Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pradeep Kumar Bolla | -- | 2690 | 2023-12-18 16:48:41 | | | |
| 2 | Rita Xu | Meta information modification | 2690 | 2023-12-19 03:14:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Corrie, L.; Ajjarapu, S.; Banda, S.; Parvathaneni, M.; Bolla, P.K.; Kommineni, N. HPMCAS-Based Amorphous Solid Dispersions in Clinic. Encyclopedia. Available online: https://encyclopedia.pub/entry/52888 (accessed on 07 February 2026).
Corrie L, Ajjarapu S, Banda S, Parvathaneni M, Bolla PK, Kommineni N. HPMCAS-Based Amorphous Solid Dispersions in Clinic. Encyclopedia. Available at: https://encyclopedia.pub/entry/52888. Accessed February 07, 2026.
Corrie, Leander, Srinivas Ajjarapu, Srikanth Banda, Madhukiran Parvathaneni, Pradeep Kumar Bolla, Nagavendra Kommineni. "HPMCAS-Based Amorphous Solid Dispersions in Clinic" Encyclopedia, https://encyclopedia.pub/entry/52888 (accessed February 07, 2026).
Corrie, L., Ajjarapu, S., Banda, S., Parvathaneni, M., Bolla, P.K., & Kommineni, N. (2023, December 18). HPMCAS-Based Amorphous Solid Dispersions in Clinic. In Encyclopedia. https://encyclopedia.pub/entry/52888
Corrie, Leander, et al. "HPMCAS-Based Amorphous Solid Dispersions in Clinic." Encyclopedia. Web. 18 December, 2023.
Copy Citation
Therapeutic candidates with low solubility have become increasingly common in pharmaceutical research pipelines. Several techniques such as hot melt extrusion, spray drying, supercritical fluid technology, electrospinning, KinetiSol, etc., have been devised to improve either or both the solubility and dissolution to enhance the bioavailability of these active substances belonging to BCS Class II and IV.
amorphous solid dispersions (ASDs)
hydroxypropylmethylcellulose acetate succinate (HPMCAS)
clinical trials
1. Introduction
Active pharmaceutical ingredient (API) solubility and bioavailability have been key impediments to the development of more efficient drug delivery techniques for many decades [1]. Various techniques for overcoming this challenge have been offered in the literature, including amorphous solid dispersions (ASDs) [2], nanosuspension [3], polymeric nanoparticles [4][5][6][7][8], liposomes [9], liquid–solid compacts [10][11], solid lipid nanoparticles [12][13][14], self-emulsifying drug delivery systems [15] and other delivery systems [16][17][18][19]. ASDs have been described as the most effective technique for increasing the solubility and dissolution rate, and, as a result, the oral bioavailability of poor water-soluble drugs throughout the last few decades [20]. Because ASDs improve apparent solubility, they also increase apparent permeability, allowing for the oral administration of medicines with low-aqueous solubility [21][22][23][24].
ASDs are single-phase systems in which drug molecules are disseminated or dissolved in one or more polymeric carriers [25]. When compared to pure amorphous drugs, the inclusion of polymeric carriers provides various advantages, including long-term storage stability and improved dissolution capabilities [26][27]. Hydroxypropylmethylcellulose acetate succinate (HPMCAS) has been widely used in ASD formulations as a polymeric carrier. HPMCAS is an effective polymer for the formulation of ASD with dual functionality: solubilization of poorly soluble drugs, promotion of rapid dissolution in the intestinal medium, and prevention of subsequent precipitation of drugs by maintaining supersaturation concentration of the drug molecules [28][29][30][31][32][33]. Several processes, including hot melt extrusion (HME) and spray drying, are used to manufacture ASDs [34]; however, the use of these techniques are dependent on the qualities of the API, desired product attributes and appropriate processing window. Among these approaches, hot melt extrusion (HME) is a supplementary pharmaceutical production technology that is frequently employed by industrial and academic researchers to address API solubility difficulties. In recent years, similar to the lyophilization technique, HME has been used as a drying technique for the solidification or removal of moisture from nanosuspension [35][36]. Though lyophilization is a widely employed drying technique for pharmaceuticals, its usage is associated with time and cost constraints.
2. Industrial Scale Manufacturing Techniques
2.1. Hot Melt Extrusion
Hot melt extrusion (HME) and spray drying (SD) are the most commonly used techniques for the development of ASDs (Figure 1). Hot melt extrusion is one of the most efficient solid dispersion manufacturing processes. The API and the polymer matrix are blended together in this procedure to create a physical combination that may be extruded under various circumstances [37]. Processing parameters, for example, feed rate, shear force, temperature, die geometry, barrel design, screw speed and other variables should be taken into account when using this method [38]. These parameters could have a considerable impact on the final product’s quality. HME has various advantages over other conventional approaches, including a continuous, one-step, solvent-free operation with fewer processing steps. Furthermore, it does not require compression and can increase the bioavailability as well as increase the biodistribution of the drug at the molecular level [39]. However, some of the restrictions that could limit its application in scaling up and technology transfer are as follows: the need for a larger energy input as compared to other approaches, as well as the likely exclusion of some thermo-labile chemicals due to the high processing temperatures involved [40]. HME has been used successfully to combine various pharmaceutical drug delivery techniques, such as conversion to salts/prodrug approach [41], lipid-based delivery systems [42], immediate and modified release tablets [43] and 3D printing [44][45].
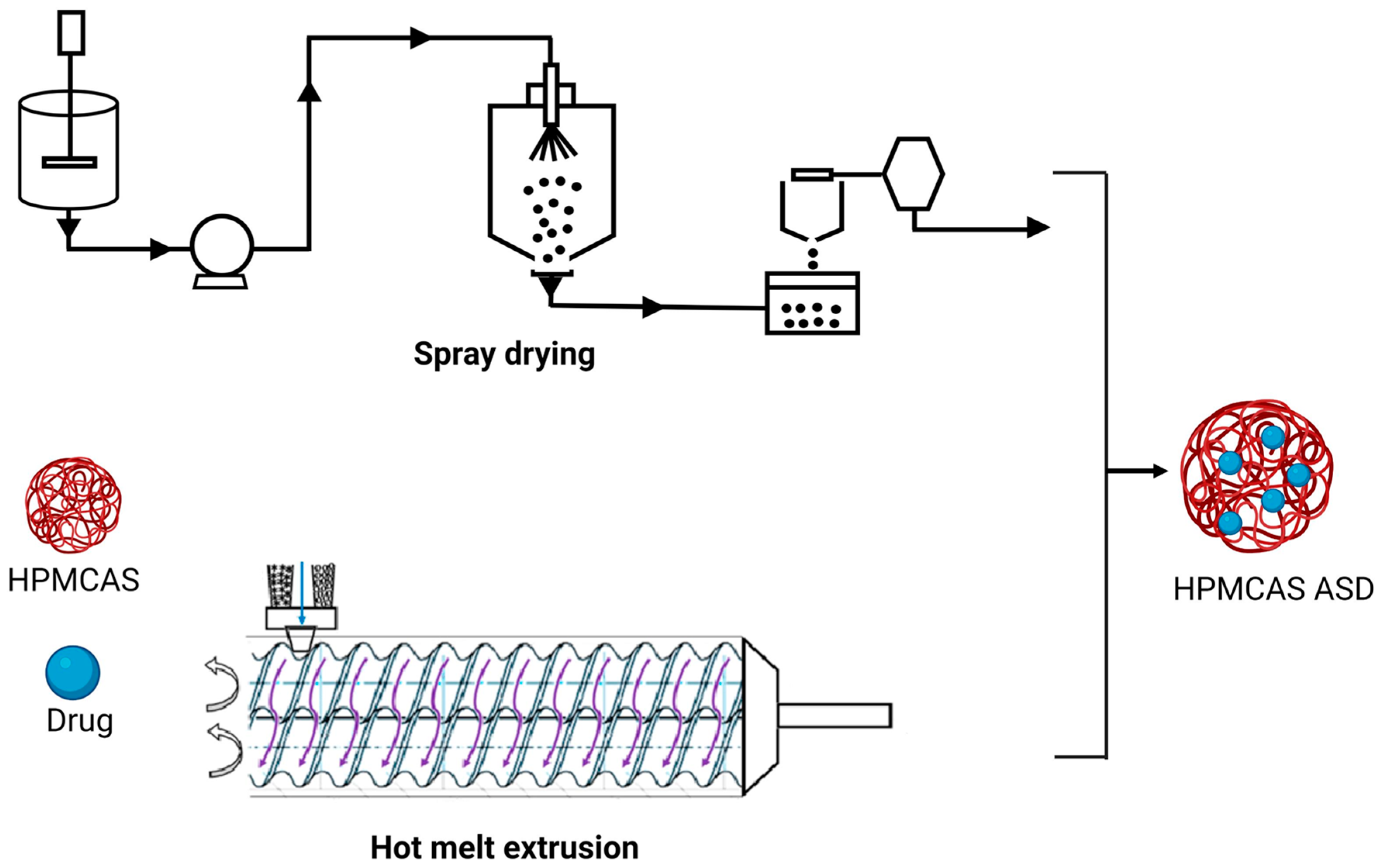
Figure 1. Schematic representation of HME and SD in ASD manufacturing.
The barrel, feeder and screw parts are the major components of the extruder in HME [46][47]. The extruder barrel is made up of a feeding portion, a venting section and a closed segment design. To soften or reduce the viscosity of the polymer, each part of the barrel can be heated [48]. The feeder facilitates the transmission of material to the barrel. In the HME process, the starvation feeder with a screw speed independent of feed rate is most typically utilized. Screw elements aid in the mixing, transferring, and ultimately pushing of the melt through a die. The screw design makes it easier to set up multiple screw configurations to generate low or high shear. The conveying components aid in pushing the solid material within the barrel, whereas the kneading elements aid in the kneading process [49]. In HME, the crystalline API is dissolved (when the processing temperature is above the melting point of API) or molecularly dispersed (when the processing temperature is below the melting point of API) within the molten polymer owing to the thermal and mechanical energies generated by the rotating screws and heated barrel, respectively [50]. When using HME for ASD, it is necessary to ensure the processing temperature does not degrade the drug or polymer [38]. The HME process can be broken down into the following steps: feeding, melting and plasticizing, conveying and mixing, venting, stripping, and downstream processing [44][45]. While employing HME for ASD production, processing parameters of HME, such as barrel temperature, screw speed, feed rate, barrel design, die geometry and shear force, should be considered [42].
2.2. Spray Drying
The spray-drying process requires numerous phases involving diverse components. The feed solution/suspension is first introduced into the drying chamber through a nozzle. Droplets are atomized when they escape the nozzle tip and come into contact with the drying fluid, which is hot gas (typically air) inside the drying chamber. The residence duration within the drying chamber is determined by the process parameters and the size of the equipment and can normally last a few milliseconds. At the dynamic droplet surface, energy/mass transfer occurs during the transit through the drying chamber. Finally, using a cyclone, the dried material is separated from the drying area and collected in a collection vessel. The exhaust gases are filtered via HEPA filters. The feed pump utilized is determined by the viscosity of the feed material and the type of atomizer system used [51][52][53][54][55].
When employing this atomization setup, make sure to use a drying chamber with a large enough diameter. Material adherence to the drying chamber walls may limit its application for costly drugs and active moieties. The droplet size distribution produced by the atomization setup dictates the residence time required in the chamber as well as its dimensions. The character of the gas flow (turbulent or laminar) will also influence droplet residence time and final product moisture content. The need for strict inter-batch control of the temperature and humidity of the drying air is especially important for spray drying of amorphous systems [54]. Particles are gathered after drying, utilizing certain design elements and separating mechanisms. The relatively small particle size utilized in medications necessitates this. Particle collection might occur in the bottom of the drying chamber, where it must be scrapped [56]. Bag filters and cyclones are common particle separating devices. In pharmaceutical contexts, typical cyclones are reverse-flow, gas-solid separators in which centrifugal force causes the separation of two phases with differing masses [57].
Spray-drying ASDs are created by evaporating a solvent or a solvent mixture from a drug and polymeric carrier solution. Atomization of the feed stock solution or suspension, droplet-gas contact, droplet drying and particle creation, and separation of dried solid particles from the drying medium (wet drying gas) are all processes in the SD process [58]. To eliminate any leftover solvent, the powder may need to be dried again. While spray drying ASDs, the following critical processing variables should be considered: solution viscosity, total solid content in the spray solution, input and outlet temperatures, nozzle selection and drying gas flow rate [59][60][61][62].
3. Marketed HPMCAS ASD Formulations
The increased Food and Drug Administration (FDA) approval of ASD products in recent years implies that ASD technology can be used to improve the dissolution rate and bioavailability of poorly soluble pharmaceuticals. Over the previous 12 years, the FDA has approved more than 20 ASD products. HPMCAS is used in eight ASD products.
3.1. Erleada®
Erleada® is available in two strengths (60 mg and 240 mg) as an immediate-release tablet formulation of apalutamide [63]. It is indicated for non-metastatic, castration-resistant prostate cancer. Apalutamide is practically insoluble at physiological pH. The preparation process includes, firstly, the preparation of the ASD of apalutamide by using HPMCAS through spray drying [64][65]. The manufacturing process includes pre-blending of the ASD, granulation, extra-granular blending, lubrication, compression and film coating of tablets. A mean Cmax of 6 µg/mL and 100 µg h/mL was achieved at steady state [66][67].
3.2. Trikafta®
Trikafta® is an immediate-release triple fixed-dose tablet formulation of elexacaftor, tezacaftor and ivacaftor. It is used in the treatment of cystic fibrosis in patients with F50del allele mutation. All three active ingredients have low solubility [68]. Tezacaftor is a BCS Class II molecule, whereas elexacaftor and ivacaftor can be classified as either BCS Class II or IV molecules based on available information [69]. Elexacaftor has a solubility of less than 0.1 mg/mL in pH buffers ranging from 1.0 to 8.0. Tezacaftor has a solubility of 0.004 mg/mL in pH 1.0, 0.003 mg/mL in pH 4.5 and 0.005 mg/mL in pH 6.8. Trikafta is prepared by a continuous process involving spray drying, blending, granulation, compression of granules into core tablets and film coating of tablets [66]. A new formulation, granules, was later approved in 2021 for the same combination prepared through the same manufacturing process [70][71].
3.3. Delstrigo®
Delstrigo® is a fixed-dose combination tablet containing Doravirine (100 mg)/lamivudine (300 mg)/tenofovir disoproxil fumarate (300 mg) indicated for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment history. Delstrigo® is a bilayer tablet, comprising an ASD formulation of doravirine in the first layer, and lamivudine, tenofovir disoproxil fumarate are separately co-granulated in the second layer [72]. Doravirine is a non-hygroscopic, crystalline powder, which is practically insoluble in water, considered as a Biopharmaceutical Classification System (BCS) class II compound. Lamivudine is soluble in water, sparingly soluble in methanol, slightly soluble in ethanol, and is considered a BCS class III compound. Tenofovir disoproxil fumarate is a slightly hygroscopic powder, slightly soluble in water and sparingly soluble in pH buffers 2.0–8.0. It is considered a BCS class III compound. The manufacturing process consists of spray drying, blending, lubrication, roller compaction, tablet compression and film coating. Briefly, a spray-dried intermediate (SDI) of Doravirine is manufactured by spray drying a solution of HPMCAS and doravirine dissolved in a mixture of water and organic solvent. The SDI is blended and roller compacted after combining with excipients to produce doravirine granules. Lamivudine and tenofovir disoproxil fumarate are combined with excipients, blended and roller compacted to produce the granules. Then, the granules are compressed into a bilayer tablet core and film coated [73].
3.4. Symdeko®
Symdeko® is a fixed-dose combination of tezacaftor (100 mg)/ivacaftor (150 mg) and ivacaftor (150 mg), indicated for the treatment of patients with cystic fibrosis. Tezacaftor is a crystalline material, practically insoluble in water and buffer solutions from pH 1.0 to pH 9.0. Ivacaftor, active in the combination drug product, has poor dissolution and bioavailability properties in its crystalline form; thus, preparation of an ASD using HPMCAS via spray drying was employed to overcome the solubility and dissolution-limited bioavailability [74].
The oral bioavailability of tezacaftor was not determined in humans; however, it was moderate in rats and dogs (~40 to 50%). Absorption of tezacaftor does not vary during fasting or when consumed with fatty foods. In the case of ivacaftor, absorption increases three-fold with fat containing foods. The bioavailability of the crystalline drug and HPMCAS ASD dosed as oral suspension was evaluated using a vehicle composed of methyl cellulose/SLS/water (0.5/0.5/99%). The crystalline form had a bioavailability of 3–6%, whereas solid dispersion showed a bioavailability of around 100%, demonstrating that absorption was dependent on solubility [75].
3.5. Orkambi®
Orkambi® is a fixed-dose combination immediate-release tablet comprising lumacaftor (200 mg) and ivacaftor (125 mg) for the treatment of cystic fibrosis. Both lumacaftor and ivacaftor are practically insoluble in water with an aqueous solubility of 0.02 mg/mL and <0.05 mg/mL, respectively. Therefore, it is important to develop the amorphous form of active substances in order to improve solubility limited oral bioavailability. In the initial phase-I trials, the bioavailability of a capsule formulation of lumacaftor following oral dose in healthy fasted males showed a higher oral bioavailability with Cmax and AUC0-inf values 1.4 times higher compared to suspension formulation, and the median Tmax values for capsule and suspension formulation were 3 h and 4 h, respectively, indicating the capsule formulation was absorbed more slowly than the suspension. Following multiple oral-dose administrations of ivacaftor in combination with lumacaftor, the exposure of ivacaftor increased approximately 2.5 to 3.4-fold when administered with food containing fat [76].
3.6. Noxafil® Delayed Release Tablets
The Noxafil® is a delayed release tablet of posaconazole, developed using HME technology to alleviate the inconsistent pharmacokinetics and variable oral bioavailability associated with the oral suspension form of posaconazole. Posaconazole is a broad-spectrum antifungal agent used for prophylaxis and the treatment of fungal infections. A pH sensitive, enteric polymer (HPMCAS) was used to prevent the release of posaconazole in the acidic gastric environment of the stomach, allowing for release in the intestinal site. This delayed release mechanism significantly improved oral bioavailability of posaconazole and achieved higher plasma drug levels with less variability for tablet ASD formulations compared to the suspension formulation. Furthermore, patient’s fed/fasting state effect or consequent administration of medications had no discernible influence on the delayed release of tablet formulation [77][78].
3.7. Kalydeco®
Kalydeco® is a spray-dried ASD formulation of ivacaftor, indicated for the treatment of cystic fibrosis. When administered in crystalline form, it had an oral bioavailability of 3–6% in rats due to solubility-limited oral absorption. The ASD of ivacaftor was formulated using HPMCAS to overcome the solubility limitations and improve formulation stability. The ASD formulation of ivacaftor exhibited superior solubility (67.4 μg/mL) compared to the solubility of its crystalline polymorph B (1 μg/mL). Furthermore, the ASD formulation demonstrated relative bioavailability of 100% compared to crystalline ivacaftor [79]. After single-dose oral administration to healthy adult volunteers in a fed state, the mean AUC and Cmax observed were 10,600 ng h/mL and 768 ng/mL, respectively [80][81].
3.8. Zelboraf™
Zelboraf™ is the ASD formulation containing vemurafenib in HPMCAS-LF (30:70, w/w), produced by the solvent/antisolvent precipitation process. The process involves dissolving the drug, ionic polymer (HPMCAS) in N, N-dimethylacetamide, then the solution is precipitated by transferring it into acidified aqueous media. The precipitates are then filtered, washed repeatedly to remove the trace levels of acid and solvent content, vacuum dried and milled to obtain an amorphous powder intermediate known as microprecipitated bulk powder. This amorphous powder provides excellent physical stability with improved oral bioavailability.
The vemurafenib dissolution from its solid dispersions is ~30 times more than crystalline vemurafenib, resulting in approximately five times higher vemurafenib plasma concentrations. During phase-I clinical trials, no tumor regression was observed with conventional vemurafenib formulation at a dose as high as 1600 mg due to the limitations of poor solubility and low oral bioavailability. When patients are treated with ASD of vemurafenib, a substantial tumor regression was achieved as a result of enhanced formulation performance [82].
3.9. Incivek®
Incivek® is a tablet ASD formulation of telaprevir produced using the spray-drying technique. It is an immediate-release tablet containing 375 mg of telaprevir with a total target weight of 1 g.
Telaprevir is a hepatitis C protease inhibitor that is used to treat genotype 1 chronic hepatitis C in conjunction with peginterferon alfa and ribavirin. Telaprevir is most likely absorbed in the small intestine; nevertheless, absolute bioavailability in humans has not been determined. Telaprevir bioavailability is influenced by food. In phase-3 studies, when a 375 mg tablet formulation was administered to healthy subjects, a three- to four-fold increase in AUC and Cmax of telaprevir was seen in a fed condition compared to a fasted state. The crystalline form of telaprevir has an aqueous solubility of 4.7 μg/mL and does not ionize between pH 1 and 7, suggesting the poor bioavailability of the drug. Hence, product development focused on converting the crystalline form of telaprevir to a stable amorphous form utilizing HPMCAS and spray-drying technique. The manufacturing process involves the addition of a stabilizing polymer (HPMCAS) to the spray-drying mixture containing drug and organic solvents, then secondary drying of the mixture to remove any residual solvent, tableting with extra granular excipients, compressing the tablets, and finally coating the tablets with a film coating [83].
References
- Reddy, A.B.; Reddy, N.D. Development of Multiple-Unit Floating Drug Delivery System of Clarithromycin: Formulation, in vitro Dissolution by Modified Dissolution Apparatus, in vivo Radiographic Studies in Human Volunteers. Drug Res. 2017, 67, 412–418.
- Mamidi, H.K.; Rohera, B.D. Application of Thermodynamic Phase Diagrams and Gibbs Free Energy of Mixing for Screening of Polymers for Their Use in Amorphous Solid Dispersion Formulation of a Non-Glass-Forming Drug. J. Pharm. Sci. 2021, 110, 2703–2717.
- Karri, V.; Butreddy, A.; Dudhipala, N. Fabrication of Efavirenz Freeze Dried Nanocrystals: Formulation, Physicochemical Characterization, In Vitro and Ex Vivo Evaluation. Adv. Sci. Eng. Med. 2015, 7, 385–392.
- Sarkar, C.; Kommineni, N.; Butreddy, A.; Kumar, R.; Bunekar, N.; Gugulothu, K. PLGA Nanoparticles in Drug Delivery. In Nanoengineering of Biomaterials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 217–260.
- Butreddy, A.; Gaddam, R.P.; Kommineni, N.; Dudhipala, N.; Voshavar, C. PLGA/PLA-Based Long-Acting Injectable Depot Microspheres in Clinical Use: Production and Characterization Overview for Protein/Peptide Delivery. Int. J. Mol. Sci. 2021, 22, 8884.
- Bolla, P.K.; Gote, V.; Singh, M.; Yellepeddi, V.K.; Patel, M.; Pal, D.; Gong, X.; Sambalingam, D.; Renukuntla, J. Preparation and characterization of lutein loaded folate conjugated polymeric nanoparticles. J. Microencapsul. 2020, 37, 502–516.
- Rodriguez, V.A.; Bolla, P.K.; Kalhapure, R.S.; Boddu, S.H.S.; Neupane, R.; Franco, J.; Renukuntla, J. Preparation and Characterization of Furosemide-Silver Complex Loaded Chitosan Nanoparticles. Processes 2019, 7, 206.
- Bolla, P.K.; Gote, V.; Singh, M.; Patel, M.; Clark, B.A.; Renukuntla, J. Lutein-Loaded, Biotin-Decorated Polymeric Nanoparticles Enhance Lutein Uptake in Retinal Cells. Pharmaceutics 2020, 12, 798.
- Jyothi, V.G.S.; Bulusu, R.; Rao, B.V.K.; Pranothi, M.; Banda, S.; Bolla, P.K.; Kommineni, N. Stability characterization for pharmaceutical liposome product development with focus on regulatory considerations: An update. Int. J. Pharm. 2022, 624, 122022.
- Butreddy, A.; Dudhipala, N. Enhancement of Solubility and Dissolution Rate of Trandolapril Sustained Release Matrix Tablets by Liquisolid Compact Approach. Asian, J. Pharm. 2015, 9, 290–297.
- Mamidi, H.K.; Mishra, S.M.; Rohera, B.D. Application of modified SeDeM expert diagram system for selection of direct compression excipient for liquisolid formulation of Neusilin® US2. J. Drug Deliv. Sci. Technol. 2021, 64, 102506.
- Bolla, P.K.; Kalhapure, R.S.; Rodriguez, V.A.; Ramos, D.V.; Dahl, A.; Renukuntla, J. Preparation of solid lipid nanoparticles of furosemide-silver complex and evaluation of antibacterial activity. J. Drug Deliv. Sci. Technol. 2019, 49, 6–13.
- Amis, T.M.; Renukuntla, J.; Bolla, P.K.; Clark, B.A. Selection of Cryoprotectant in Lyophilization of Progesterone-Loaded Stearic Acid Solid Lipid Nanoparticles. Pharmaceutics 2020, 12, 892.
- Corrie, L.; Gundaram, R.; Kukatil, L. Formulation and evaluation of Cassia tora phytosomal gel using central composite design. Recent Innov. Chem. Eng. 2021, 14, 347–357.
- Kallakunta, V.R.; Bandari, S.; Jukanti, R.; Veerareddy, P.R. Oral self emulsifying powder of lercanidipine hydrochloride: Formulation and evaluation. Powder Technol. 2012, 221, 375–382.
- Butreddy, A.; Narala, A.; Dudhipala, N. Formulation and characterization of Liquid Crystalline Hydrogel of Agomelatin: In vitro and Ex vivo evaluation. J. Appl. Pharm. Sci. 2015, 5, 110–114.
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481.
- Bolla, P.K.; Meraz, C.A.; Rodriguez, V.A.; Deaguero, I.; Singh, M.; Yellepeddi, V.K.; Renukuntla, J. Clotrimazole Loaded Ufosomes for Topical Delivery: Formulation Development and In-Vitro Studies. Molecules 2019, 24, 3139.
- Pasika, S.R.; Bulusu, R.; Rao, B.V.K.; Kommineni, N.; Bolla, P.K.; Kala, S.G.; Godugu, C. Nanotechnology for Biomedical Applications. In Nanomaterials; Singh, D.K., Singh, S., Singh, P., Eds.; Springer Nature Singapore: Singapore, 2023; pp. 297–327.
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075.
- Hancock, B.C.; Parks, M. What is the True Solubility Advantage for Amorphous Pharmaceuticals? Pharm. Res. 2000, 17, 397–404.
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int. J. Pharm. 2020, 586, 119560.
- Corrie, L.; Kaur, J.; Awasthi, A.; Vishwas, S.; Gulati, M.; Saini, S.; Kumar, B.; Pandey, N.K.; Gupta, G.; Dureja, H.; et al. Multivariate Data Analysis and Central Composite Design-Oriented Optimization of Solid Carriers for Formulation of Curcumin-Loaded Solid SNEDDS: Dissolution and Bioavailability Assessment. Pharmaceutics 2022, 14, 2395.
- Guner, G.; Amjad, A.; Berrios, M.; Kannan, M.; Bilgili, E. Nanoseeded Desupersaturation and Dissolution Tests for Elucidating Supersaturation Maintenance in Amorphous Solid Dispersions. Pharmaceutics 2023, 15, 450.
- Zhang, J.; Guo, M.; Luo, M.; Cai, T. Advances in the development of amorphous solid dispersions: The role of polymeric carriers. Asian, J. Pharm. Sci. 2023, 18, 100834.
- Jermain, S.V.; Brough, C.; Williams, R.O., 3rd. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2018, 535, 379–392.
- Nguyen, H.T.; Van Duong, T.; Taylor, L.S. Impact of Gastric pH Variations on the Release of Amorphous Solid Dispersion Formulations Containing a Weakly Basic Drug and Enteric Polymers. Mol. Pharm. 2023, 20, 1681–1695.
- Butreddy, A. Hydroxypropyl methylcellulose acetate succinate as an exceptional polymer for amorphous solid dispersion formulations: A review from bench to clinic. Eur. J. Pharm. Biopharm. 2022, 177, 289–307.
- Butreddy, A.; Sarabu, S.; Almutairi, M.; Ajjarapu, S.; Kolimi, P.; Bandari, S.; Repka, M.A. Hot-melt extruded hydroxypropyl methylcellulose acetate succinate based amorphous solid dispersions: Impact of polymeric combinations on supersaturation kinetics and dissolution performance. Int. J. Pharm. 2022, 615, 121471.
- Choudhari, M.; Damle, S.; Saha, R.N.; Dubey, S.K.; Singhvi, G. Emerging Applications of Hydroxypropyl Methylcellulose Acetate Succinate: Different Aspects in Drug Delivery and Its Commercial Potential. AAPS PharmSciTech. 2023, 24, 188.
- Nguyen, H.T.; Van Duong, T.; Jaw-Tsai, S.; Bruning-Barry, R.; Pande, P.; Taneja, R.; Taylor, L.S. Fed- and Fasted-State Performance of Pretomanid Amorphous Solid Dispersions Formulated with an Enteric Polymer. Mol. Pharm. 2023, 20, 3170–3186.
- Lalge, R.; Kumar, N.S.K.; Suryanarayanan, R. Understanding the Effect of Nucleation in Amorphous Solid Dispersions through Time–Temperature Transformation. Mol. Pharm. 2023, 20, 4196–4209.
- Cao, J.; Zhang, S.; Hao, Y.; Fan, K.; Wang, L.; Zhao, X.; He, X. Amorphous solid dispersion preparation via coprecipitation improves the dissolution, oral bioavailability, and intestinal health enhancement properties of magnolol. Poult. Sci. 2023, 102, 102676.
- Mendonsa, N.; Almutairy, B.; Kallakunta, V.R.; Sarabu, S.; Thipsay, P.; Bandari, S.; Repka, M.A. Manufacturing strategies to develop amorphous solid dispersions: An overview. J. Drug Deliv. Sci. Technol. 2019, 55, 101459.
- Gajera, B.Y.; Shah, D.A.; Dave, R.H. Investigating a Novel Hot Melt Extrusion-Based Drying Technique to Solidify an Amorphous Nanosuspension Using Design of Experiment Methodology. Aaps Pharmscitech 2018, 19, 3778–3790.
- Kalhapure, R.S.; Bolla, P.; Dominguez, D.C.; Dahal, A.; Boddu, S.H.; Renukuntla, J. FSE–Ag complex NS: Preparation and evaluation of antibacterial activity. IET Nanobiotechnology 2018, 12, 836–840.
- Butreddy, A.; Bandari, S.; Repka, M.A. Quality-by-design in hot melt extrusion based amorphous solid dispersions: An industrial perspective on product development. Eur. J. Pharm. Sci. 2020, 158, 105655.
- Sarabu, S.; Butreddy, A.; Bandari, S.; Batra, A.; Lawal, K.; Chen, N.N.; Kogan, M.; Bi, V.; Durig, T.; Repka, M.A. Preliminary investigation of peroxide levels of Plasdone™ copovidones on the purity of atorvastatin calcium amorphous solid dispersions: Impact of plasticizers on hot melt extrusion processability. J. Drug Deliv. Sci. Technol. 2022, 70, 103190.
- Butreddy, A.; Sarabu, S.; Dumpa, N.; Bandari, S.; Repka, M.A. Extended release pellets prepared by hot melt extrusion technique for abuse deterrent potential: Category-1 in-vitro evaluation. Int. J. Pharm. 2020, 587, 119624.
- Butreddy, A.; Sarabu, S.; Bandari, S.; Dumpa, N.; Zhang, F.; Repka, M.A. Polymer-Assisted Aripiprazole–Adipic Acid Cocrystals Produced by Hot Melt Extrusion Techniques. Cryst. Growth Des. 2020, 20, 4335–4345.
- Butreddy, A.; Almutairi, M.; Komanduri, N.; Bandari, S.; Zhang, F.; Repka, M.A. Multicomponent crystalline solid forms of aripiprazole produced via hot melt extrusion techniques: An exploratory study. J. Drug Deliv. Sci. Technol. 2021, 63, 102529.
- Sarabu, S.; Kallakunta, V.R.; Butreddy, A.; Janga, K.Y.; Ajjarapu, S.; Bandari, S.; Zhang, F.; Murthy, S.N.; Repka, M.A. A One-Step Twin-Screw Melt Granulation with Gelucire 48/16 and Surface Adsorbent to Improve the Solubility of Poorly Soluble Drugs: Effect of Formulation Variables on Dissolution and Stability. Aaps Pharmscitech 2021, 22, 79.
- Nyavanandi, D.; Kallakunta, V.R.; Sarabu, S.; Butreddy, A.; Narala, S.; Bandari, S.; Repka, M.A. Impact of hydrophilic binders on stability of lipid-based sustained release matrices of quetiapine fumarate by the continuous twin screw melt granulation technique. Adv. Powder Technol. 2021, 32, 2591–2604.
- Dumpa, N.; Butreddy, A.; Wang, H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 120501.
- Nukala, P.K.; Palekar, S.; Patki, M.; Patel, K. Abuse Deterrent Immediate Release Egg-Shaped Tablet (Egglets) Using 3D Printing Technology: Quality by Design to Optimize Drug Release and Extraction. Aaps Pharmscitech 2019, 20, 80.
- Mamidi, H.K.; Palekar, S.; Nukala, P.K.; Mishra, S.M.; Patki, M.; Fu, Y.; Supner, P.; Chauhan, G.; Patel, K. Process optimization of twin-screw melt granulation of fenofibrate using design of experiment (DoE). Int. J. Pharm. 2021, 593, 120101.
- Nukala, P.K.; Palekar, S.; Patki, M.; Fu, Y.; Patel, K. Multi-dose oral abuse deterrent formulation of loperamide using hot melt extrusion. Int. J. Pharm. 2019, 569, 118629.
- Butreddy, A.; Sarabu, S.; Bandari, S.; Batra, A.; Lawal, K.; Chen, N.N.; Bi, V.; Durig, T.; Repka, M.A. Influence of Plasdone™ S630 Ultra—An Improved Copovidone on the Processability and Oxidative Degradation of Quetiapine Fumarate Amorphous Solid Dispersions Prepared via Hot-Melt Extrusion Technique. AAPS Pharmscitech. 2021, 22, 196.
- Butreddy, A.; Nyavanandi, D.; Narala, S.; Austin, F.; Bandari, S. Application of Hot Melt Extrusion Technology in the Development of Abuse-Deterrent Formulations: An Overview. Curr. Drug Deliv. 2021, 18, 4–18.
- Bandari, S.; Nyavanandi, D.; Kallakunta, V.R.; Janga, K.Y.; Sarabu, S.; Butreddy, A.; Repka, M.A. Continuous twin screw granulation—An advanced alternative granulation technology for use in the pharmaceutical industry. Int. J. Pharm. 2020, 580, 119215.
- Poozesh, S.; Bilgili, E. Scale-up of pharmaceutical spray drying using scale-up rules: A review. Int. J. Pharm. 2019, 562, 271–292.
- Thybo, P.; Hovgaard, L.; Lindeløv, J.S.; Brask, A.; Andersen, S.K. Scaling Up the Spray Drying Process from Pilot to Production Scale Using an Atomized Droplet Size Criterion. Pharm. Res. 2008, 25, 1610–1620.
- Ziaee, A.; Albadarin, A.B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300–318.
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50.
- Corrie, L.; Gulati, M.; Awasthi, A.; Vishwas, S.; Kaur, J.; Khursheed, R.; Kumar, R.; Kumar, A.; Imran, M.; Chellappan, D.; et al. Polysaccharide, fecal microbiota, and curcumin-based novel oral colon-targeted solid self-nanoemulsifying delivery system: Formulation, characterization, and in-vitro anticancer evaluation. Mater. Today Chem. 2022, 26, 101165.
- Cal, K.; Sollohub, K. Spray Drying Technique. I: Hardware and Process Parameters. J. Pharm. Sci. 2010, 99, 575–586.
- E Snyder, H. Pharmaceutical spray drying: Solid-dose process technology platform for the 21st century. Ther. Deliv. 2012, 3, 901–912.
- Vasconcelos, T.; Marques, S.; das Neves, J.; Sarmento, B. Amorphous solid dispersions: Rational selection of a manufacturing process. Adv. Drug Deliv. Rev. 2016, 100, 85–101.
- Paudel, A.; Ayenew, Z.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284.
- Dobry, D.E.; Settell, D.M.; Baumann, J.M.; Ray, R.J.; Graham, L.J.; Beyerinck, R.A. A Model-Based Methodology for Spray-Drying Process Development. J. Pharm. Innov. 2009, 4, 133–142.
- Li, J.; Patel, D.; Wang, G. Use of Spray-Dried Dispersions in Early Pharmaceutical Development: Theoretical and Practical Challenges. AAPS J. 2017, 19, 321–333.
- Corrie, L.; Gulati, M.; Singh, S.K.; Kapoor, B.; Khursheed, R.; Awasthi, A.; Vishwas, S.; Chellappan, D.K.; Gupta, G.; Jha, N.K.; et al. Recent updates on animal models for understanding the etiopathogenesis of polycystic ovarian syndrome. Life Sci. 2021, 280, 119753.
- E Badowski, M.; Burton, B.; Shaeer, K.M.; Dicristofano, J. Oral oncolytic and antiretroviral therapy administration: Dose adjustments, drug interactions, and other considerations for clinical use. Drugs Context 2019, 8, 212550.
- Saladi, V.N.; Kammari, B.R.; Mandad, P.R.; Krishna, G.R.; Sajja, E.; Thirumali, R.S.; Marutapilli, A.; Mathad, V.T. Novel Pharmaceutical Cocrystal of Apalutamide, a Nonsteroidal Antiandrogen Drug: Synthesis, Crystal Structure, Dissolution, Stress, and Excipient Compatibility. Cryst. Growth Des. 2022, 22, 1130–1142.
- Tan, D.K.; Davis, D.A.; Miller, D.A.; Williams, R.O.; Nokhodchi, A. Innovations in Thermal Processing: Hot-Melt Extrusion and KinetiSol® Dispersing. Aaps Pharmscitech 2020, 21, 312.
- Tambe, S.; Jain, D.; Meruva, S.K.; Rongala, G.; Juluri, A.; Nihalani, G.; Mamidi, H.K.; Nukala, P.K.; Bolla, P.K. Recent Advances in Amorphous Solid Dispersions: Preformulation, Formulation Strategies, Technological Advancements and Characterization. Pharmaceutics 2022, 14, 2203.
- USFDA. Erleada (Apalutamide), NDA/BLA Multi-Disciplinary Review and Evaluation NDA 210951. 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210951Orig1s000MultidisciplineR.pdf (accessed on 30 September 2023).
- Becq, F.; Mirval, S.; Carrez, T.; Lévêque, M.; Billet, A.; Coraux, C.; Sage, E.; Cantereau, A. The rescue of F508del-CFTR by elexacaftor/tezacaftor/ivacaftor (Trikafta) in human airway epithelial cells is underestimated due to the presence of ivacaftor. Eur. Respir. J. 2022, 59, 2100671.
- Stegemann, S.; Moreton, C.; Svanbäck, S.; Box, K.; Motte, G.; Paudel, A. Trends in oral small-molecule drug discovery and product development based on product launches before and after the Rule of Five. Drug Discov. Today 2023, 28, 103344.
- Cheng, A.; Baker, O.; Hill, U. Elexacaftor, tezacaftor and ivacaftor: A case of severe rash and approach to desensitisation. BMJ Case Rep. 2022, 15, e247042.
- USFDA. Trikafta® Product Quality Review. 2017. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212273Orig1s000ChemR.pdf (accessed on 30 September 2023).
- Panmai, S.; Tatavarti, A.; Farrington, A.M.; Biyyala, V.; Allain, L.R.; Nefliu, M.; Lamm, M.; Klinzing, G.R.; Ren, J. Pharmaceutical Compositions Containing Doravirine, Tenofovir Disoproxil Fumarate and Lamivudine. U.S. Patent 10,842,751, 24 November 2020. Available online: https://patents.google.com/patent/US10603282B2/en (accessed on 30 September 2023).
- EMA. Delstrigo: Summary of Product Characteristics. 2023. Available online: https://www.medicines.org.uk/emc/product/9694/smpc/print (accessed on 30 September 2023).
- Szabó, E.; Démuth, B.; Galata, D.L.; Vass, P.; Hirsch, E.; Csontos, I.; Marosi, G.; Nagy, Z.K. Continuous Formulation Approaches of Amorphous Solid Dispersions: Significance of Powder Flow Properties and Feeding Performance. Pharmaceutics 2019, 11, 654.
- Hughes, D.L. Patent Review of Synthetic Routes and Crystalline Forms of the CFTR-Modulator Drugs Ivacaftor, Lumacaftor, Tezacaftor, and Elexacaftor. Org. Process. Res. Dev. 2019, 23, 2302–2322.
- Elborn, J.S.; Ramsey, B.W.; Boyle, M.P.; Konstan, M.W.; Huang, X.; Marigowda, G.; Waltz, D.; E Wainwright, C. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: A pooled analysis. Lancet Respir. Med. 2016, 4, 617–626.
- Cornely, O.A.; Duarte, R.F.; Haider, S.; Chandrasekar, P.; Helfgott, D.; Jiménez, J.L.; Candoni, A.; Raad, I.; Laverdiere, M.; Langston, A.; et al. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J. Antimicrob. Chemother. 2015, 71, 718–726.
- Kraft, W.K.; Chang, P.S.; van Iersel, M.L.P.S.; Waskin, H.; Krishna, G.; Kersemaekers, W.M. Posaconazole Tablet Pharmacokinetics: Lack of Effect of Concomitant Medications Altering Gastric pH and Gastric Motility in Healthy Subjects. Antimicrob. Agents Chemother. 2014, 58, 4020–4025.
- Miller, D.A.; Ellenberger, D.; Gil, M. Spray-Drying Technology. In Formulating Poorly Water Soluble Drugs; Williams, R.O., III, Watts, A., Miller, D., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 437–525.
- TGA. Kalydeco—Australian Prescribing Information. 2023. Available online: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent=&id=CP-2015-PI-01372-1&d=20230930172310101 (accessed on 30 September 2023).
- EMA. Kalydeco—Summary of Product Characteristics. 2023. Available online: https://www.ema.europa.eu/en/documents/product-information/kalydeco-epar-product-information_en.pdf (accessed on 30 September 2023).
- Shah, N.; Sandhu, H.; Phuapradit, W.; Pinal, R.; Iyer, R.; Albano, A.; Chatterji, A.; Anand, S.; Choi, D.S.; Tang, K.; et al. Development of novel microprecipitated bulk powder (MBP) technology for manufacturing stable amorphous formulations of poorly soluble drugs. Int. J. Pharm. 2012, 438, 53–60.
- Mosquera-Giraldo, L.I.; Taylor, L.S. Glass–Liquid Phase Separation in Highly Supersaturated Aqueous Solutions of Telaprevir. Mol. Pharm. 2015, 12, 496–503.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
19 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




