
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Virginia Venezia | -- | 3160 | 2023-12-18 10:16:35 | | | |
| 2 | Fanny Huang | Meta information modification | 3160 | 2023-12-19 06:26:38 | | | | |
| 3 | Fanny Huang | + 2 word(s) | 3162 | 2023-12-22 06:58:19 | | | | |
| 4 | Fanny Huang | Meta information modification | 3162 | 2023-12-22 06:59:27 | | |
Video Upload Options
Energy and environmental issues are of great importance in the present era. The transition to renewable energy sources necessitates technological, political, and behavioral transformations. Hydrogen is a promising solution, and many countries are investing in the hydrogen economy. The incorporation of hydrogen for efficient energy transport and storage and its integration into the transport sector are crucial measures. However, to fully develop a hydrogen-based economy, the sustainability and safety of hydrogen in all its applications must be ensured.
1. Introduction
2. Hydrogen Production from Bio-Alcohols: Focus on Bioethanol
2.1. Reforming
During POx, the fuel reacts with a limited supply of air or pure oxygen. The exothermic nature of the reaction means that it releases heat, avoiding the need for external heating. It is important to control the amount of air or oxygen supplied during partial oxidation to achieve the desired products and avoid complete oxidation, which would result in a reduced amount of hydrogen produced [23].
| Technology | Temperature | Pressure | Feed Ratio | Ref. |
|---|---|---|---|---|
| Steam Reforming | 450–650 °C | 2–25 bar | Water/Ethanol = 3–6 | [7][22] |
| Partial Oxidation | 200–400 °C | 1–10 bar | Air/Ethanol = 4 | [41][44] |
| Autothermal Reforming | 600–900 °C | 1–10 bar | Ethanol/Air/Water = 1/0.3–0.5/2–3 | [41][45] |
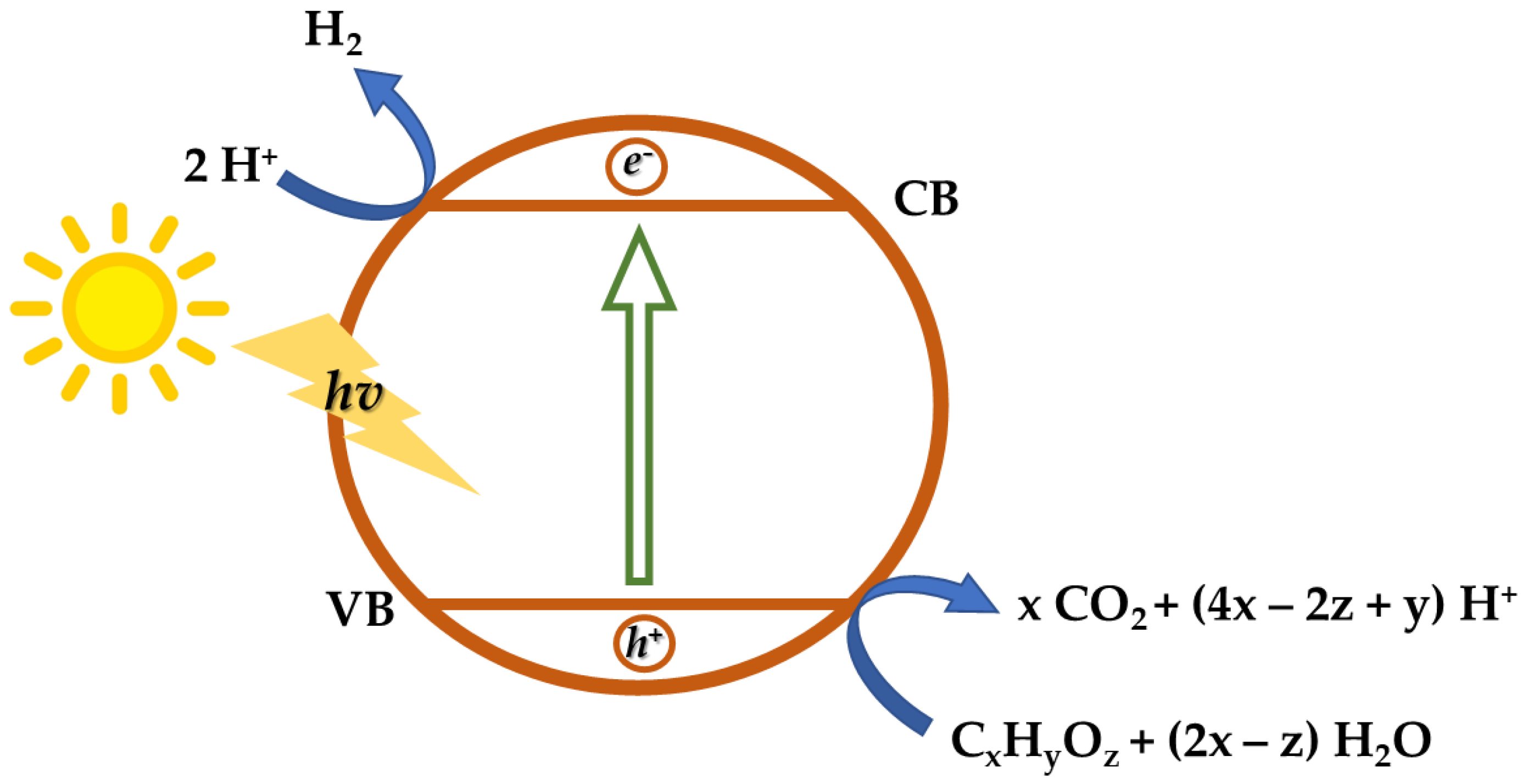
2.2. Photocatalysis
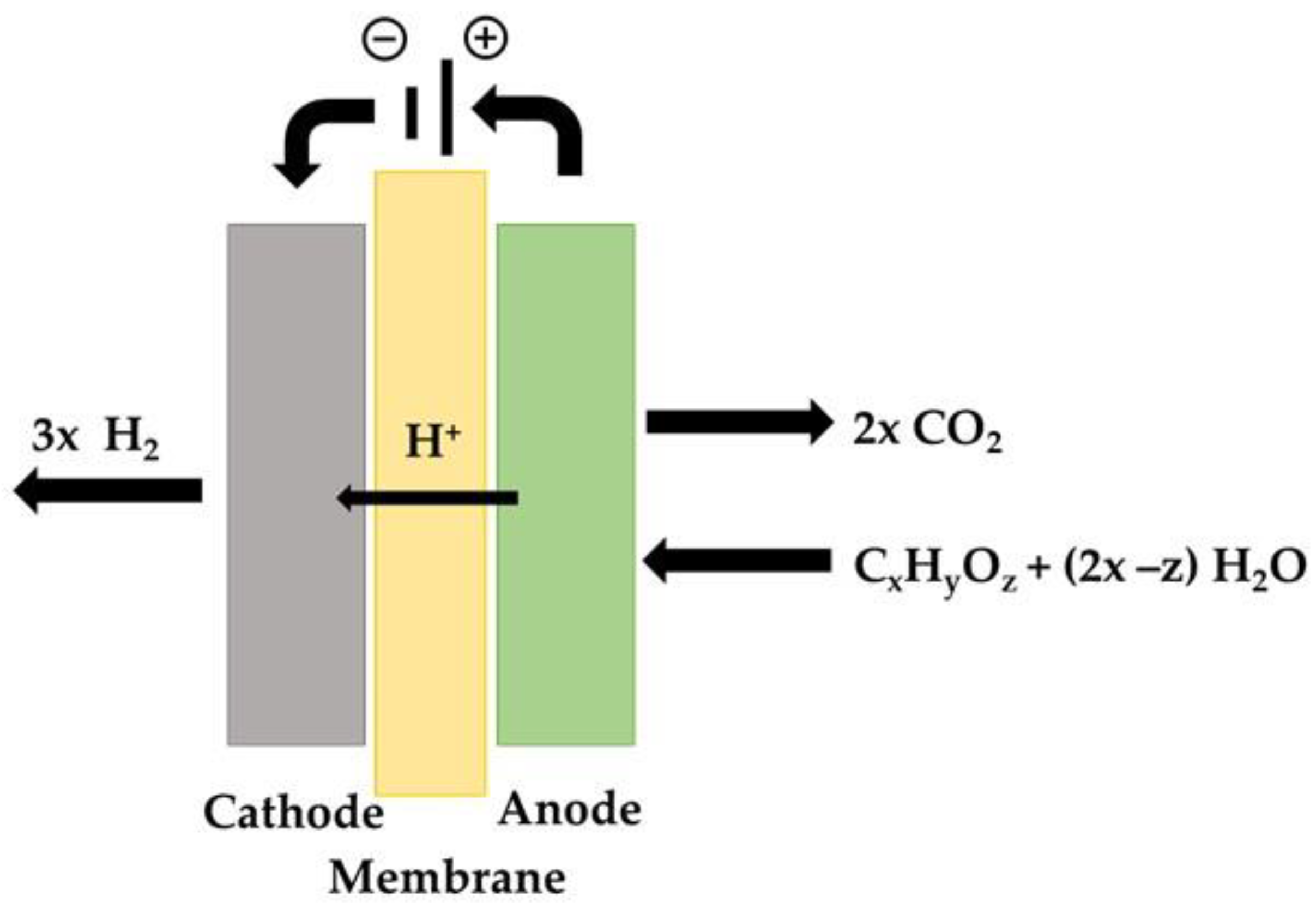
2.3. Electrolysis
| H2 Production Process from Ethanol |
Advantages | Disadvantages | Performances | Ref. |
|---|---|---|---|---|
| Steam Reforming |
|
|
H2 Yield: 60–95% EtOH Conversion: 90–100% H2 Selectivity: 70–90% @Ni/Al2O3, 250–500 °C, H2O/EtOH = 3–6 H2 production cost: 1.58–2.6 USD/kgH2 |
[18][44][88][89] |
| Partial Oxidation |
|
|
H2 Selectivity: 60–97% EtOH Conversion: @Noble metal/Al2O3, 500–600 °C, H2O/O2/EtOH = 3/0.3/1 |
[90][91] |
| Autothermal Reforming |
|
|
H2 Yield: 47–94% EtOH Conversion: 84–97% @Rh/Al2O3 H2 Selectivity: 40–60% @Pt/Al2O3 400–600 °C, H2O/O2/EtOH = 3/0.3/1 |
[92][93] |
| Photocatalysis |
|
|
H2 production rate: 30–34 mmol/gh @Pt/TiO2 |
[94][95] |
| Electrocatalysis |
|
|
H2 volume: 102 mL @Pt/C, 20 °C j = 50 mA/cm−2 |
[71] |
References
- Fayyazbakhsh, A.; Bell, M.L.; Zhu, X.; Mei, X.; Koutný, M.; Hajinajaf, N.; Zhang, Y. Engine Emissions with Air Pollutants and Greenhouse Gases and Their Control Technologies. J. Clean. Prod. 2022, 376, 134260.
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report. of the Intergovernmental Panel on Climate Change; Arias, P., Bustamante, M., Elgizouli, I., Flato, G., Howden, M., Méndez-Vallejo, C., Pereira, J.J., Pichs-Madruga, R., Rose, S.K., Saheb, Y., et al., Eds.; IPCC: Geneva, Switzerland, 2023.
- Bouckaert, S.; Pales, A.F.; McGlade, C.; Remme, U.; Wanner, B.; Varro, L.; D’Ambrosio, D.; Spencer, T. Net Zero by 2050—A Roadmap for the Global Energy Sector; International Energy Agency: Paris, France, 2021; Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 18 November 2023).
- Schmidt, J.; Gruber, K.; Klingler, M.; Klöckl, C.; Ramirez Camargo, L.; Regner, P.; Turkovska, O.; Wehrle, S.; Wetterlund, E. A New Perspective on Global Renewable Energy Systems: Why Trade in Energy Carriers Matters. Energy Environ. Sci. 2019, 12, 2022–2029.
- Ahmed, M.R.; Barua, T.; Das, B.K. A Comprehensive Review on Techno-Environmental Analysis of State-of-the-Art Production and Storage of Hydrogen Energy: Challenges and Way Forward. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 5905–5937.
- Eljack, F.; Kazi, M.K. Prospects and Challenges of Green Hydrogen Economy via Multi-Sector Global Symbiosis in Qatar. Front. Sustain. 2020, 1, 612762.
- Xiang, H.; Xin, R.; Prasongthum, N.; Natewong, P.; Sooknoi, T.; Wang, J.; Reubroycharoen, P.; Fan, X. Catalytic Conversion of Bioethanol to Value-Added Chemicals and Fuels: A Review. Resour. Chem. Mater. 2022, 1, 47–68.
- Culaba, A.B.; Mayol, A.P.; San Juan, J.L.G.; Ubando, A.T.; Bandala, A.A.; Concepcion, R.S.; Alipio, M.; Chen, W.H.; Show, P.L.; Chang, J.S. Design of Biorefineries towards Carbon Neutrality: A Critical Review. Bioresour. Technol. 2023, 369, 128256.
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation 2021, 7, 268.
- Melendez, J.R.; Mátyás, B.; Hena, S.; Lowy, D.A.; El Salous, A. Perspectives in the Production of Bioethanol: A Review of Sustainable Methods, Technologies, and Bioprocesses. Renew. Sustain. Energy Rev. 2022, 160, 112260.
- Bušić, A.; Mardetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Šantek, M.I.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311.
- IRENA. Available online: https://www.irena.org/Energy-Transition/Technology/Transportation-costs/Bioethanol (accessed on 15 November 2023).
- Suer, J.; Traverso, M.; Jäger, N. Carbon Footprint Assessment of Hydrogen and Steel. Energies 2022, 15, 9468.
- Bepari, S.; Kuila, D. Steam Reforming of Methanol, Ethanol and Glycerol over Nickel-Based Catalysts—A Review. Int. J. Hydrogen Energy 2020, 45, 18090–18113.
- Kappis, K.; Papavasiliou, J.; Avgouropoulos, G. Methanol Reforming Processes for Fuel Cell Applications. Energies 2021, 14, 8442.
- Xu, X.; Shuai, K.; Xu, B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen. Catalysts 2017, 7, 183.
- Ogo, S.; Sekine, Y. Recent Progress in Ethanol Steam Reforming Using Non-Noble Transition Metal Catalysts: A Review. Fuel Process. Technol. 2020, 199, 106238.
- Anil, S.; Indraja, S.; Singh, R.; Appari, S.; Roy, B. A Review on Ethanol Steam Reforming for Hydrogen Production over Ni/Al2O3 and Ni/CeO2 Based Catalyst Powders. Int. J. Hydrogen Energy 2022, 47, 8177–8213.
- Shtyka, O.; Dimitrova, Z.; Ciesielski, R.; Kedziora, A.; Mitukiewicz, G.; Leyko, J.; Maniukewicz, W.; Czylkowska, A.; Maniecki, T. Steam Reforming of Ethanol for Hydrogen Production: Influence of Catalyst Composition (Ni/Al2O3, Ni/Al2O3–CeO2, Ni/Al2O3–ZnO) and Process Conditions. React. Kinet. Mech. Catal. 2021, 132, 907–919.
- Adeniyi, A.G.; Ighalo, J.O. A Review of Steam Reforming of Glycerol. Chem. Pap. 2019, 73, 2619–2635.
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from Renewables: A Case Study of Glycerol Reforming. Catalysts 2019, 9, 722.
- Azizan, M.T.; Aqsha, A.; Ameen, M.; Syuhada, A.; Klaus, H.; Abidin, S.Z.; Sher, F. Catalytic Reforming of Oxygenated Hydrocarbons for the Hydrogen Production: An Outlook. Biomass Convers. Biorefinery 2020, 13, 8441–8464.
- Makaryan, I.A.; Salgansky, E.A.; Arutyunov, V.S.; Sedov, I.V. Non-Catalytic Partial Oxidation of Hydrocarbon Gases to Syngas and Hydrogen: A Systematic Review. Energies 2023, 16, 2916.
- Balopi, B.; Moyo, M.; Gorimbo, J. Autothermal Reforming of Bio-Ethanol: A Short Review of Strategies Used to Synthesize Coke-Resistant Nickel-Based Catalysts. Catal. Lett. 2022, 152, 3004–3016.
- Baruah, R.; Dixit, M.; Basarkar, P.; Parikh, D.; Bhargav, A. Advances in Ethanol Autothermal Reforming. Renew. Sustain. Energy Rev. 2015, 51, 1345–1353.
- Wang, W.; Wang, Y.Q. Thermodynamic Analysis of Steam Reforming of Ethanol for Hydrogen Generation. Int. J. Energy Res. 2008, 32, 1432–1443.
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2005, 19, 2098–2106.
- Vicente, J.; Ereña, J.; Montero, C.; Azkoiti, M.J.; Bilbao, J.; Gayubo, A.G. Reaction Pathway for Ethanol Steam Reforming on a Ni/SiO2 Catalyst Including Coke Formation. Int. J. Hydrogen Energy 2014, 39, 18820–18834.
- Xu, W.; Liu, Z.; Johnston-Peck, A.C.; Senanayake, S.D.; Zhou, G.; Stacchiola, D.; Stach, E.A.; Rodriguez, J.A. Steam Reforming of Ethanol on Ni/CeO2: Reaction Pathway and Interaction between Ni and the CeO2 Support. ACS Catal. 2013, 3, 975–984.
- Deng, Y.; Li, S.; Appels, L.; Zhang, H.; Sweygers, N.; Baeyens, J.; Dewil, R. Steam Reforming of Ethanol by Non-Noble Metal Catalysts. Renew. Sustain. Energy Rev. 2023, 175, 113184.
- Grzybek, G.; Greluk, M.; Tarach, K.; Pyra, K.; Słowik, G.; Rotko, M.; Góra-Marek, K. Bioethanol Steam Reforming over Cobalt-Containing USY and ZSM-5 Commercial Zeolite Catalysts. Front. Mater. 2020, 7, 597528.
- Sohn, H.; Ozkan, U.S. Cobalt-Based Catalysts for Ethanol Steam Reforming: An Overview. Energy Fuels 2016, 30, 5309–5322.
- Erdohelyi, A.; Raskó, J.; Kecskés, T.; Tóth, M.; Dömök, M.; Baán, K. Hydrogen Formation in Ethanol Reforming on Supported Noble Metal Catalysts. Catal. Today 2006, 116, 367–376.
- Frusteri, F.; Freni, S.; Spadaro, L.; Chiodo, V.; Bonura, G.; Donato, S.; Cavallaro, S. H2 Production for MC Fuel Cell by Steam Reforming of Ethanol over MgO Supported Pd, Rh, Ni and Co Catalysts. Catal. Commun. 2004, 5, 611–615.
- Dalena, F.; Giglio, E.; Marino, A.; Aloise, A.; Giorgianni, G.; Migliori, M.; Giordano, G. Steam Reforming of Bioethanol Using Metallic Catalysts on Zeolitic Supports: An Overview. Catalysts 2022, 12, 617.
- Montero, C.; Remiro, A.; Valle, B.; Oar-Arteta, L.; Bilbao, J.; Gayubo, A.G. Origin and Nature of Coke in Ethanol Steam Reforming and Its Role in Deactivation of Ni/La2O3-αAl2O3 Catalyst. Ind. Eng. Chem. Res. 2019, 58, 14736–14751.
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A Review on Reforming Bio-Ethanol for Hydrogen Production. Int. J. Hydrogen Energy 2007, 32, 3238–3247.
- Song, H.; Zhang, L.; Watson, R.B.; Braden, D.; Ozkan, U.S. Investigation of Bio-Ethanol Steam Reforming over Cobalt-Based Catalysts. Catal. Today 2007, 129, 346–354.
- Contreras, J.L.; Salmones, J.; Colín-Luna, J.A.; Nuño, L.; Quintana, B.; Córdova, I.; Zeifert, B.; Tapia, C.; Fuentes, G.A. Catalysts for H2 Production Using the Ethanol Steam Reforming (a Review). Int. J. Hydrogen Energy 2014, 39, 18835–18853.
- Salge, J.R.; Deluga, G.A.; Schmidt, L.D. Catalytic Partial Oxidation of Ethanol over Noble Metal Catalysts. J. Catal. 2005, 235, 69–78.
- Vita, A.; Pino, L.; Italiano, C.; Palella, A. Steam Reforming, Partial Oxidation, and Autothermal Reforming of Ethanol for Hydrogen Production in Conventional Reactors. In Ethanol: Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 159–191. ISBN 9780128114582.
- Youn, M.H.; Seo, J.G.; Lee, H.; Bang, Y.; Chung, J.S.; Song, I.K. Hydrogen Production by Auto-Thermal Reforming of Ethanol over Nickel Catalysts Supported on Metal Oxides: Effect of Support Acidity. Appl. Catal. B 2010, 98, 57–64.
- Gutierrez, A.; Karinen, R.; Airaksinen, S.; Kaila, R.; Krause, A.O.I. Autothermal Reforming of Ethanol on Noble Metal Catalysts. Int. J. Hydrogen Energy 2011, 36, 8967–8977.
- Chen, W.H.; Biswas, P.P.; Ong, H.C.; Hoang, A.T.; Nguyen, T.B.; Dong, C. Di A Critical and Systematic Review of Sustainable Hydrogen Production from Ethanol/Bioethanol: Steam Reforming, Partial Oxidation, and Autothermal Reforming. Fuel 2023, 333, 126526.
- Chen, W.H.; Biswas, P.P.; Ubando, A.T.; Park, Y.K.; Ashokkumar, V.; Chang, J.S. Design of Experiment for Hydrogen Production from Ethanol Reforming: A State-of-the-Art Review. Fuel 2023, 342, 127871.
- Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-Driven Hydrogen Production: Recent Advances, Challenges, and Future Perspectives. ACS Energy Lett. 2022, 7, 1043–1065.
- Gupta, A.; Likozar, B.; Jana, R.; Chanu, W.C.; Singh, M.K. A Review of Hydrogen Production Processes by Photocatalytic Water Splitting—From Atomistic Catalysis Design to Optimal Reactor Engineering. Int. J. Hydrogen Energy 2022, 47, 33282–33307.
- Yao, Y.; Gao, X.; Li, Z.; Meng, X. Photocatalytic Reforming for Hydrogen Evolution: A Review. Catalysts 2020, 10, 335.
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544.
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic Hydrogen Production Using Metal Doped TiO2: A Review of Recent Advances. Appl. Catal. B 2019, 244, 1021–1064.
- Bowker, M. Sustainable Hydrogen Production by the Application of Ambient Temperature Photocatalysis. Green. Chem. 2011, 13, 2235–2246.
- Escobedo, S.; de Lasa, H. Synthesis and Performance of Photocatalysts for Photocatalytic Hydrogen Production: Future Perspectives. Catalysts 2021, 11, 1505.
- Karimi Estahbanati, M.R.; Feilizadeh, M.; Attar, F.; Iliuta, M.C. Current Developments and Future Trends in Photocatalytic Glycerol Valorization: Photocatalyst Development. Ind. Eng. Chem. Res. 2020, 59, 22330–22352.
- Rueda-Navarro, C.M.; Ferrer, B.; Baldoví, H.G.; Navalón, S. Photocatalytic Hydrogen Production from Glycerol Aqueous Solutions as Sustainable Feedstocks Using Zr-Based UiO-66 Materials under Simulated Sunlight Irradiation. Nanomaterials 2022, 12, 3808.
- Augustin, A.; Chuaicham, C.; Shanmugam, M.; Vellaichamy, B.; Rajendran, S.; Hoang, T.K.A.; Sasaki, K.; Sekar, K. Recent Development of Organic-Inorganic Hybrid Photocatalysts for Biomass Conversion into Hydrogen Production. Nanoscale Adv. 2022, 4, 2561–2582.
- Shimura, K.; Yoshida, H. Heterogeneous Photocatalytic Hydrogen Production from Water and Biomass Derivatives. Energy Environ. Sci. 2011, 4, 2467–2481.
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Photocatalytic Degradation of Organic Pollutants with Simultaneous Production of Hydrogen. Catal. Today 2007, 124, 94–102.
- Karimi Estahbanati, M.R.; Babin, A.; Feilizadeh, M.; Nayernia, Z.; Mahinpey, N.; Iliuta, M.C. Photocatalytic Conversion of Alcohols to Hydrogen and Carbon-Containing Products: A Cleaner Alcohol Valorization Approach. J. Clean. Prod. 2021, 318, 128546.
- Sola, A.C.; Homs, N.; Ramírez de la Piscina, P. Photocatalytic H2 Production from Ethanol (Aq) Solutions: The Effect of Intermediate Products. Int. J. Hydrogen Energy 2016, 41, 19629–19636.
- Barba-Nieto, I.; Caudillo-Flores, U.; Gómez-Cerezo, M.N.; Kubacka, A.; Fernández-García, M. Boosting Pt/TiO2 Hydrogen Photoproduction through Zr Doping of the Anatase Structure: A Spectroscopic and Mechanistic Study. Chem. Eng. J. 2020, 398, 125665.
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986.
- Yang, Y.Z.; Chang, C.H.; Idriss, H. Photo-Catalytic Production of Hydrogen Form Ethanol over M/TiO2 Catalysts (M = Pd, Pt or Rh). Appl. Catal. B 2006, 67, 217–222.
- Rusinque, B.; Escobedo, S.; de Lasa, H. Photocatalytic Hydrogen Production under Near-UV Using Pd-Doped Mesoporous TiO2 and Ethanol as Organic Scavenger. Catalysts 2019, 9, 33.
- Serafin, J.; Ouzzine, M.; Sreńscek-Nazzal, J.; Llorca, J. Photocatalytic Hydrogen Production from Alcohol Aqueous Solutions over TiO2-Activated Carbon Composites Decorated with Au and Pt. J. Photochem. Photobiol. A Chem. 2022, 425, 113726.
- Ibrahim, N.S.; Leaw, W.L.; Mohamad, D.; Alias, S.H.; Nur, H. A Critical Review of Metal-Doped TiO2 and Its Structure–Physical Properties–Photocatalytic Activity Relationship in Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 28553–28565.
- Vitiello, G.; Clarizia, L.; Abdelraheem, W.; Esposito, S.; Bonelli, B.; Ditaranto, N.; Vergara, A.; Nadagouda, M.; Dionysiou, D.D.; Andreozzi, R.; et al. Near UV-Irradiation of CuOx-Impregnated TiO2 Providing Active Species for H2 Production Through Methanol Photoreforming. ChemCatChem 2019, 11, 4314–4326.
- Clarizia, L.; Russo, D.; Di Somma, I.; Andreozzi, R.; Marotta, R. Hydrogen Generation through Solar Photocatalytic Processes: A Review of the Configuration and the Properties of Effective Metal-Based Semiconductor Nanomaterials. Energies 2017, 10, 1624.
- Karimi Estahbanati, M.R.; Mahinpey, N.; Feilizadeh, M.; Attar, F.; Iliuta, M.C. Kinetic Study of the Effects of PH on the Photocatalytic Hydrogen Production from Alcohols. Int. J. Hydrogen Energy 2019, 44, 32030–32041.
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454.
- Coutanceau, C.; Baranton, S. Electrochemical Conversion of Alcohols for Hydrogen Production: A Short Overview. Wiley Interdiscip. Rev. Energy Environ. 2016, 5, 388–400.
- Lamy, C.; Jaubert, T.; Baranton, S.; Coutanceau, C. Clean Hydrogen Generation through the Electrocatalytic Oxidation of Ethanol in a Proton Exchange Membrane Electrolysis Cell (PEMEC): Effect of the Nature and Structure of the Catalytic Anode. J. Power Sources 2014, 245, 927–936.
- Pethaiah, S.S.; Sadasivuni, K.K.; Jayakumar, A.; Ponnamma, D.; Tiwary, C.S.; Sasikumar, G. Methanol Electrolysis for Hydrogen Production Using Polymer Electrolyte Membrane: A Mini-Review. Energies 2020, 13, 5879.
- Caravaca, A.; Sapountzi, F.M.; De Lucas-Consuegra, A.; Molina-Mora, C.; Dorado, F.; Valverde, J.L. Electrochemical Reforming of Ethanol-Water Solutions for Pure H 2 Production in a PEM Electrolysis Cell. Int. J. Hydrogen Energy 2012, 37, 9504–9513.
- Yu, J.; González-Cobos, J.; Dappozze, F.; Grimaldos-Osorio, N.; Vernoux, P.; Caravaca, A.; Guillard, C. First PEM Photoelectrolyser for the Simultaneous Selective Glycerol Valorization into Value-Added Chemicals and Hydrogen Generation. Appl. Catal. B 2023, 327, 122465.
- De Lucas-Consuegra, A.; Calcerrada, A.B.; De La Osa, A.R.; Valverde, J.L. Electrochemical Reforming of Ethylene Glycol. Influence of the Operation Parameters, Simulation and Its Optimization. Fuel Process. Technol. 2014, 127, 13–19.
- Liu, Y.P.; Zhao, S.F.; Guo, S.X.; Bond, A.M.; Zhang, J.; Zhu, G.; Hill, C.L.; Geletii, Y.V. Electrooxidation of Ethanol and Methanol Using the Molecular Catalyst 10−. J. Am. Chem. Soc. 2016, 138, 2617–2628.
- Asiri, H.A.; Anderson, A.B. Mechanisms for Ethanol Electrooxidation on Pt(111) and Adsorption Bond Strengths Defining an Ideal Catalyst. J. Electrochem. Soc. 2015, 162, F115–F122.
- Lima, F.H.B.; Gonzalez, E.R. Ethanol Electro-Oxidation on Carbon-Supported Pt-Ru, Pt-Rh and Pt-Ru-Rh Nanoparticles. Electrochim. Acta 2008, 53, 2963–2971.
- Zhang, B.W.; Sheng, T.; Wang, Y.X.; Qu, X.M.; Zhang, J.M.; Zhang, Z.C.; Liao, H.G.; Zhu, F.C.; Dou, S.X.; Jiang, Y.X.; et al. Platinum-Cobalt Bimetallic Nanoparticles with Pt Skin for Electro-Oxidation of Ethanol. ACS Catal. 2017, 7, 892–895.
- Rizo, R.; Sebastián, D.; Lázaro, M.J.; Pastor, E. On the Design of Pt-Sn Efficient Catalyst for Carbon Monoxide and Ethanol Oxidation in Acid and Alkaline Media. Appl. Catal. B 2017, 200, 246–254.
- Silva, J.C.M.; De Souza, R.F.B.; Parreira, L.S.; Neto, E.T.; Calegaro, M.L.; Santos, M.C. Ethanol Oxidation Reactions Using SnO2@Pt/C as an Electrocatalyst. Appl. Catal. B 2010, 99, 265–271.
- Rizo, R.; Bergmann, A.; Timoshenko, J.; Scholten, F.; Rettenmaier, C.; Jeon, H.S.; Chen, Y.T.; Yoon, A.; Bagger, A.; Rossmeisl, J.; et al. Pt-Sn-Co Nanocubes as Highly Active Catalysts for Ethanol Electro-Oxidation. J. Catal. 2021, 393, 247–258.
- Lamy, C.; Rousseau, S.; Belgsir, E.M.; Coutanceau, C.; Léger, J.M. Recent Progress in the Direct Ethanol Fuel Cell: Development of New Platinum-Tin Electrocatalysts. Electrochim. Acta 2004, 49, 3901–3908.
- Yang, G.; Zhang, Q.; Yu, H.; Peng, F. Platinum-Based Ternary Catalysts for the Electrooxidation of Ethanol. Particuology 2021, 58, 169–186.
- Kowal, A.; Li, M.; Shao, M.; Sasaki, K.; Vukmirovic, M.B.; Zhang, J.; Marinkovic, N.S.; Liu, P.; Frenkel, A.I.; Adzic, R.R. Ternary Pt/Rh/SnO2 Electrocatalysts for Oxidizing Ethanol to CO2. Nat. Mater. 2009, 8, 325–330.
- Rodríguez-Gómez, A.; Dorado, F.; Sánchez, P.; de la Osa, A.R. Boosting Hydrogen and Chemicals Production through Ethanol Electro-Reforming on Pt-Transition Metal Anodes. J. Energy Chem. 2022, 70, 394–406.
- Serrano-Jiménez, J.; de la Osa, A.R.; Rodríguez-Gómez, A.; Sánchez, P.; Romero, A.; de Lucas-Consuegra, A. Electro-Reforming of Bioethanol Produced by Sugar Fermentation on a Pt-Ni Anodic Catalyst Supported on Graphene Nanoplatelets. J. Environ. Chem. Eng. 2023, 11, 109703.
- Khamhaeng, P.; Laosiripojana, N.; Assabumrungrat, S.; Kim-Lohsoontorn, P. Techno-Economic Analysis of Hydrogen Production from Dehydrogenation and Steam Reforming of Ethanol for Carbon Dioxide Conversion to Methanol. Int. J. Hydrogen Energy 2021, 46, 30891–30902.
- Khamhaeng, P.; Kim-Lohsoontorn, P. Performance and Cost Analysis of Hydrogen Production from Steam Reforming and Dehydrogenation of Ethanol. In IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2020; Volume 991.
- Tóth, M.; Varga, E.; Oszkó, A.; Baán, K.; Kiss, J.; Erdohelyi, A. Partial Oxidation of Ethanol on Supported Rh Catalysts: Effect of the Oxide Support. J. Mol. Catal. A Chem. 2016, 411, 377–387.
- Chiu, W.-C.; Horng, R.-F.; Chou, H.-M. Hydrogen Production from an Ethanol Reformer with Energy Saving Approaches over Various Catalysts. Int. J. Hydrogen Energy 2013, 38, 2760–2769.
- Hung, C.C.; Chen, S.L.; Liao, Y.K.; Chen, C.H.; Wang, J.H. Oxidative Steam Reforming of Ethanol for Hydrogen Production on M/Al2O3. Int. J. Hydrogen Energy 2012, 37, 4955–4966.
- Silva Júnior, M.E.; Palm, M.O.; Duarte, D.A.; Catapan, R.C. Catalytic Pt/Al2O3 Monolithic Foam for Ethanol Reforming Fabricated by the Competitive Impregnation Method. ACS Omega 2023, 8, 6507–6514.
- Jovic, V.; Al-Azri, Z.H.N.; Chen, W.T.; Sun-Waterhouse, D.; Idriss, H.; Waterhouse, G.I.N. Photocatalytic H2 Production from Ethanol-Water Mixtures over Pt/TiO2 and Au/TiO2 Photocatalysts: A Comparative Study. Top. Catal. 2013, 56, 1139–1151.
- Al-Azri, Z.H.N.; Jovic, V.; Chen, W.-T.; Sun-Waterhouse, D.; Metson, J.B.; Waterhouse, G.I.N. Performance Evaluation of Pd/TiO2 and Pt/TiO2 for Hydrogen Production from ethanol-Water Mixtures. Int. J. Nanotechnol. 2014, 11, 695–703.
- Di Benedetto, A.; Portarapillo, M.; Landi, G.; Luciani, G. Process for Green Hydrogen Production. WO Patent WO2023105545A1, 7 December 2022.
- Di Nardo, A.; Portarapillo, M.; Russo, D.; Luciani, G.; Landi, G.; Ruoppolo, G.; Pezzella, A.; Di Benedetto, A. Cyan Hydrogen Process: A New Route for Simultaneous Hydrogen Production and Carbon Valorisation. ACS Omega, 2023; in press.




