the inverse Simpson index was greater in samples from women who experienced premature delivery, as reported in other studies with women of various racial backgrounds [61]. L. iners was predominant in women who delivered at term or preterm, with increasing abundance in successive trimesters. Next in relative abundance were L. crispatus in the term group and L. jensenii in the preterm group. Other taxa more abundant in the preterm group than in the term group were Lachnospiraceae BVAB1, Prevotella cluster 2, S. amnii, Dialister cluster 51; P. amnii, Clostridiales BVAB2; Coriobacteriaceae, Dialister micraerophilus, and Parvimonas.
Other Adverse Outcomes
The most frequent disease outcomes of S. agalactiae colonisation during pregnancy are infant meningitis or sepsis, which are accompanied by high mortality risk. Other infrequent outcomes include stillbirths, maternal infections, and prematurity; however, given the wide prevalence of this genital infection, the potential annual burden of GBS-associated PTB has been estimated for the first time at 50,000 births, with a wide uncertainty range [62].
A pre-pregnancy health examination program included 89 women whose pregnancy outcomes were followed up for 1 year. Vaginal swabs were collected, 16S rRNA genes were sequenced, and M. hominis colonisation was confirmed by qPCR. Cox models were used to estimate the fecundability odds ratio (FOR) for women with M. hominis [63]. The prevalence of M. hominis was 22.47% (20/89) with a relatively low abundance. The Simpson index of the Mycoplasma-positive group was significantly lower than that of the negative group (p = 0.003), suggesting that microbiome diversity appeared to increase with M. hominis positivity. The relative abundance of M. hominis was negatively correlated with L. crispatus (p = 0.024), but positively correlated with G. vaginalis, A. vaginae, and P. bivia (p < 0.05 for all). The cumulative one-year pregnancy rate for the Mycoplamas-positive group was lower than that in the negative group (58.96% vs. 66.76%, p = 0.029). After controlling for potential confounders, the risk of pregnancy in the positive group was reduced by 38% when compared with the negative group [63].
3.2. Longitudinal Studies
Longitudinal studies of the vaginal microbiome examine the abundance of the microbial taxa and their change throughout pregnancy in the context of a dynamic environment. Childbearing may be considered a pro-inflammatory condition in which the vaginal microbiota might possess immunomodulation properties based on the modification of bacterial species; in particular, lactobacilli seem to play a key role in this process [32]. The vaginal bacterial composition of pregnant women exhibits considerable convergence across different populations, becoming less rich and diverse compared to the vaginal bacterial populations of non-pregnant women. During pregnancy, Lactobacillus taxa become the predominant bacteria in the vagina in most women of various racial backgrounds, leading to a decrease in alpha-diversity [30].
The vaginal ecosystem of 64 Caucasian women with normal pregnancies was characterised in the first, second, and third trimester. Advancing in the pregnancy, there was a significant decrease in cases of bacterial vaginosis and an increase in cases with normal microbiota. The relative abundance of lactobacilli increased from 50% in the first trimester, to 73.4% in the second trimester, and to 79.7% in the third trimester. This shift was associated with marked changes in the vaginal metabolome: several metabolites, such as lactate, glycine, phenylalanine, leucine, and isoleucine, ordinarily found in healthy vaginas, reached their highest concentrations at the end of pregnancy. Concomitantly, the abundance of microbiota present in bacterial vaginosis decreased throughout the pregnancy, and there was a progressive reduction in the levels of metabolites such as biogenic amines, alcohols, propionate, and acetate associated with dysbiosis. The levels of cytokines IL-6 and IL-8 were positively correlated, and their lowest concentrations were measured in the second trimester. A total of 19 women had a Candida infection, with 10 cases throughout the pregnancy; associated with this infection were higher levels of IL-8, 4-hydroxyphenyllactate, choline, and O- acetylcholine, as well as a higher concentration of leukocytes [64].
A prospective study with weekly sampling of the vaginal microbiome was conducted with 40 pregnant women, of which 11 delivered preterm. The vagitypes identified corresponded well with the five described for non-pregnant women. The taxonomic composition and diversity of the microbiota was generally stable in each individual with some vagitype transitions during the pregnancy; the Lactobacillus-dominated vagitypes I, II, III, and V were more stable than those of vagitype IV. Pregnancies with vagitype IV exhibited a stronger association with PTB, at every time window during gestation. G. vaginalis was strongly associated with PTB; a high Ureaplasma abundance combined with a low abundance of Lactobacillus was associated with PTB as well. Twenty-five women provided a postpartum sample; their microbiota indicated that delivery generally was accompanied by a significant, sudden, and durable increase in bacterial community diversity, albeit not in all cases [65].
An exploratory longitudinal investigation of the vaginal microbiota composition of eight Mexican women with healthy pregnancies collected samples in the third trimester of pregnancy and, subsequently, at childbirth at term. The vaginal microbiota was dominated by the Lactobacillus genus at both time points. There were no statistically significant differences in relative abundances, absolute read count, bacterial richness, community diversity, evenness, and beta-diversity between the third trimester of pregnancy and the time of childbirth. Nonetheless, compared to the third trimester of pregnancy, a trend was observed of higher absolute read counts for the genera Gardnerella, Faecalibaculum, Ileibacterium, and Lactococcus, and lower absolute read counts of Lactobacillus spp. at childbirth, but these changes in absolute read counts did not result in significant statistical differences between the microbial populations at both times. The results suggest that the vaginal bacterial composition is stable, and Lactobacillus genus is the dominant taxa in Mexican women’s vagina at the third trimester of pregnancy and at childbirth [32].
4. Conclusions
In healthy pregnancies, the bacterial communities in the vagina have a relatively lower number of taxa than in non-pregnant women, and are dominated by a few species, namely, those of the genus Lactobacillus, although other taxa such as A. vaginae, G. vaginalis, and Prevotella spp. are also found in normal pregnancies. Interestingly, although the smaller number of taxa in individuals commonly results in a lower alpha-diversity, marked differences in vagitypes between individuals effect a higher beta-diversity during pregnancy. A relationship has been found between the vaginal microbiome and racial background; women of Hispanic or African ancestry harbour more anaerobic flora in their microbiota than women of Asian or Caucasian backgrounds who commonly have Lactobacillus spp. as the dominant taxa. Besides racial background, other factors that modulate the vaginal microbiota include maternal age, previous pregnancies, blood pressure, behavioural habits, and various environmental factors.
Epidemiological studies provide evidence that the urogenital microbiota is linked to obstetric diseases. A marker of pregnancy complications is the proliferation in the genital microbiota to the significant abundance or dominance of aerobes such as Acinetobacter baumannii, E. coli, E. faecalis, S. aureus, and S. agalactiae [66][67], or anaerobes like A. vaginae, Clostridiales BVAB 1-3, D. microaerophilus, G. vaginalis, M. hominis, P. timonensis, and U. urealyticum.
Investigations on the changes induced in the vaginal bacterial populations by pregnancy in health and disease indicated that the vaginal microbiota is stable in the absence of infections. These studies confirmed that considerable changes in the vaginal community composition occur immediately following pregnancy, as well as postpartum.
The stability of the vaginal microbiome during a healthy pregnancy is such that alterations in the bacterial flora dominated by Lactobacillus spp. reflect the status of various obstetric conditions and are predictive biomarkers for certain pregnancy-adverse outcomes. Thus, it has been proposed that the composition of the vaginal microbiome may be a useful prognostic indicator of preterm labour and serve as a monitoring tool for tocolytic treatment to prevent PTB [68].
Nonetheless, much more needs to be learned about the pathogenicity and mechanisms of host defence to these micro-organisms. Future investigations will serve to elucidate the functional effect of the microbial communities and/or specific bacterial species on homeostasis and disease during pregnancy. A better understanding of the host–micro-organism interactions might reveal new opportunities for disease prevention, therapy, and improving women’s quality of life and overall health.
In addition, the complex gut microbiomes are involved in host immunity, metabolism, digestion, and the functioning of the nervous system, and are important for the health of the mother and child. During pregnancy, changes may occur naturally in the microbiomes of the oral cavity, intestine, and breast milk. Changes in the structure and composition of the gut microbiomes with an increase in the abundance of various genera of micro-organisms, e.g., Acinetobacter, Actinobacter, Klebsiella, Rothia, etc., and a decrease in others, e.g., Bacteroides, Bifidobacterium, Eubacterium, etc., can manifest in pregnancy complications such as gestational diabetes, gestational obesity, pre-eclampsia, diseases of the digestive tract, and autoimmune disease. The relationships between imbalances of the maternal gut microbiomes and their physiological effects during pregnancy are starting to be elucidated, but many more investigations are required to provide a comprehensive picture that would serve to foster maternal and offspring health [69].
References
- UN Inter-agency Group for Child Mortality Estimation. Levels & Trends in Child Mortality, Report 2021; UNICEF: New York, NY, USA, 2021; Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 15 July 2022).
- Editorial: Miscarriage-Worldwide reform of care is needed. Lancet 2021, 397, 1507.
- UN Inter-Agency Group for Child Mortality Estimation. A Neglected Tragedy: The Global Burden of Stillbirth Report 2020; UNICEF: New York, NY, USA, 2020; Available online: https://www.unicef.org/media/84851/file/UN-IGME-the-global-burden-of-stillbirths-2020.pdf (accessed on 30 July 2022).
- Walani, S.R. Global burden of preterm birth. Int. J. Gynecol. Obstet. 2020, 150, 31–33.
- Baskaradoss, J.K.; Geevarghese, A.; Al Dosari, A.A.F. Causes of adverse pregnancy outcomes and the role of maternal periodontal status. Open Dent. J. 2012, 6, 79–84.
- Hussein, J.; Ugwumadu, A.; Witkin, S.S. Editor’s Choice. Br. J. Obst. Gynaecol. 2011, 118, i–ii.
- Pomar, L.; Baud, D. Emerging virus infections in adverse pregnancy outcomes. Viruses 2022, 14, 285.
- Chaudhry, S.A.; Koren, G. Hepatitis A infection during pregnancy. Can. Fam. Physician 2015, 61, 963–964.
- Sirilert, S.; Tongsong, T. Hepatitis B virus infection in pregnancy: Immunological response, natural course and pregnancy outcomes. J. Clin. Med. 2021, 10, 2926.
- Wu, C.; Wu, X.; Xia, J. Hepatitis E virus infection during pregnancy. Virol. J. 2020, 17, 73.
- Wang, R.; Yan, W.; Du, M.; Tao, L.; Liu, J. The effect of influenza virus infection on pregnancy outcomes: A systematic review and meta-analysis of cohort studies. Int. J. Infect. Dis. 2021, 105, 567–578.
- Ragusa, R.; Platania, A.; Cuccia, M.; Zappalà, G.; Giorgianni, G.; D’Agati, P.; Bellia, M.A.; Marranzano, M. Measles and pregnancy: Immunity and immunization-What can be learned from observing complications during an epidemic year. J. Pregnancy 2020, 2020, 6532868.
- Canadian Paediatric Society. Rubella (German measles) in Pregnancy. Paediatr. Child Health 2007, 12, 798.
- Shi, T.-L.; Huang, L.-J.; Xiong, Y.-Q.; Zhong, Y.-Y.; Yang, J.-J.; Fu, T.; Lei, X.-F.; Chen, Q. The risk of herpes simplex virus and human cytomegalovirus infection during pregnancy upon adverse pregnancy outcomes: A meta-analysis. J. Clin. Virol. 2018, 104, 48–55.
- Njue, A.; Coyne, C.; Margulis, A.V.; Wang, D.; Marks, M.A.; Russell, K.; Das, R.; Sinha, A. The role of congenital cytomegalovirus infection in adverse birth outcomes: A review of the potential mechanisms. Viruses 2021, 13, 20.
- Condrat, C.E.; Filip, L.; Gherghe, M.; Cretoiu, D.; Suciu, N. Maternal HPV infection: Effects on pregnancy outcome. Viruses 2021, 13, 2455.
- World Health Organisation. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. 2013. Available online: https://www.who.int/hiv/pub/guidelines/arv2013/download/en/ (accessed on 30 July 2022).
- Yeruva, T.; Rajkumar, H.; Donugama, V. Vaginal lactobacilli profile in pregnant women with normal & abnormal vaginal flora. Indian J. Med. Res. 2017, 146, 534–540.
- Godoy-Vitorino, F.; Romaguera, J.; Zhao, C.; Vargas-Robles, D.; Ortiz-Morales, G.; Vázquez-Sánchez, F.; Sanchez-Vázquez, M.; de la Garza-Casillas, M.; Martinez-Ferrer, M.; White, J.R.; et al. Cervicovaginal fungi and bacteria associated with cervical intraepithelial neoplasia and high-risk human papillomavirus infections in a Hispanic population. Front. Microbiol. 2018, 9, 2533.
- Maki, Y.; Fujisaki, M.; Sato, Y.; Sameshima, H. Candida chorioamnionitis leads to preterm birth and adverse fetal-neonatal outcome. Infect. Dis. Obstet. Gynecol. 2017, 2017, 9060138.
- Garrison, A.; Boivin, M.; Khoshnood, B.; Courtin, D.; Alao, J.; Mireku, M.; Ibikounle, M.; Massougbodji, A.; Cot, M.; Bodeau-Livinec, F. Soil-transmitted helminth infection in pregnancy and long-term child neurocognitive and behavioral development: A prospective mother-child cohort in Benin. PLoS Negl. Trop. Dis. 2021, 15, e0009260.
- Murenjekwa, W.; Makasi, R.; Ntozini, R.; Chasekwa, B.; Mutasa, K.; Moulton, L.H.; Tielsch, J.M.; Humphrey, J.H.; Smith, L.E.; Prendergast, A.J.; et al. Determinants of urogenital schistosomiasis among pregnant women and its association with pregnancy outcomes, neonatal deaths, and child growth. J. Infect. Dis. 2021, 223, 1433–1444.
- Weill, A.; Bernigaud, C.; Mokni, M.; Gil, S.; Elefant, E.; Chosidow, O. Scabies-infested pregnant women: A critical therapeutic challenge. PLoS Negl. Trop. Dis. 2021, 15, e0008929.
- Chua, C.L.L.; Hasang, W.; Rogerson, S.J.; Teo, A. Poor birth outcomes in malaria in pregnancy: Recent insights into mechanisms and prevention approaches. Front. Immunol. 2021, 12, 621382.
- Thompson, J.M.; Eick, S.M.; Dailey, C.; Dale, A.P.; Mehta, M.; Nair, A.; Cordero, J.F.; Welton, M. Relationship between pregnancy-associated malaria and adverse pregnancy outcomes: A systematic review and meta-analysis. J. Trop. Pediatr. 2020, 66, 327–338.
- Kwizera, A.; Ntasumumuyange, D.; Small, M.; Rulisa, S.; Moscovitz, A.N.; Magriples, U. Assessment of perinatal outcomes of pregnant women with severe versus simple malaria. PLoS ONE 2021, 16, e0247053.
- Arranz-Solis, D.; Mukhopadhyay, D. Toxoplasma effectors that affect pregnancy outcomes. Trends Parasitol. 2021, 27, 283–295.
- Li, X.L.; Wei, H.X.; Zhang, H.; Peng, H.J.; Lindsay, D.S. A meta-analysis on risks of adverse pregnancy outcomes in Toxoplasma gondii infection. PLoS ONE 2014, 9, e97775.
- Van Gerwen, O.T.; Craig-Kuhn, M.C.; Jones, A.T.; Schroeder, J.A.; Deaver, J.; Buekens, P.; Kissinger, P.J.; Muzny, C.A. Trichomoniasis and adverse birth outcomes: A systematic review and meta-analysis. Br. J. Obst. Gynaecol. 2021, 128, 1907–1915.
- Gupta, P.; Singh, M.P.; Goyal, K. Diversity of vaginal microbiome in pregnancy: Deciphering the obscurity. Front. Public Health 2020, 8, 326.
- Amabebe, E.; Anumba, D.O.C. The vaginal microenvironment: The physiologic role of Lactobacilli. Front. Med. 2018, 5, 181.
- González-Sánchez, A.; Reyes-Lagos, J.J.; Peña-Castillo, M.A.; Nirmalkar, N.; García-Mena, J.; Pacheco-López, G. Vaginal microbiota is stable and mainly dominated by Lactobacillus at third trimester of pregnancy and active childbirth: A longitudinal study of ten Mexican women. Curr. Microbiol. 2022, 79, 230.
- Serrano, M.G.; Parikh, H.I.; Brooks, J.P.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bpkhari, Y.A.; Bradley, S.P.; et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019, 25, 1001–1011.
- Chen, X.; Lu, Y.; Chen, T.; Li, R. The female vaginal microbiome in health and bacterial vaginosis. Front. Cell. Infect. Microbiol. 2021, 11, 631972.
- van der Veer, C.; Hertzberger, R.Y.; Bruisten, S.M.; Tytgat, H.L.P.; Swanenburg, J.; de Kat Angelino-Bart, A.; Schuren, F.; Molenaar, D.; Reid, G.; de Vries, H.; et al. Comparative genomics of human Lactobacillus crispatus isolates reveals genes for glycosylation and glycogen degradation: Implications for in vivo dominance of the vaginal microbiota. Microbiome 2019, 7, 49.
- Edwards, V.L.; Smith, S.B.; McComb, E.J.; Tamarelle, J.; Ma, B.; Humphrys, M.S.; Gajer, P.; Gwilliam, K.; Schaefer, A.M.; Lai, S.K.; et al. The cervicovaginal microbiota-host interaction modulates Chlamydia trachomatis infection. mBio 2019, 10, e01548-19.
- Al-Memar, M.; Bobdiwala, S.; Fourie, H.; Mannino, R.; Lee, Y.S.; Smith, A.; Marchesi, J.R.; Timmerman, D.; Bourne, T.; Bennett, P.R.; et al. The association between vaginal bacterial composition and miscarriage: A nested case–control study. Br. J. Obst. Gynaecol. 2020, 127, 264–274.
- Kanmaz, A.G.; İnan, A.H.; Beyan, E.; Budak, A. The effects of threatened abortions on pregnancy outcomes. Ginekol. Pol. 2019, 90, 195–200.
- Christiansen, O.B.; Steffensen, R.; Nielsen, H.S.; Varming, K. Multifactorial etiology of recurrent miscarriage and its scientific and clinical implications. Gynecol. Obstet. Investig. 2008, 66, 257–267.
- Giakoumelou, S.; Wheelhouse, N.; Cuschieri, K.; Entrican, G.; Howie, S.E.; Horne, A.W. The role of infection in miscarriage. Hum. Reprod. Update 2016, 22, 116–133.
- Shahid, M.; Quinlivan, J.A.; Peek, M.; Castaño-Rodríguez, N.; Mendz, G.L. Is there an association between the vaginal microbiome and first trimester miscarriage? A prospective observational study. J. Obs. Gyn. Res. 2022, 48, 119–128.
- Fu, M.; Zhang, X.; Liang, Y.; Lin, S.; Qian, W.; Fan, S. Alterations in vaginal microbiota and associated metabolome in women with recurrent implantation failure. mBio 2020, 1, e03242-19.
- Grewal, K.; Lee, Y.S.; Smith, A.; Brosens, J.J.; Bourne, T.; Al-Memar, M.; Kundu, S.; MacIntyre, D.A.; Bennett, P. Chromosomally normal miscarriage is associated with vaginal dysbiosis and local inflammation. BMC Med. 2022, 20, 38.
- Valenti, P.; Rosa, L.; Capobianco, D.; Lepanto, M.S.; Schiavi, E.; Cutone, A.; Paesano, R.; Mastromarino, P. Role of lactobacilli and lactoferrin in the mucosal cervicovaginal defense. Front. Immunol. 2018, 9, 376.
- Kuon, R.J.; Togawa, R.; Vomstein, K.; Weber, M.; Goeggl, T.; Strowitzki, T.; Markert, U.R.; Zimmermann, S.; Daniel, V.; Dalpke, A.H.; et al. Higher prevalence of colonization with Gardnerella vaginalis and Gram-negative anaerobes in patients with recurrent miscarriage and elevated peripheral natural killer cells. J. Reprod. Immunol. 2017, 120, 15–19.
- Nija, R.J.; Sanju, S.; Sidharthan, N.; Mony, U. Extracellular trap by blood cells: Clinical implications. Tissue Eng. Regen. Med. 2020, 17, 141–153.
- Tong, M.; Abrahams, V.M. Neutrophils in preterm birth: Friend or foe? Placenta 2020, 102, 17–20.
- Omeljaniuk, W.J.; Jabłońska, E.; Garley, M.; Pryczynicz, A.; Ratajczak-Wrona, W.; Socha, K.; Borawska, M.H.; Charkiewicz, A.E. Biomarkers of neutrophil extracellular traps (NETs) and nitric oxide-(NO)-dependent oxidative stress in women who miscarried. Sci. Rep. 2020, 10, 13088.
- Hug, L.; You, D.; Blencowe, H.; Mishra, A.; Wang, Z.; Fix, M.J.; Wakefield, J.; Moran, A.C.; Gaigbe-Togbe, V.; Suzuki, E.; et al. Global, regional, and national estimates and trends in stillbirths from 2000 to 2019: A systematic assessment. Lancet 2021, 398, 772–785.
- Eunice Kennedy Shriver National Institute of Child Health and Human Development. What Are Possible Causes of Stillbirth? Available online: https://www.nichd.nih.gov/health/topics/stillbirth/topicinfo/causes# (accessed on 22 December 2022).
- Megli, C.J.; Coyne, C.B. Infections at the maternal–fetal interface: An overview of pathogenesis and defence. Nat. Rev. Microbiol. 2022, 20, 67–82.
- Stillbirth, Cleveland Clinic. 2023. Available online: https://my.clevelandclinic.org/health/diseases/9685-stillbirth (accessed on 15 February 2023).
- Page, J.M.; Bardsley, T.; Thorsten, V.; Allshouse, A.A.; Varner, M.W.; Debbink, M.P.; Dudley, D.J.; Saade, G.R.; Goldenberg, R.L.; Stoll, B.; et al. Stillbirth associated with infection in a diverse U.S. cohort. Obstet. Gynecol. 2019, 134, 1187–1196.
- Cao, G.; Liu, J.; Liu, M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990–2019. JAMA Pediatr. 2022, 176, 787–796.
- Ohuma, E.O.; Moller, A.-B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271.
- Cappelletti, M.; Presicce, P.; Kallapur, S.G. Immunobiology of acute chorioamnionitis. Front. Immunol. 2020, 11, 649.
- Gomez-Lopez, N.; Romero, R.; Tarca, A.L.; Miller, D.; Panaitescu, B.; Schwenkel, G.; Gudicha, D.W.; Hassan, S.S.; Pacora, P.; Jung, E.; et al. Gasdermin D: Evidence of pyroptosis in spontaneous preterm labor with sterile intra-amniotic inflammation or intra-amniotic infection. Am. J. Reprod. Immunol. 2019, 82, e13184.
- Zhang, X.; Zhai, Q.; Wang, J.; Ma, X.; Xing, B.; Fan, H.; Gao, Z.; Zhao, F.; Liu, W. Variation of the vaginal microbiome during and after pregnancy in Chinese women. Genom. Proteom. Bioinform. 2022, 20, 7.
- Sun, S.; Serrano, M.G.; Fettweis, J.M.; Basta, P.; Rosen, E.; Ludwig, K.; Sorgen, A.A.; Blakley, I.C.; Wu, M.C.; Nancy Dole, N. Race, the vaginal microbiome, and spontaneous preterm birth. mSystems 2022, 7, 3.
- Petrova, M.I.; Reid, G.; Vaneechoutte, M.; Lebeer, S. Lactobacillus iners: Friend or foe? Trends Microbiol. 2017, 25, 182–191.
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021.
- Gonçalves, B.P.; Procter, S.R.; Paul, P.; Chandna, J.; Lewin, A.; Seedat, F.; Koukounari, A.; Dangor, Z.; Leahy, S.; Santhanam, S.; et al. Group B streptococcus infection during pregnancy and infancy: Estimates of regional and global burden. Lancet Glob. Health 2022, 10, e807–e819.
- Hong, X.; Yin, J.; Wang, W.; Zhao, F.; Ding, X.; Yu, H.; Zhang, X.; Huang, B. The associations between low abundance of Mycoplasma hominis and female fecundability: A pregnancy-planning cohort study. BMC Microbiol. 2022, 22, 121.
- Marangoni, A.; Laghi, L.; Zagonari, S.; Patuelli, G.; Zhu, C.; Foschi, C.; Morselli, S.; Pedna, M.F.; Sambri, V. New insights into vaginal environment during pregnancy. Front. Mol. Biosci. 2021, 8, 656844.
- Di Giulio, D.B.; Callahan, B.J.; McMurdie, P.J.; Costello, E.K.; Lyella, D.J.; Robaczewska, A.; Suna, C.L.; Goltsman, D.S.A.; Wong, R.J.; Shawa, G.; et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 2015, 112, 11060–11065.
- Nguyen, A.T.C.; Le Nguyen, N.T.; Hoang, T.T.A.; Nguyen, T.T.; Tran, T.T.Q.; Tran, D.N.T.; Nguyen, A.T.K.; Tran, L.M.; Nguyen, D.H.C.; Le, T.M.; et al. Aerobic vaginitis in the third trimester and its impact on pregnancy outcomes. BMC Pregnancy Childbirth 2022, 22, 432.
- Ma, X.; Wu, M.; Wang, C.; Li, H.; Fan, A.; Wang, Y.; Cha, H.; Xue, F. The pathogenesis of prevalent aerobic bacteria in aerobic vaginitis and adverse pregnancy outcomes: A narrative review. Reprod. Health 2022, 19, 21.
- Sakabe, Y.; Nishizawa, H.; Kato, A.; Noda, Y.; Ohwaki, A.; Yoshizawa, H.; Kato, T.; Sekiya, T.; Fujii, T.; Kurahashi, H. Longitudinal study of the vaginal microbiome in pregnancies involving preterm labor. Fujita Med. J. 2022, 8, 96–101.
- Yao, Y.; Cai, X.; Chen, C.; Fang, H.; Zhao, Y.; Fei, W.; Chen, F.; Zheng, C. The Role of microbiomes in pregnant women and offspring: Research progress of recent years. Front. Pharmacol. 2020, 11, 643.
More
Information
Subjects:
Obstetrics & Gynaecology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
476
Revisions:
3 times
(View History)
Update Date:
22 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




