Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Chemical
Energy and environmental issues are of great importance in the present era. The transition to renewable energy sources necessitates technological, political, and behavioral transformations. Hydrogen is a promising solution, and many countries are investing in the hydrogen economy. The incorporation of hydrogen for efficient energy transport and storage and its integration into the transport sector are crucial measures. However, to fully develop a hydrogen-based economy, the sustainability and safety of hydrogen in all its applications must be ensured.
- hydrogen
- production
- bio-alcohol
1. Introduction
Population growth as well as industrialization have been accompanied by a huge increase in energy consumption, causing severe environmental impact in terms of fossil fuel depletion, pollution, and increased content of greenhouse gases [1]. The grey range with its central black line draws a corresponding estimate of the human-caused share of historical warming. Coloured areas show the global surface temperature projections, and thick coloured central lines show the median estimate as a function of cumulative CO2 emissions for different scenarios, ranging from very low (SSP1-1.9), low (SSP1-2.6), intermediate (SSP2-4.5), high (SSP3-7.0), to very high (SSP5-8.5) [2].
To limit human-caused global warming to a specific level, we need to restrain the cumulative CO2 emissions, reaching net zero or net negative CO2 emissions, along with reductions of other GHG emissions. To this end in the Paris Agreement countries agreed to reach the following targets: a reduction of 50–52% of CO2 emissions by 2030; carbon pollution-free electricity by 2035; net ZERO GHG emissions by 2050 [3].
To reach these goals, a complete energetic transition towards renewable, cleaner, and more sustainable sources is required, with the most important strategy being the development of H2 technologies and their widespread use in human activities. H2 is intrinsically clean since it only produces water, thus contributing to decarbonization. H2 can be produced by different means, including renewable sources. It releases a significant amount of heat when burned without pollution. Furthermore, it can be exploited in a huge range of applications, replacing traditional fossil fuels and including the transport, heat, industry, and electricity sectors [4]. Finally, because of the plethora of production technologies, its availability is not affected by outside influences [5]. Despite the significant technological potential and the possibility of transitioning to a low-carbon economy, hydrogen as an energy carrier remains limited, with only 6% of H2 production being used for this purpose. Most of the hydrogen is instead used as an intermediate in industrial chemical production, emphasizing its constrained role in meeting global energy demands [6].
To reach the goal of widespread use of H2, all the related technologies must be developed and optimized to meet sustainable targets. Figure 1 shows the H2 cycle, evidencing that it is based on four cornerstones: production, storage, transportation, and use. Each step must be managed to eventually produce clean energy; H2 must be produced, and then the first action is the optimization of H2 production unit. Produced H2 must be stored and transported in a green, sustainable, and safe way. Finally, H2 should be used for clean and efficient energy production through high-performance devices that ensure significant energy conversion rates by using fuel cells.
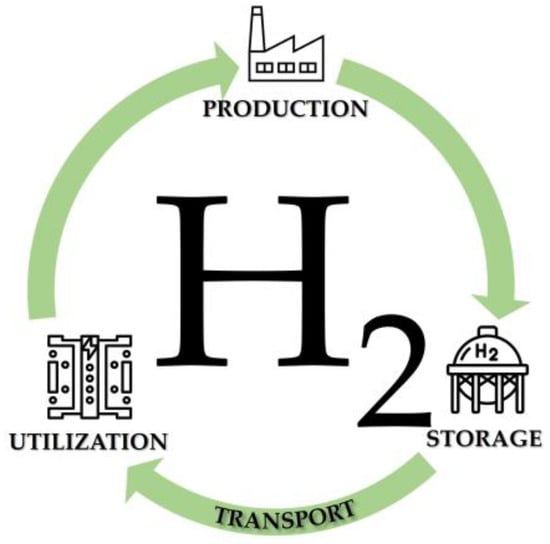
Figure 1. The H2 cycle.
2. Hydrogen Production from Bio-Alcohols: Focus on Bioethanol
Biowaste-derived alcohols represent an important alternative feedstock for carbon-neutral hydrogen production. Since they can be produced directly from renewable biomass, they have a sustainable feature [8]. CO2 emissions from producing hydrogen using bioderived sources are offset by the CO2 previously absorbed by these sources, resulting in net zero CO2 emissions [9].
Bioethanol is traditionally made from plant by-products such as sugarcane, corn, and wheat (first-generation). However, lignocellulosic (second-generation) and algal biomass (third-generation) are now explored due to food sustainability concerns [10]. However, second-generation raw materials necessitate further procedures, including pre-treating lignocellulose, to generate fermentable substances. The challenge remains in maintaining the cost-effectiveness of these processes. Additionally, research in the third-generation sector is still in its early stages due to limited investment and design hurdles [11]. Annual production of bioethanol is steadily increasing, with a projected global output and consumption of nearly 134.5 billion liters by 2024. The cost of raw materials has a significant influence on bioethanol production expenses, accounting for between 40 and 75% of the total cost [12]. At current pricing, the anticipated cost of advanced bioethanol is between USD 1.04 and USD 1.45 per liter of gasoline equivalent (LGE) [13]. Minimising greenhouse gas emissions, ensuring energy security, and promoting agricultural practices in rural areas are all measures that can assist in offsetting the production of bioethanol.
Researchers have investigated a variety of methods to manufacture hydrogen from light alcohols, such as steam reforming (SR), partial oxidation (POx), autothermal reforming (ATR), photocatalysis (PC), and electrocatalysis (EC).
2.1. Reforming
Steam reforming (SR) is a highly advanced technique used in the petrochemical industry to convert natural gas, primarily methane, into hydrogen. However, it results in 8–13 Kg CO2eq/KgH2 emissions, both as a product reaction and for heating the reactor due to the reaction endothermicity [14]. Today, the research aims to expand the capabilities and adaptability of SR technology beyond its dependence on natural gas. One approach involves investigating alternative reactants, such as oxygenated hydrocarbons such as methanol [15,16,17], ethanol [15,18,19,20], glycerol [21,22], dimethyl ether, acetone, and acetic acid, as well as heavier hydrocarbons ranging from C3 to C10 [23].
For oxygenated hydrocarbons, the stoichiometric SR reaction (1) is as follows: The CO can be further transformed to produce additional hydrogen and carbon dioxide through the exothermic water-gas shift (WGS) reaction (2).
CO + H2O → CO2 + H2
Another reforming technology is partial oxidation (POx) (3), in which partial combustion of the fuel produces hydrogen.
During POx, the fuel reacts with a limited supply of air or pure oxygen. The exothermic nature of the reaction means that it releases heat, avoiding the need for external heating. It is important to control the amount of air or oxygen supplied during partial oxidation to achieve the desired products and avoid complete oxidation, which would result in a reduced amount of hydrogen produced [24].
Autothermal reforming (ATR) (4) is a combination of both partial oxidation (exothermic) and steam reforming processes (endothermic) to supply the required heat and enable self-sustained reforming.
Fuel is first oxidized to heat up the reactor and then reacts with steam to produce hydrogen-rich syngas [25]. ATR has the potential to achieve high hydrogen yield and hydrogen selectivity because syngas can be produced from air or H2O. By using the POx to produce heat and the SR to increase hydrogen production, the ATR technique produces a thermally neutral process [26].
In the case of ethanol, ethanol SR (ESR), POx, and ATR reactions are reported in the following (5)–(7) and are accompanied by the water-gas shift reaction (WGS) (2) to produce hydrogen:
C2H5OH + H2O → 2 CO + 4 H2
C2H5OH + ½ O2 → CO + 3 H2
C2H5OH + 2 H2O + ½ O2 → 2 CO2 + 5 H2
In Table 1 are reported the operating conditions (temperature, pressure, feed ratio) of the ethanol reforming technologies.
The reaction pathway of ESR has been extensively studied, and the mechanism complexity is widely reported [27,28,29] since different parallel reactions occur simultaneously with hydrogen production. The product distribution depends on both the nature of the catalyst (i.e., active sites, support, precursor, preparation methods) and the reaction conditions, e.g., temperature, water/ethanol ratio, and feed flow rate. The complex set of by-products includes carbon monoxide (CO), methane (CH4), ethane (C2H6), ethylene (C2H4), and acetaldehyde (CH3CHO) [30].
Supported metal catalysts are commonly employed for ethanol steam reforming (ESR), in which the support plays a crucial role in activating steam to produce oxygen. Oxygen stabilizes the metal particles present in the catalyst and promotes the gasification of coke, while the metal phases break the hydrocarbon bonds [8]. Non-noble metal-based catalysts are widely employed for ethanol reforming due to their relatively low cost and good activity for C-C and C-H bond cleavage [31]. Among Ni, Co, Fe, and Cu species, the highest activity and selectivity for hydrogen and the lowest formation of CH4 are achieved by Co [32,33]. Noble metal-based catalysts (Pt, Pd, Rh, and Ru) have high activity and stability in ESR, but the resource scarcity and relatively high costs represent an obstacle for their large-scale application. In general, Rh-based catalysts emerge as the most active and selective for H2 production, even at low temperatures [34,35,36]. The reaction is highly influenced by the kind of support: basic solids encourage the dehydrogenation to acetaldehyde, while acidic ones cause the dehydration to ethylene, a key precursor to coke in ESR [37]. The most investigated and widely adopted support is Al2O3, but due to its acidic nature, it induces coke formation. The selectivity of H2 for Co-based catalysts decreased in the following order: Co/Al2O3 > Co/ZrO2 > Co/MgO > Co/SiO2 > Co/C [38], while the higher activity and hydrogen yield are achieved by Co/ZrO2 [39]. The Rh/Al2O3 catalyst showed the highest selectivity for H2, while Rh/CeO2–ZrO2 exhibited the highest yield in H2 [40].
For the ethanol POx, Salge et al. [41] reported that the H2 yield increases in the order Rh–Ru > Rh > Pd > Pt for an alumina-supported catalyst and that Rh/CeO2 appears as the most stable catalyst with a H2 selectivity, thanks to the promotion of WGS and the limitation of ethanol dehydration by ceria [42].
Also, in the case of ethanol ATR, Youn et al. [43] showed that nickel catalysts supported by the basic supports, ZrO2 and TiO2, had improved catalytic performance (hydrogen yield) and resistance to coke formation. Moreover, Gutierrez et al. [44] demonstrated that the Rh-containing catalysts are more suitable than the commercial Ni/Al2O3 catalysts for the ATR of ethanol.
Table 1. Operating conditions (temperature, pressure, feed ratio) of the ethanol reforming technologies for hydrogen production.
| Technology | Temperature | Pressure | Feed Ratio | Ref. |
|---|---|---|---|---|
| Steam Reforming | 450–650 °C | 2–25 bar | Water/Ethanol = 3–6 | [8,23] |
| Partial Oxidation | 200–400 °C | 1–10 bar | Air/Ethanol = 4 | [42,45] |
| Autothermal Reforming | 600–900 °C | 1–10 bar | Ethanol/Air/Water = 1/0.3–0.5/2–3 | [42,46] |
2.2. Photocatalysis
Photo-assisted hydrogen production processes, a promising way to utilize solar energy and water or other renewable feedstocks, have received significant attention over the last few years [47]. Photocatalysis is a process in which light is absorbed by a semiconducting material to generate electron-hole pairs. When the energy of the irradiated light photon is greater than the band-gap energy of the semiconductor, the electrons migrate to the conduction band (CB) with positive holes left on the valence band (VB). Holes can oxidize either water or organic/inorganic compounds and split them into H+ protons on VB; electrons reduce the H+ species to produce H2 on CB (Figure 2). However, the charge carrier reaction with the target substance competes with the recombination of an electron and a hole [48,49]. If bio-available oxygenated compounds are employed as sacrificial agents, this approach can be seen as nearly carbon-neutral since the CO2 generated can be transformed back into biomass via plant photosynthesis [49,50]. A variety of organic compounds have been investigated, such as methanol [51,52], ethanol [53], glycerol [54,55], and raw biomass [56]. Compared to pure water splitting, the lower Gibbs free energy change leads to more efficient hydrogen production [57], and the formation of CO2 as the product of the oxidation half reaction instead of O2 decreases the recombination reaction [58]. The fundamental reactions are reported in (8)–(9) and in Figure 2.
CxHyOz + (2x − z) H2O → (4x − 2z + y) H+ + x CO2
2 H+ + 2 e− → H2
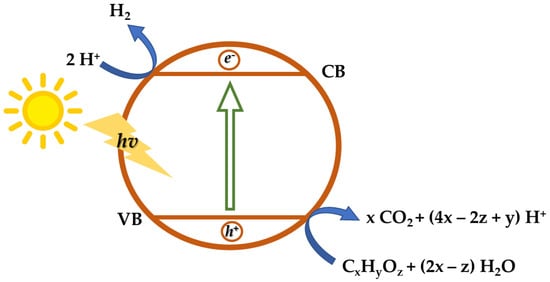
Figure 2. Schematic representation of the photocatalytic process of bio-alcohols and oxygenated hydrocarbons.
For ethanol photoreforming, several mechanisms [57] were proposed. Among those, the most reliable considers the direct oxidation by photoexcited holes in acetaldehyde (10) and the simultaneous reduction of the H+ to H2 (9). The acetaldehyde is then converted to CH4 and CO. Through the WSR reaction (7), CO and CH4 produce CO2 and other H2 [59,60,61].
C2H5OH + 2 H+ → CH3CHO + 2 H+
TiO2-based photocatalysts have received the most attention among all photocatalysts, largely because of their favored characteristics, including an energy band location that can drive a variety of redox processes and, more importantly, H+ reduction, good chemical stability, and low toxicity [62]. However, the low efficiency for hydrogen production is due to fast electron-hole recombination in the bulk or on the surface of semiconductor particles. A promising approach to avoiding that is the deposition of metal nanoparticles on the surface of a photocatalyst that tend to take the electron and avoids recombination [63]. Noble metals including Au, Pd, and Pt are preferred for this purpose, even though Cu species have been proposed as a more economic choice [64,65,66,67]. Other key factors that influence hydrogen production are solution pH, alcohol concentration, temperature, and catalyst load [68]. Increasing the alcohol concentration initially increases the rate, but excessive amounts hinder water adsorption and decrease photocatalytic activity. The same rate trend occurs in relation to the catalyst load: at low catalyst loads, photon adsorption is not complete, while high catalyst loads lead to non-uniform adsorption of light photons. The solution pH affects the reaction rate due to the agglomeration of photocatalyst particles and to variation of the surface charge, which influence the absorption of H+ and alcohols [69]. Estahbanati et al. [59] reported that the maximum amount of hydrogen produced is around a pH of 4 in the presence of Pt/TiO2 for the use of ethanol, methanol, and glycerol as sacrificial agents. The thermal activation energies in these systems are typically less than 30 kJ mol−1, indicating milder activation steps compared to conventional catalytic reactions, such as product desorption and photoexcited carrier migration.
2.3. Electrolysis
Nowadays, water electrolysis represents the most developed sustainable and clean hydrogen production process, but it covers only 4% of industrial hydrogen production processes globally due to economic issues [70]. In fact, even when utilizing noble metal-based catalysts (Pt, Ru, Ir), the low kinetics of water oxidation, which implies high cell voltage (i.e., high energy consumption), makes this technique expensive and not competitive with the main hydrogen production method from natural gas [71]. Therefore, using bio-alcohols as hydrogen carriers in an electrolysis cell can be an interesting alternative, since their reversible oxidation potentials are much lower than that of water (ca. 0.1 V against 1.23 V under standard conditions), so the cell voltages or hydrogen production are lower (and so is the energy consumption) [72]. Methanol [73], ethanol [74], glycerol [75], and ethylene glycol [76] have been considered for hydrogen production through their electrolysis in proton exchange membrane electrolysis cells (PEMECs).
Figure 3 shows the principal of electrochemical decomposition of a bio-alcohol on a PEMEC. Taking ethanol as an example, it is fed to the anode and completely oxidized in the presence of water, releasing CO2 and protons (H+) (11). The protons that reach the cathode compartment, after crossing over the membrane, are reduced to H2 (12).
C2H5OH + 3 H2O → 2 CO2 + 12 H+ + 12 e−
12 H+ + 12 e− → 6 H2
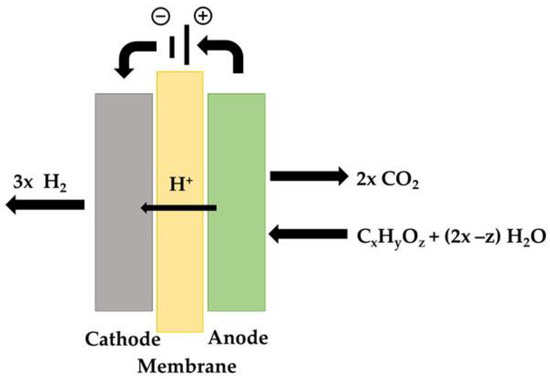
Figure 3. Schematic representation of the electrolysis process of bio-alcohols and oxygenated hydrocarbons.
This corresponds to the electrochemical reforming of ethanol into hydrogen and carbon dioxide, according to the overall reaction:
C2H5OH + 3 H2O → 6 H2 + 2 CO2
Both water and ethanol electrolysis require external energy; however, the energy needed for ethanol is less than that for water (ΔH = +58 kJ/mole H2 for ethanol vs. ΔH = +286 kJ/mole H2 for water) [72]. The lowering of cell voltage is required to boost the energy efficiency of an alcohol PEMEC. The decrease in cell voltage corresponds directly to the increase in the rate of electrochemical reactions happening at both electrodes, with a particular emphasis on the anode, where alcohol oxidation takes place [71]. Unfortunately, the electrooxidation of ethanol is a slow process with overpotentials of 0.3–0.4 V. Furthermore, platinum is known to be rapidly poisoned on its surface by adsorbed species (COads) formed by alcohol dissociative adsorption [77]. To prevent this phenomenon, one approach is to modify the electrode by introducing oxygenated species (e.g., OHads) that are generated through the dissociation of water at lower potentials. This modification facilitates the complete oxidation of COads species to carbon dioxide [78]. It has generally been found that combining Pt with other metals, such as Ni, Ru [74,79], Rh [71,72], Co [80], and Sn [81,82,83,84], in bi- and tri-metallic [85,86] catalysts increase the electrochemical performance of the anode, lowering the rate of catalyst deactivation. Nickel-containing Pt-based catalysts have demonstrated better electrochemical performance compared to other well-studied promoters such as Ru, especially at high potentials, while also being cost-effective [87]. A Pt-Ni-based anodic catalyst supported by graphene nanopatterns has been recently developed by Serrano-Jiménez et al. [88], achieving competitive current density and energy consumption.
As reported above, if ethanol is produced from renewable sources or organic waste, the process can contribute to a carbon-neutral balance. Moreover, bioethanol is relatively easy to obtain from renewable sources and thus has abundant availability. Table 2 shows the advantages and disadvantages, costs, and efficiency of the different investigated methods for hydrogen production from bio-ethanol.
Table 2. Pros and cons, costs, and yield for hydrogen production by ethanol.
| H2 Production Process from Ethanol |
Advantages | Disadvantages | Performances | Ref. |
|---|---|---|---|---|
| Steam Reforming |
|
|
H2 Yield: 60–95% EtOH Conversion: 90–100% H2 Selectivity: 70–90% @Ni/Al2O3, 250–500 °C, H2O/EtOH = 3–6 H2 production cost: 1.58–2.6 USD/kgH2 |
[19,45,89,90] |
| Partial Oxidation |
|
|
H2 Selectivity: 60–97% EtOH Conversion: @Noble metal/Al2O3, 500–600 °C, H2O/O2/EtOH = 3/0.3/1 |
[91,92] |
| Autothermal Reforming |
|
|
H2 Yield: 47–94% EtOH Conversion: 84–97% @Rh/Al2O3 H2 Selectivity: 40–60% @Pt/Al2O3 400–600 °C, H2O/O2/EtOH = 3/0.3/1 |
[93,94] |
| Photocatalysis |
|
|
H2 production rate: 30–34 mmol/gh @Pt/TiO2 |
[95,96] |
| Electrocatalysis |
|
|
H2 volume: 102 mL @Pt/C, 20 °C j = 50 mA/cm−2 |
[72] |
The production of hydrogen from ethanol entails several distinct technologies, each with their own advantages and challenges. The decision on which method to utilize depends on factors such as scalability, economics, and efficiency. Therefore, it is essential to continue research and development in order to overcome limitations. Sustainable hydrogen production is of utmost importance. Progress in catalyst development, process optimization, and cost reduction may revolutionize ethanol-based hydrogen production, making it economically feasible and environmentally sustainable.
In this scenario, researchers propose a novel hydrogen production process, recently patented [97], so-called cyan hydrogen, from bio-alcohols and sodium metaborate, a by-product of little industrial interest, as a valid alternative. This name is inspired by a combination of the green and blue processes, due to the key role played by water and the low carbon content in the gas phase, respectively. The developed process is constituted by a sequential discontinuous step in which bio-alcohols and water are alternatively fed to the sodium metaborate. Recently, researchers demonstrated the feasibility of the process in the presence of ethanol [98]. The process results in the simultaneous production of a hydrogen-rich stream (98% v/v) and a polymeric compound with a repetitive carbon pattern, −CH2−CH2−, i.e., polyethylene structure.
This entry is adapted from the peer-reviewed paper 10.3390/en16237908
This entry is offline, you can click here to edit this entry!
