Tryptase is the most abundant granule-derived serine protease that is mainly produced by mast cells (MCs) and to a much lesser extent by basophils
[1][2][3]. In humans, five isoforms can be found: α-, β-, γ-, δ-, and ε-tryptase
[4][5][6]. However, only α- and β-tryptase are clinically relevant and are the most abundant
[7]. Resting MCs constitutively secrete monomeric pro-tryptase
[8]. In the case of MC degranulation (e.g., during anaphylaxis), MCs release mature tetrameric tryptase. The tryptase assay commercially available from Thermo Fisher
® (Waltham, MA, USA) ImmunoCAP measures all of these isoforms in monomeric and tetrameric form (i.e., total serum tryptase)
[9].
The predominant indication for tryptase measurement is to document conditions related to systemic MC activation (MCA) (e.g., during anaphylaxis or episodes of mast cell activation syndromes). In this regard, paired sampling is of utmost importance, namely, one during the event (i.e., ideally 30–120 min after the onset of symptoms)—defined as acute serum tryptase (aST)—and one at least 24 h after the event—defined as baseline tryptase (bST). According to the consensus formula, an aST ≥ [(1.2 × bST) + 2], depicts MCA
[10][11]. Tryptase can also be a useful marker in suspected primary MC disorders (PMCDs). If bST is more than 20 ng/mL, a minor criterion for the diagnosis of systemic mastocytosis (SM) is met
[12][13][14]. Documented MCA based on paired tryptase samples is also a validated and required criterion in mast cell activation syndrome (MCAS). Hereditary alpha-tryptasemia (HαT) is an autosomal dominant genetic trait of an increased copy number of the alpha-coding (of ≥2)
TPSAB1-gene
[15][16], which may account for elevated serum tryptase in the absence of clonal mast cell disorders. The clinical relevance of HαT is still under debate since not everyone with this trait experiences mediator-related symptoms or anaphylaxis
[17][18]. However, it seems to be a disease-modifying trait in anaphylaxis and PMCD
[19][20][21].
2. Tryptase for Suspected Mast Cell Disorders
Serum tryptase measures the total concentration of different isoforms of tryptase, mostly α- and β-tryptase. Both can be either in a monomeric (immature) or a tetrameric (mature) form. The monomeric forms are secreted constitutively and can have some variability
[23]. During the degranulation of MCs (e.g., anaphylaxis), the mature tetrameric forms of tryptase are released, resulting in an increase in total tryptase
[2].
The interpretation of tryptase is dependent on the context. In acute settings, such as suspected anaphylaxis or MC mediator-related symptoms, an aST should ideally be measured from 30 up to 120 min after the start of symptoms since this will correspond with the peak value
[24]. However, it may be still measured up to 4 h after a systemic hypersensitivity reaction. Even if the determination of tryptase is not directly available, the serum of the patient should be obtained within this widow. The sample can always be stored or shipped for analysis afterward since tryptase is rather stable
[25]. A bST should be taken at least 24 h afterward. Paired analysis of both values allows one to determine if MCA had taken place and should always be checked
[26]. Different approaches have been proposed such as a delta tryptase > 3 ng/mL or a rise of 35% in tryptase
[27][28]. In perioperative anaphylaxis, the consensus formula showed the best sensitivity and specificity
[11]. Overall, the most used and validated approach is the consensus formula.
Using the consensus formula, the aST should have an increase of 20% + 2 ng/mL compared with the bST value
[10]. Generally, the severity and magnitude of hypotension in anaphylaxis correlate with the height of tryptase
[29][30]. Recently, an aST/bST ratio above 1.685 has been proposed to increase specificity in patients with ISM and/or HαT as these patients can depict a variability in bST without MCAS
[23]. However, this requires further validation. For the time being, the consensus formula is still widely used as a criterion and considered as the golden standard
[10]. On the other hand, some patients might clearly experience anaphylaxis but not fulfill the criteria for MCA due to a lack of elevation in tryptase. This is especially true for patients with food-induced anaphylaxis
[31][32], in which only a 30% increase in tryptase seems to suffice
[33]. It is possible that other mediators such as platelet-activating factor (PAF), prostaglandin D2, leukotriene E4, histamine, or other cytokines might be more sensitive in these patients
[34][35][36][37]. Note that even when MCA is depicted, no conclusion can be drawn about the mechanism (IgE-mediated or non-IgE-mediated) responsible for MC degranulation
[38][39].
An elevated bST can be found in different scenarios that are listed in
Table 1. The most common reason is HαT (91%), followed by chronic renal failure (7%) and hematologic malignancies and mastocytosis (1%)
[8][19]. HαT is a genetic trade in which there is an increased copy number of the alpha-coding
TPSAB1-gene. In the majority of cases, it involves a duplication, although more copies (e.g., a quintuplication
[40]) have been described. It is the cause of an elevated bST in about 90% of patients and is found in up to 6% of the general population
[8][22]. The trait is inherited in an autosomal dominant way. The majority are healthy individuals and will not develop any MC-related conditions
[41]. One hypothesis is that the pathological potency of HαT is caused by active heterotetrametric α/β-tryptase and that these are more abundant in patients with a higher α/β ratio
[42]. However, more research is needed on this topic. Actually, an increased bST up to 15 ng/mL without any mediator-related symptoms or anaphylaxis is no reason for further evaluation
[43]. On the other hand, mastocytosis should be suspected in patients with elevated bST if other common causes of elevated bST are excluded or if the bST exceeds the predicted value based on the number of
TPSAB1 replications. One way to calculate estimated tryptase is to divide the bST by 1 + the extra copy numbers of the alpha-tryptase gene
[43]. Another way is by using an online calculator tool (
https://bst-calculater.niaid.nih.gov/ (accessed on 13 November 2023)) developed by Chovanek et al.
[44].
Table 1. Most common etiologies of elevated bST. HαT: hereditary alpha tryptasemia; SM: systemic mastocytosis; SCF: stem cell factor.
| HαT |
| Chronic renal failure |
| Obesity |
| Hematologic malignancy (especially myeloid neoplasms) |
| SM |
| Chronic parasitic infections (e.g., helminthic infections) |
| Administration of SCF |
| Rare genetic mutations (e.g., GATA2 or PLCG2) |
| Elderly |
| Cardiovascular disease |
| False positive (due to interference with the immunoassay) |
In some cases, patients with HαT do present with a clinical picture dominated by mediator-related symptoms (i.e., vibratory urticaria, flushing, abdominal cramps, headache, dysautonomia, etc.)
[15][16][45]. Some patients might meet the criteria for MCAS
[46][47]. However, it is debated whether HαT should be considered as a separate clinical phenotype of MCAS. There is a consensus that HαT is a risk modifier of severe anaphylaxis
[21][48][49][50][51][52][53]. However, a recent study did not find a difference in the prevalence of HαT in patients with or without anaphylaxis with underlying SM
[54], and more studies with opposing results exist
[55].
A bST ≥ 20 ng/mL is a minor criterion for the diagnosis of SM. Importantly, if another hematological neoplasm is present, this criterion is no longer valid, and in the case of HαT, the tryptase level should be adjusted
[56]. Moreover, the bST is included in different scoring systems to assess the risk for underlying PMCD in patients presenting with severe anaphylaxis without having typical signs of mastocytosis lesions in the skin. The most commonly used and validated is the REMA-score (Red Española de Mastocitosis (Spanish Network on Mastocytosis))
[57], which was initially developed for
Hymenoptera venom allergy and later extended to other causes of anaphylaxis. Other scoring systems used to predict clonality are the NICAS score (National Institute for Health and Care Excellence)
[58] and Karolinska score
[59], which can be used for patients with idiopathic or unprovoked anaphylaxis. These tools use similar parameters such as sex and clinical symptoms. They differ in the cut-off for tryptase and the presence of the
KIT D816V mutation in peripheral blood, of which the latter is only included in NICAS. Regarding bST, the REMA score includes < 15 (−1 point) and > 25 ng/mL (+2 points); while Karolinska uses < 11.4 ng/mL (−1 point) and >20 ng/mL (+2 points), and finally, NICAS uses only 11.4 ng/mL as cut off
[60]. However, tryptase is only one element in the diagnosis. One should be cautious that patients with SM and anaphylaxis seem to have a lower bST in contrast to patients with SM without anaphylaxis
[61][62][63][64][65]. Note that the validity of
KIT D816V detection is highly dependent on the analytic performance characteristics of the applied molecular assay. It is therefore recommended to use a highly sensitive technique such as an allele-specific oligonucleotide quantitative reverse transcriptase polymerase chain reaction (ASO-qPCR)
[66] or digital droplet PCR (ddPCR)
[67]. Importantly, even with a highly sensitive test, a negative result for the
KIT D816V mutation in peripheral blood would not exclude underlying PMCD, since this has a low negative predictive value
[68][69]. Thus, the diagnosis might be challenging, especially since the clinical presentation can be very heterogeneous
[70]. Importantly, tryptase in patients with SM is not correlated with symptom severity
[71]. It is however correlated with disease severity since tryptase serves a B-finding if it is above 200 ng/mL (adjusted for the
TPSAB1 status)
[56][72].
Non-MC disorders can give rise to bST, such as impaired renal function
[73]. Probably, this is due to an elevated SCF since tryptase is not cleared by kidneys
[74]. An increase in SCF can induce MC hyperplasia and thus give rise to elevated bST
[75]. Tryptase also tends to rise with age
[76]. Moreover, obesity can be a cause of elevated bST
[77], as well as helminthic infections, hematological malignancies, cardiovascular disease, (nummular) eczema, or rare genetic mutations (e.g., GATA2 or PLAID)
[8][78][79]. Even alcohol consumption
[80] or tobacco smoking
[81] can decrease or elevate bST, respectively. Also, a case of a patient with Gaucher Disease type 1, a lysosomal storage disorder, was reported to have an elevated tryptase level (up to 80 ng/mL) that improved upon initiation of enzyme replacement therapy
[82]. Finally, interference with the immunoassay may lead to a false positive result (e.g., by heterophilic antibodies
[83]).
Acute tryptase can be used to prove MCA in MCAS
[84]. For a complete differential diagnosis of mediator-related symptoms
[85] or management of these symptoms
[86], the reader is referred elsewhere. The most commonly used biomarker is paired serum tryptase since the consensus formula has been validated. For the diagnosis of MCAS, other biomarkers such as histamine, prostaglandins, leukotrienes, or metabolites can be used. However, one should keep in mind that these metabolites have yet to be validated
[34], although an increase of 1.3 in leukotriene E4, 2,3-dinor-11b-prostaglandinF2a, and n-methylhistamine has been proposed as a possible sign of MCA
[87]. Additionally, diamine oxidase has been shown to significantly increase during anaphylaxis and has a longer half-life compared with tryptase, making this an interesting marker for further evaluation
[88].
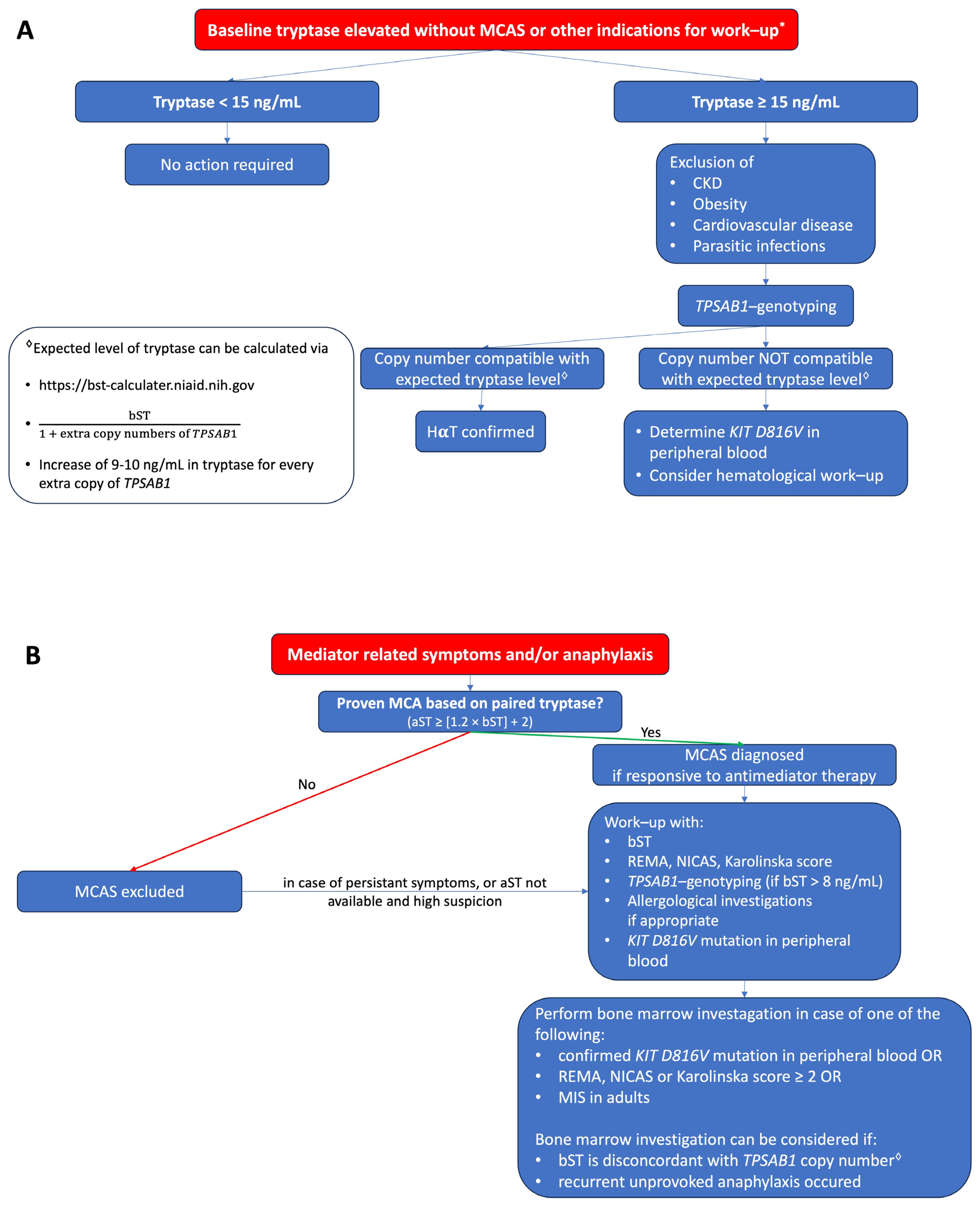
A proposed algorithm in how to evaluate patients with elevated tryptase without or with mediator related symptoms is shown in figure 1A and figure 1B, respectively.
Figure 1. (A) Proposed algorithm for patients with elevated bST. (B) Proposed algorithm for patients with mediator-related symptoms. MCAS: mast cell activation syndrome; MCA: mast cell activation; CKD: chronic kidney disease; HαT: hereditary alpha tryptasemia; bST: baseline serum tryptase; aST: acute serum tryptase; MIS: mastocytosis in the skin. * Indications for work-up include unexplained osteoporosis or MIS in adults.