Tryptase has proven to be a very useful and specific marker to demonstrate mast cell activation and degranulation when an acute (i.e., within 4 h after the event) and baseline value (i.e., at least 24 h after the event) are compared and meet the consensus formula (i.e., an increase of 20% + 2). The upper limit of normal determined by the manufacturer is 11.4 ng/mL; however, this boundary has been the subject of debate. According to ECNM and AIM experts, the normal range of baseline tryptase should be 1 to 15 ng/mL. A genetic trait, hereditary alpha tryptasemia, characterized by an increased alpha coding TPSAB1 copy number is associated with a baseline value above 8 ng/mL. Elevated tryptase can also be found in chronic kidney disease, obesity, and hematological neoplasms. A tryptase > 20 ng/mL serves as a minor criterion to diagnose systemic mastocytosis and an increase in tryptase > 20% + 2 during an acute event is a required criterion in the diagnosis of mast cell activation syndrome.
- mastocytosis
- mast cell activation syndrome (MCAS)
- tryptase
1. Introduction
2. Tryptase for Suspected Mast Cell Disorders
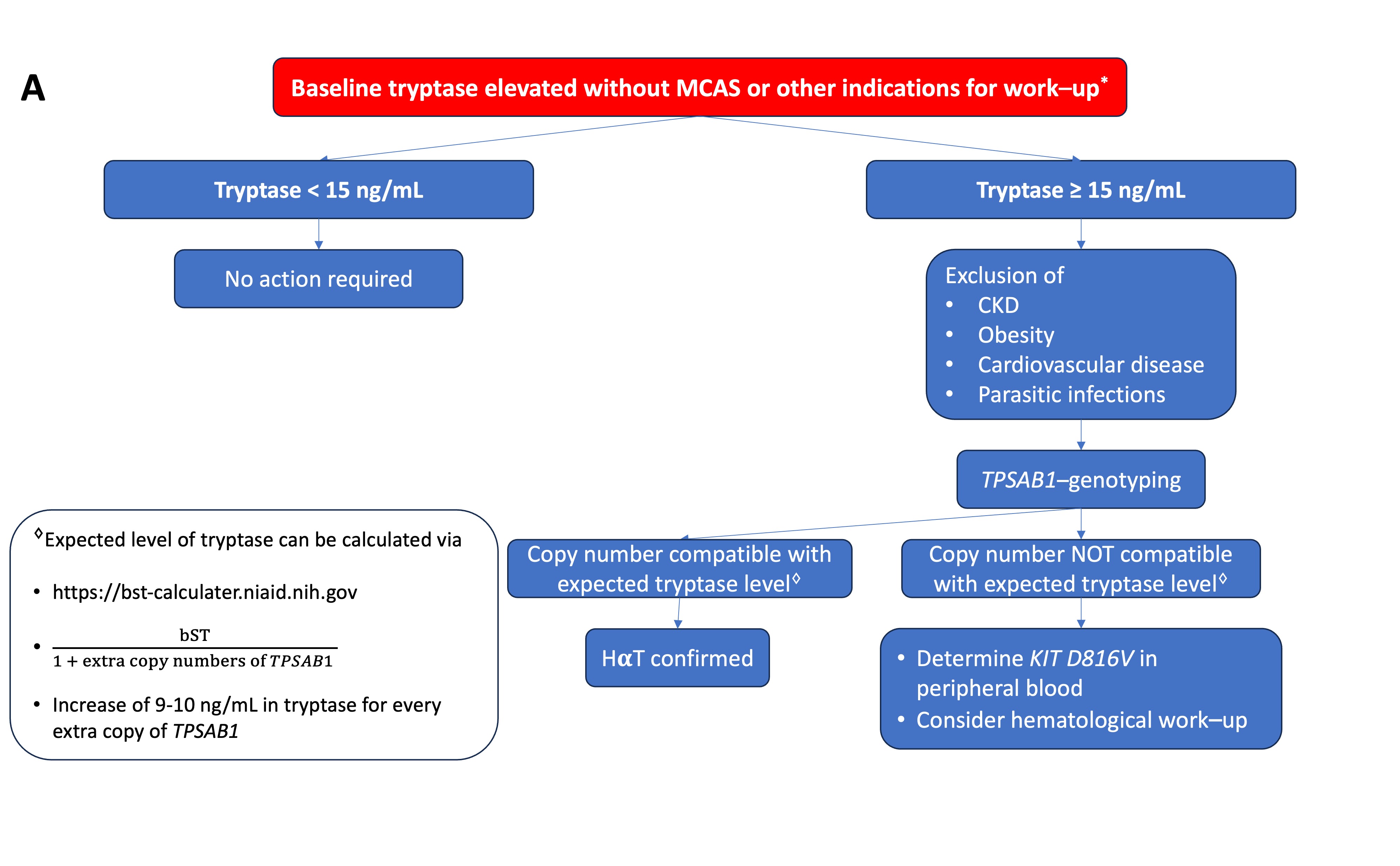
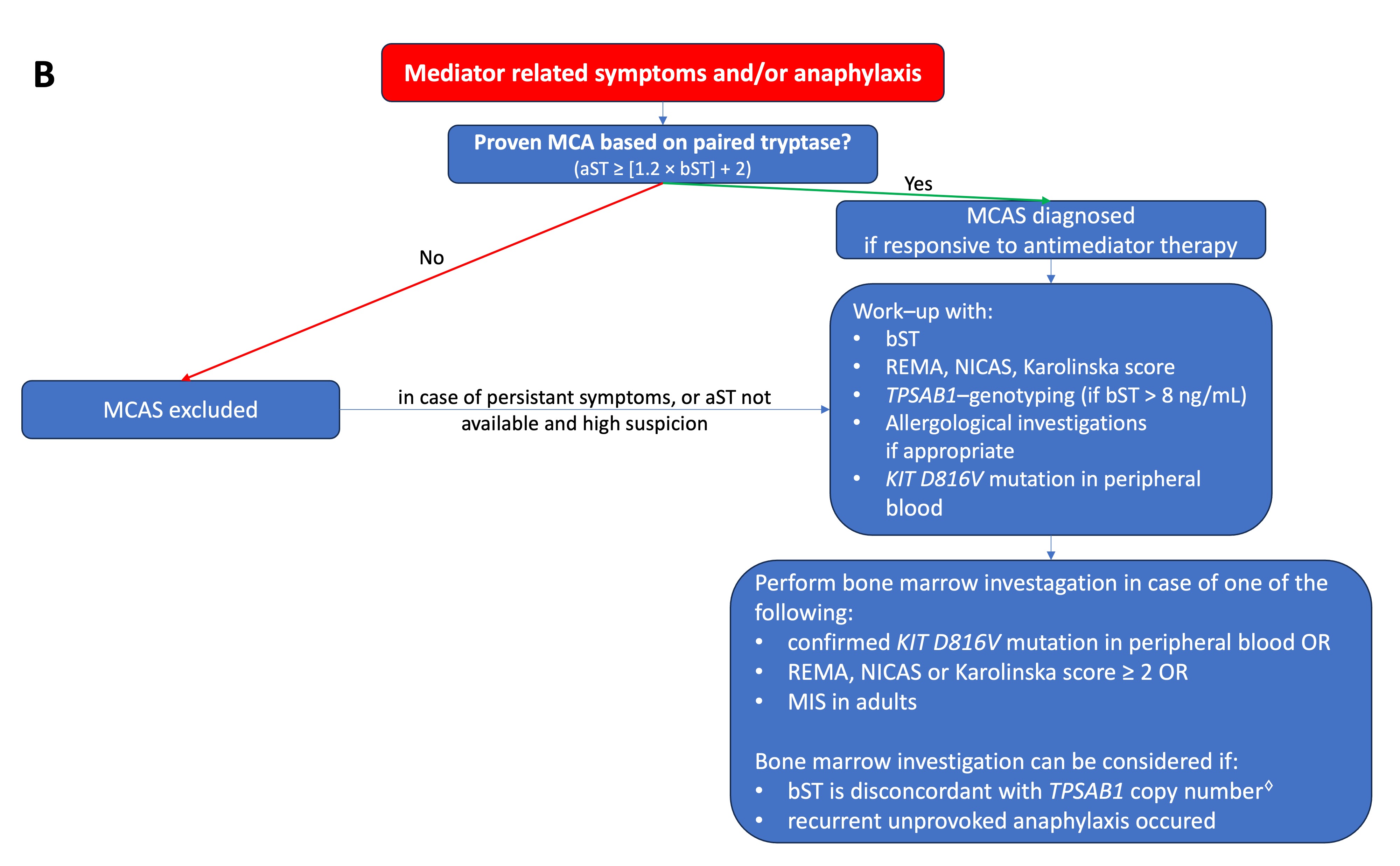
Serum tryptase measures the total concentration of different isoforms of tryptase, mostly α- and β-tryptase. Both can be either in a monomeric (immature) or a tetrameric (mature) form. The monomeric forms are secreted constitutively and can have some variability [23]. During the degranulation of MCs (e.g., anaphylaxis), the mature tetrameric forms of tryptase are released, resulting in an increase in total tryptase [2]. The interpretation of tryptase is dependent on the context. In acute settings, such as suspected anaphylaxis or MC mediator-related symptoms, an aST should ideally be measured from 30 up to 120 min after the start of symptoms since this will correspond with the peak value [24]. However, it may be still measured up to 4 h after a systemic hypersensitivity reaction. Even if the determination of tryptase is not directly available, the serum of the patient should be obtained within this widow. The sample can always be stored or shipped for analysis afterward since tryptase is rather stable [25]. A bST should be taken at least 24 h afterward. Paired analysis of both values allows one to determine if MCA had taken place and should always be checked [26]. Different approaches have been proposed such as a delta tryptase > 3 ng/mL or a rise of 35% in tryptase [27][28][27,28]. In perioperative anaphylaxis, the consensus formula showed the best sensitivity and specificity [11]. Overall, the most used and validated approach is the consensus formula. Using the consensus formula, the aST should have an increase of 20% + 2 ng/mL compared with the bST value [10]. Generally, the severity and magnitude of hypotension in anaphylaxis correlate with the height of tryptase [29][30][29,30]. Recently, an aST/bST ratio above 1.685 has been proposed to increase specificity in patients with ISM and/or HαT as these patients can depict a variability in bST without MCAS [23]. However, this requires further validation. For the time being, the consensus formula is still widely used as a criterion and considered as the golden standard [10]. On the other hand, some patients might clearly experience anaphylaxis but not fulfill the criteria for MCA due to a lack of elevation in tryptase. This is especially true for patients with food-induced anaphylaxis [31][32][31,32], in which only a 30% increase in tryptase seems to suffice [33]. It is possible that other mediators such as platelet-activating factor (PAF), prostaglandin D2, leukotriene E4, histamine, or other cytokines might be more sensitive in these patients [34][35][36][37][34,35,36,37]. Note that even when MCA is depicted, no conclusion can be drawn about the mechanism (IgE-mediated or non-IgE-mediated) responsible for MC degranulation [38][39][38,39]. An elevated bST can be found in different scenarios that are listed in Table 1. The most common reason is HαT (91%), followed by chronic renal failure (7%) and hematologic malignancies and mastocytosis (1%) [8][19][8,19]. HαT is a genetic trade in which there is an increased copy number of the alpha-coding TPSAB1-gene. In the majority of cases, it involves a duplication, although more copies (e.g., a quintuplication [40]) have been described. It is the cause of an elevated bST in about 90% of patients and is found in up to 6% of the general population [8][22][8,22]. The trait is inherited in an autosomal dominant way. The majority are healthy individuals and will not develop any MC-related conditions [41]. One hypothesis is that the pathological potency of HαT is caused by active heterotetrametric α/β-tryptase and that these are more abundant in patients with a higher α/β ratio [42]. However, more research is needed on this topic. Actually, an increased bST up to 15 ng/mL without any mediator-related symptoms or anaphylaxis is no reason for further evaluation [43]. On the other hand, mastocytosis should be suspected in patients with elevated bST if other common causes of elevated bST are excluded or if the bST exceeds the predicted value based on the number of TPSAB1 replications. One way to calculate estimated tryptase is to divide the bST by 1 + the extra copy numbers of the alpha-tryptase gene [43]. Another way is by using an online calculator tool (https://bst-calculater.niaid.nih.gov/ (accessed on 13 November 2023)) developed by Chovanek et al. [44].| HαT |
|---|
| Chronic renal failure |
| Obesity |
| Hematologic malignancy (especially myeloid neoplasms) |
| SM |
| Chronic parasitic infections (e.g., helminthic infections) |
| Administration of SCF |
| Rare genetic mutations (e.g., GATA2 or PLCG2) |
| Elderly |
| Cardiovascular disease |
| False positive (due to interference with the immunoassay) |
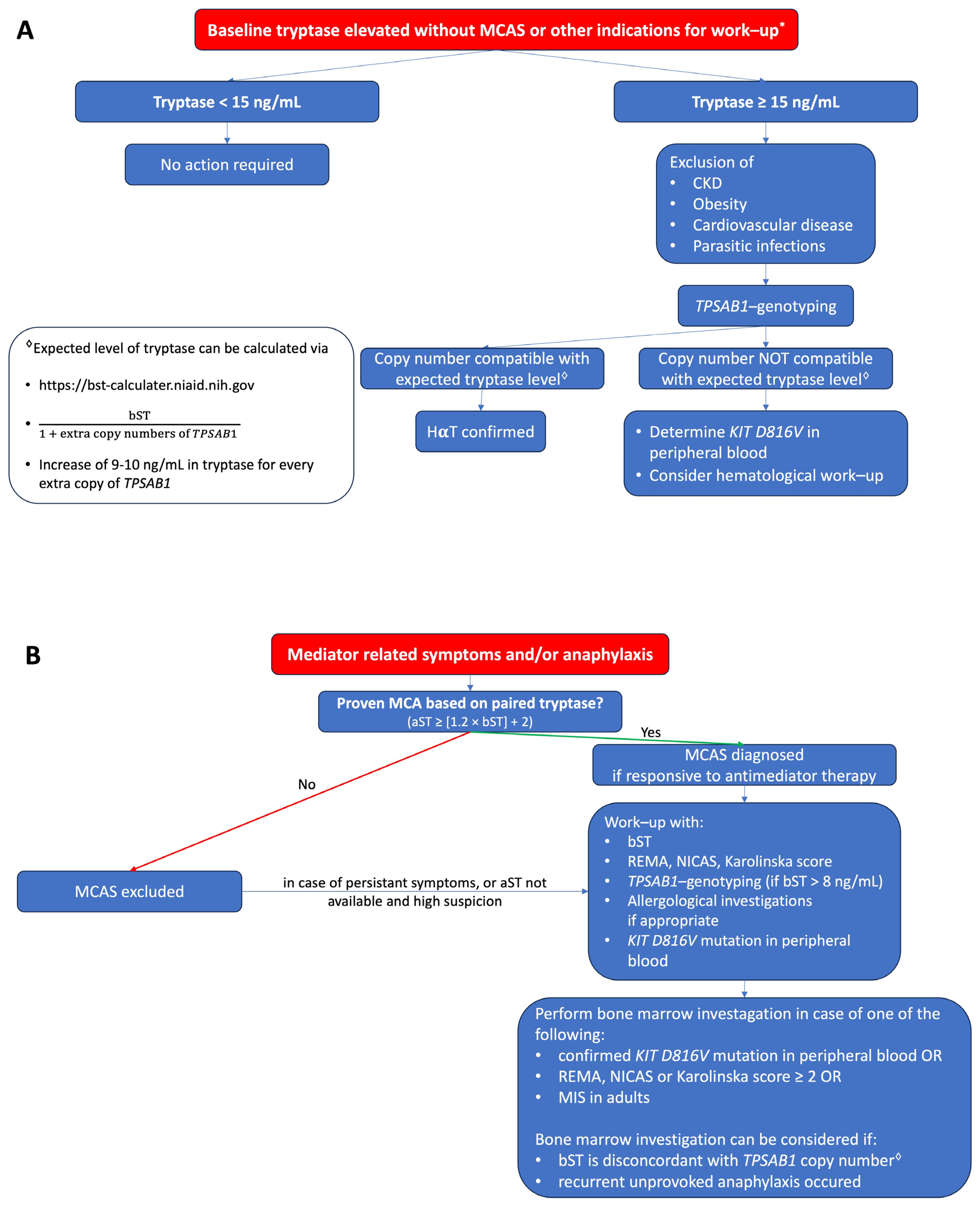 Figure 1. (A) Proposed algorithm for patients with elevated bST. (
Figure 1. (A) Proposed algorithm for patients with elevated bST. (proposed algorithm in patients with elevated bST; Figure 1B
) Proposed algorithm for patients with mediator-related symptoms. MCAS: mast cell activation syndrome; MCA: mast cell activation; CKD: chronic kidney disease; HαT: hereditary alpha tryptasemia; bST: baseline serum tryptase; aST: acute serum tryptase; MIS: mastocytosis in the skin. * Indications for work-up include unexplained osteoporosis or MIS in adults.proposed algorithm in patients with mediator related symptoms; MCAS: mast cell activation syndrome; MCA: mast cell activation; CKD: chronic kidney disease; HaT: hereditary alpha tryptasemia; bST: baseline serum tryptase; aST: acute serum tryptase; MIS: mastocytosis in the skin. *Indications for work-up include unexplained osteoporosis or MIS in adults.