Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hakan Gocol | -- | 1857 | 2023-12-13 22:22:43 | | | |
| 2 | Lindsay Dong | Meta information modification | 1857 | 2023-12-19 03:30:37 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gocol, H.; Zeng, J.H.; Chang, S.; Koh, B.Y.; Nguyen, H.; Cirillo, N. Role of Arecoline in Oral Carcinogenesis. Encyclopedia. Available online: https://encyclopedia.pub/entry/52715 (accessed on 07 February 2026).
Gocol H, Zeng JH, Chang S, Koh BY, Nguyen H, Cirillo N. Role of Arecoline in Oral Carcinogenesis. Encyclopedia. Available at: https://encyclopedia.pub/entry/52715. Accessed February 07, 2026.
Gocol, Hakan, Jin Han Zeng, Sara Chang, Buo Yu Koh, Hoang Nguyen, Nicola Cirillo. "Role of Arecoline in Oral Carcinogenesis" Encyclopedia, https://encyclopedia.pub/entry/52715 (accessed February 07, 2026).
Gocol, H., Zeng, J.H., Chang, S., Koh, B.Y., Nguyen, H., & Cirillo, N. (2023, December 13). Role of Arecoline in Oral Carcinogenesis. In Encyclopedia. https://encyclopedia.pub/entry/52715
Gocol, Hakan, et al. "Role of Arecoline in Oral Carcinogenesis." Encyclopedia. Web. 13 December, 2023.
Copy Citation
Arecoline is the primary active carcinogen found in areca nut and has been implicated in the pathogenesis of oral squamous cell carcinoma (OSCC) and oral submucous fibrosis (OSF).
arecoline
nicotine
acetylcholine receptors
oral submucous fibrosis
oral cancer
mouth neoplasms
oral squamous cell carcinoma
areca nut
1. Introduction
Arecoline, an active carcinogenic alkaloid found in areca nut, has been recognized as an important factor in the pathogenesis of premalignant and malignant oral disorders [1], specifically, oral submucous fibrosis (OSF) and oral squamous cell carcinoma (OSCC). However, the precise molecular mechanisms underlying arecoline-induced OSF and OSCC development remain unclear [2][3].
OSF is characterized by progressive fibrosis and inflammation of the submucosal tissues [4]. It is a potentially malignant disorder that is associated with an increased risk of OSCC, a malignant neoplasm originating from the stratified squamous epithelium of the oral mucosa [5]. OSF progression to OSCC takes place in approximately 7–14% of patients [6]. OSCC is one of the most frequently reported malignancies in the world, especially in Taiwan and India, accounting for approximately 90% of all oral cavity cancers [7].
2. Role of Arecoline in Oral Carcinogenesis
Evidence of Increased Cell Growth and Proliferation
In vivo studies on murine models exposed to arecoline revealed the presence of squamous cell hyperplasia in the excised samples [8][9][10][11]. The introduction of arecoline-stimulated OSCC cell lines resulted in tumor growth [12]. Furthermore, certain studies explored the gene expressions associated with cellular proliferation and noted that arecoline promoted the gene expression of notch receptor 1 (NOTCH1), protein tyrosine kinase 6 (PTK6), and discoidin domain receptor tyrosine kinase 1 (DDR1) [8][13][14][15] while downregulating the tumor suppressor gene, retinoic acid receptor beta (RARB) [16].
Numerous in vitro studies have demonstrated that arecoline exposure leads to increased cell proliferation in various oral cell lines, including OSCC cells, oral keratinocytes, and gingival fibroblasts [8][13][17][18][19][20]. The levels of numerous biomarkers of cell proliferation, such as proliferating cell nuclear antigen (PCNA) and antigen kiel 67 (Ki67), were found to be elevated with arecoline treatment [8][17]. Studies that investigated gene expression found that arecoline triggers the upregulation of a wide range of genes and signaling pathways, including NOTCH1, MYC proto-oncogene (MYC), peroxiredoxin 2 (PRDX2), Wnt pathway (WNT), cysteine-rich angiogenic inducer 61 (CYR61), epidermal growth factor receptor/phosphoinositide 3-kinases (EGFR/Pl3k), and discoidin domain receptor 1 (DDR1) [8][14][15][21][22][23][24]. In contrast, the expression of tumor suppressor genes such as alcohol dehydrogenase, iron-containing 1 (ADHFE1), aldehyde dehydrogenase 1 family member A2 (ALDH1A2), dual specificity phosphatase 4 (DUSP4), and tumor protein p53 (TP53) was found to be downregulated upon exposure to arecoline [25][26][27].
Apoptosis/Cell Cycle Arrest
Caspase 8 (CASP8) was upregulated in mice models challenged with arecoline N-oxide (ANO) [11]. In vitro, arecoline induction was found to result in keratinocyte cell proliferation and inhibit apoptosis via PRDX2 gene overexpression [22], the elevation of CASP8 protein [11], the activation of mitogen-activated protein kinase 1/extracellular-signal-regulated kinase pathway (MEK1/ERK pathway) [28], and through triggering the ATM-dependent pathway, inducing arrest at mitosis [29][30]. There were observed increases in transcription factor Jun (c-jun) mRNA levels with fos proto-oncogene (c-fos) pathway activation, affecting cell cycle progression [28], but it was reported elsewhere that this did not induce c-fos mRNA expression [31].
Exposure of epithelial cells to arecoline was found to suppress viability and promote apoptosis and atrophy in a dose-dependent manner [19][32]. Arecoline inhibits epithelial cell proliferation and affects cell morphology, including cell cycle arrest in the G1/S phase and survival in a dose-dependent manner [33][34][35].
For SAS cancer cells, arecoline leads to cell death, apoptosis, and cell cycle arrest by stimulating checkpoint kinase 1 (Chk1) and checkpoint kinase 2 (Chk2) phosphorylation [36].
In fibroblasts, arecoline inhibited the expression and function of tumor protein p53 (p53) and its downstream molecules [20][27][30], as well as down-regulated cyclin-dependent kinase inhibitor 1 (p21) and cyclin-dependent kinase inhibitor 1B (p27) [37], increased carbonic anhydrase IX (CAIX) expression [38], and led to cell-cycle exit [39]. Arecoline was also found to inhibit the tissue inhibitor of metallopeptidase 1 (TIMP-1) and tissue inhibitor of metalloproteinase 2 (TIMP-2) in fibroblasts in one in vitro study [36]; however, TIMP-1 was elevated in two other studies [40][41].
Promoting Invasion (Migration/Epithelial-to-Mesenchymal Transition (EMT)/Adhesion/Invasion)
Growth and invasion were promoted through the arecoline-induced upregulation of the NOTCH1 gene in mouse models [8][14]. EMT was promoted, activating invasion, and resulting in elevated PTK6 expression with E-cadherin (E-cad) suppression [13], which is deleted in esophageal cancer 1 (DEC1) upregulation, leading to focal adhesion kinase/serine/threonine kinase (FAK/AKT) [42], and through the upregulation of keratin 17 (Krt17) [43] in murine models.
In in vitro experiments, arecoline has been found to promote EMT in oral epithelial cells through DEC1 upregulation activating FAK/AKT downstream [42], the upregulation of proteasome activator complex subunit 3 (PA28γ), and the phosphorylation of MEK-1 [44] and was found to promote the expression of EMT-related genes [20][27]. Arecoline resulted in a dose- and time-dependent increase in zinc finger protein 1 (SNAI1) expression in human oral keratinocytes (HOKs) and OECM-1 [45]. The long-term exposure of buccal mucosal fibroblasts (BMFs) resulted in the dose-dependent upregulation of transcription factor zinc-finger E box-binding homeobox 1 (ZEB1) and the upregulation of insulin-like growth factor receptor 1 (IGF-R1) [46][47].
Fibrotic Alteration/Impaired Wound Healing
Arecoline challenge resulted in squamous cell hyperplasia, increased collagen deposition and fibrotic alteration, and also increased cervical lymph node (LN) metastasis in mice [8][9][10][42][48].
Arecoline-treated BMFs resulted in a dose-dependent increase in SLUG protein, leading to the increased expression of type I collagen [49]. Collagen production was increased through heat shock protein (HSP) 47 upregulation and altered matrix metallopeptidase 1, 2, and 9 (MMP-1, MMP-2, and MMP-9) expression [36][39][41][50][51]. Extracellular matrix (ECM) synthesis and secretion was increased through the upregulation of S100A4 gene expression [52]. Plasminogen activator inhibitor-1 (PAI-1) expression was dose-dependently elevated in arecoline-treated BMFs [53] but the increased expression of α-SMA (alpha smooth muscle actin) [43] was observed in one study, while decreased expression was seen in another [54].
Immune Responses and ROS/Antioxidant Activity
One in vivo study conducted on murine models observed increased antioxidant activity in heat shock protein 27 (HSP27) when the mice were exposed to arecoline [55].
Three in vivo studies investigated the effects of arecoline on different cytokines. Elevated levels of transforming growth factor-β (TGF-β) and interleukins (IL-1α, IL-1β, IL-10, and IL-17) were observed [12][14][48]. Tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) were reduced in one of these studies [12].
Six in vitro studies observed ROS production in cells challenged with arecoline or its metabolites, including ANO, arecaidine, and arecoline N-oxide mercapturic acid (NOM) [14][19][45][56][57]. Antioxidant activities were shown to be reduced in some studies [14][57][58][59] but were increased in other studies [60][61][62].
Multiple in vitro studies have indicated the elevated expression of several inflammatory cytokines, including TGF-β, interleukins (IL-1α, IL-1β, IL-17α), serum amyloid A1 (SAA1), prostaglandin E2 (PGE2), and TNF-α [8][10][14][39][48][63][64][65][66][67]. However, one study observed a reduction in IL-6 and minimal changes in TNF-α [66]. One study mentioned the upregulation of programmed death-ligand 1 (PD-L1) in OSCC cells that were exposed to arecoline [21].
Genotoxicity and Epigenetics
The induction of DNA damage and the alteration of repair mechanisms are widely regarded as the central mechanisms responsible for arecoline-induced carcinogenesis. In vitro, arecoline stimulated an increase in O6-methyl-guanine-DNA methyltransferase (MGMT) expression in HOKs and an increase in the phosphorylation of H2AX variant histone (γH2AX) [10][17][39], as well as the induced markers of irreparable DNA double-stranded breaks in normal human oral fibroblasts and p53-binding protein 1 (53BP1) [39]. Low doses of arecoline induced elevated cell proliferation and DNA repair [17]; however, long-term and high-dose exposure reduced DNA repair [57]. Arecoline also resulted in the reduced expression of sirtuin 1 (SIRT1) mRNA [68].
In terms of epigenetic regulation, arecoline upregulated microRNA (miRNA) miR-211 expression in OSCC cell cultures [69]. miR-211-promoted OSCC was shown to repress gene transcription factor 12 (TCF12) and peroxiredoxin-like 2A (FAM213A) [69]. Arecoline exposure to cultured cells such as HOKs and OSCC cell lines led to a reduction in miR-1455 [70], miR30a, miR379, miR-203, miR-22, miR-200b, miR329, and miR410 and an increase in miR-23a, miR-886-3p, and miR-10b [20][24][25][36][65][68][70][71][72][73]. miR-23a overexpression was found to be associated with reduced double-stranded break repair [72], while miR-200 was shown to be involved in arecoline-related myofibroblast activities in BMFs and fBMFs [36].
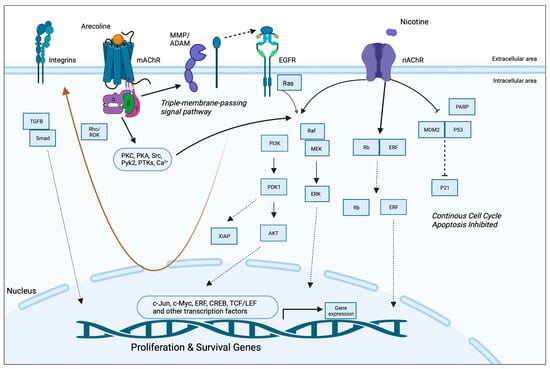
Arecoline-Mediated Acetylcholine Receptor Signaling in Oral Carcinogenesis
A study by Gareth Thomas’ group demonstrated that arecoline upregulated keratinocyte αvβ6 expression, a process modulated through the M(4) muscarinic acetylcholine receptor [63]. Arecoline-dependent αvβ6 upregulation promoted keratinocyte migration and induced invasion, raising the possibility that this mechanism may support malignant transformation. In another study, long-term nicotine-derived nitrosamine ketone (NNK) and arecoline exposure resulted in an increase in cancer stem cell properties, anti-apoptotic pathways, and a resistance to cisplatin in head and neck squamous cell carcinoma (HNSCC) cells in vitro [74]. The EGFR protein was pivotal in inducing tumor promotion and in impeding apoptosis in cancer cells by inducing phosphorylated AKT serine/threonine kinase 1 (pAKT) and nuclear factor kappa B (NFκB). While the authors pointed out that both NNK and arecoline exert agonist activity with the alpha-7-nicotinic acetylcholine receptor (α7-nAChR), the study did not directly investigate the role of nAChR in mediating the effects reported and, hence, was not included in the qualitative synthesis. Both studies, however, point to the possibility that arecoline promotes carcinogenesis via receptor-mediated mechanisms, an aspect that has not been captured in the available literature. The putative signaling pathways are depicted in Figure 1.

Figure 1. Putative pathways involving the receptor-mediated signaling of arecoline. Arecoline binds preferentially to muscarinic acetylcholine receptors (mAChR) but can also serve as a partial agonist of nicotinic receptors (nAChR). Two key molecular pathways involve EGFR and integrins. mAChR activates EGFR signaling via a so-called “triple-membrane-passing” pathway, whereby metalloproteinases cleave and activate EGF-like ligands, which, in turn, bind to EGFR and trigger downstream kinase signaling, including the Ras/Raf/MEK/ERK pathway. MAPK signaling can also be activated via canonical second messenger-mediated signals, as well as via nAChR. The two receptors also work synergistically to promote survival and inhibit apoptosis via PI3K/Akt and p21, respectively. Together, these pathways promote the expression of proliferation and survival genes, as well as migration/invasion and fibrosis/senescence via integrins and TGF-beta signaling, respectively (brown arrow). See Abbreviations part for the abbreviations and acronyms.
The Effects of Arecoline in the Oral Mucosa Could Be Mediated by the Local Cholinergic Axis
Previous research has convincingly demonstrated that both arecoline and guvacoline activate muscarinic acetylcholine receptors 1 and 3 (M1 and M3 mAChRs) [75], while only arecoline produces significant activation of the α4 nicotinic receptor and acts as a silent agonist of α7 nAChR [76]. A molecular docking simulation and antagonist co-exposure experiments also showed that arecoline has a strong affinity to muscarinic receptors M1–M4 [77]. Hence, it is likely that arecoline elicits cholinergic signals in the oral mucosa via the M2, M3, and M4 mAChR subtypes that are expressed in oral keratinocytes [78].
This keratinocyte cholinergic system has been shown to play a role in oral mucosal diseases [79] and also mediates nicotine toxicity in oral keratinocytes and in epithelial cancers [80]. It is now known that the nAChRs expressed on the cell membrane and mitochondria mediate both growth-promoting and anti-apoptotic effects synergistically. Other mechanisms associated with nicotine toxicity include the genotoxic action of reactive oxygen species [81]. With regard to mAChRs, accumulating evidence suggests that mAChR-dependent signaling pathways can promote cell proliferation and cancer progression [82].
In summary, there is increasing evidence that the non-neuronal cholinergic system in epithelial tissues is involved in carcinogenesis. Similar to the effects of nicotine, it is reasonable to speculate that AChR ligands, such as arecoline and other areca alkaloids, induce pro-tumorigenic effects in the oral mucosa via receptor-mediated signaling.
References
- Liu, Y.-J.; Peng, W.; Hu, M.-B.; Xu, M.; Wu, C.-J. The pharmacology, toxicology and potential applications of arecoline: A review. Pharm. Biol. 2016, 54, 2753–2760.
- Li, Z.; Fu, Y.; Hu, Y.; Zhu, Y.; Hu, L.; Shi, C.; Zhang, Y.; Zhang, J.; Zhou, S. Low-dose arecoline regulates distinct core signaling pathways in oral submucous fibrosis and oral squamous cell carcinoma. BMC Oral Health 2023, 23, 171.
- Zhang, P.; Chua, N.Q.E.; Dang, S.; Davis, A.; Chong, K.W.; Prime, S.S.; Cirillo, N. Molecular Mechanisms of Malignant Transformation of Oral Submucous Fibrosis by Different Betel Quid Constituents-Does Fibroblast Senescence Play a Role? Int. J. Mol. Sci. 2022, 23, 1637.
- Passi, D.; Bhanot, P.; Kacker, D.; Chahal, D.; Atri, M.; Panwar, Y. Oral submucous fibrosis: Newer proposed classification with critical updates in pathogenesis and management strategies. Natl. J. Maxillofac. Surg. 2017, 8, 89.
- Rivera, C.; Venegas, B. Histological and molecular aspects of oral squamous cell carcinoma. Oncol. Lett. 2014, 8, 7–11.
- Xie, C.; Feng, H.; Zhong, L.; Shi, Y.; Wei, Z.; Hua, Y.; Ji, N.; Li, J.; Tang, Z.; Chen, Q. Proliferative ability and accumulation of cancer stem cells in oral submucous fibrosis epithelium. Oral Dis. 2020, 26, 1255–1264.
- Markopoulos, A.K. Current aspects on oral squamous cell carcinoma. Open Dent. J. 2012, 6, 126.
- Kuo, T.M.; Nithiyanantham, S.; Lee, C.P.; Hsu, H.T.; Luo, S.Y.; Lin, Y.Z.; Yeh, K.T.; Ko, Y.C. Arecoline N-oxide regulates oral squamous cell carcinoma development through NOTCH1 and FAT1 expressions. J. Cell Physiol. 2019, 234, 13984–13993.
- Huang, L.Y.; Hsieh, Y.P.; Wang, Y.Y.; Hwang, D.Y.; Jiang, S.S.; Huang, W.T.; Chiang, W.F.; Liu, K.J.; Huang, T.T. Single-Cell Analysis of Different Stages of Oral Cancer Carcinogenesis in a Mouse Model. Int. J. Mol. Sci. 2020, 21, 8171.
- Kuo, T.M.; Luo, S.Y.; Chiang, S.L.; Yeh, K.T.; Hsu, H.T.; Wu, C.T.; Lu, C.Y.; Tsai, M.H.; Chang, J.G.; Ko, Y.C. Fibrotic Effects of Arecoline N-Oxide in Oral Potentially Malignant Disorders. J. Agric. Food Chem. 2015, 63, 5787–5794.
- Chang, P.Y.; Kuo, T.M.; Chen, P.K.; Lin, Y.Z.; Hua, C.H.; Chen, Y.C.; Ko, Y.C. Arecoline N-Oxide Upregulates Caspase-8 Expression in Oral Hyperplastic Lesions of Mice. J. Agric. Food Chem. 2017, 65, 10197–10205.
- Li, X.; Xie, X.; Gu, Y.; Zhang, J.; Song, J.; Cheng, X.; Gao, Y.; Ai, Y. Fat mass and obesity-associated protein regulates tumorigenesis of arecoline-promoted human oral carcinoma. Cancer Med. 2021, 10, 6402–6415.
- Hsieh, Y.P.; Chen, K.C.; Chen, M.Y.; Huang, L.Y.; Su, A.Y.; Chiang, W.F.; Huang, W.T.; Huang, T.T. Epigenetic Deregulation of Protein Tyrosine Kinase 6 Promotes Carcinogenesis of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 4495.
- Nithiyanantham, S.; Arumugam, S.; Hsu, H.T.; Chung, C.M.; Lee, C.P.; Tsai, M.H.; Yeh, K.T.; Luo, S.Y.; Ko, Y.C. Arecoline N-oxide initiates oral carcinogenesis and arecoline N-oxide mercapturic acid attenuates the cancer risk. Life Sci. 2021, 271, 119156.
- Chou, S.T.; Peng, H.Y.; Mo, K.C.; Hsu, Y.M.; Wu, G.H.; Hsiao, J.R.; Lin, S.F.; Wang, H.D.; Shiah, S.G. MicroRNA-486-3p functions as a tumor suppressor in oral cancer by targeting DDR1. J. Exp. Clin. Cancer Res. 2019, 38, 281.
- Lai, Z.L.; Tsou, Y.A.; Fan, S.R.; Tsai, M.H.; Chen, H.L.; Chang, N.W.; Cheng, J.C.; Chen, C.M. Methylation-associated gene silencing of RARB in areca carcinogens induced mouse oral squamous cell carcinoma. Biomed. Res. Int. 2014, 2014, 378358.
- Tu, H.F.; Chen, M.Y.; Lai, J.C.; Chen, Y.L.; Wong, Y.W.; Yang, C.C.; Chen, H.Y.; Hsia, S.M.; Shih, Y.H.; Shieh, T.M. Arecoline-regulated ataxia telangiectasia mutated expression level in oral cancer progression. Head Neck 2019, 41, 2525–2537.
- Lin, W.T.; Shieh, T.M.; Yang, L.C.; Wang, T.Y.; Chou, M.Y.; Yu, C.C. Elevated Lin28B expression is correlated with lymph node metastasis in oral squamous cell carcinomas. J. Oral Pathol. Med. 2015, 44, 823–830.
- Khan, I.; Pant, I.; Narra, S.; Radhesh, R.; Ranganathan, K.; Rao, S.G.; Kondaiah, P. Epithelial atrophy in oral submucous fibrosis is mediated by copper (II) and arecoline of areca nut. J. Cell. Mol. Med. 2015, 19, 2397–2412.
- Zheng, L.; Han, X.C.; Guo, F.; Li, N.; Jiang, C.H.; Yin, P.; Min, A.J.; Huang, L. miR-203 inhibits arecoline-induced epithelial-mesenchymal transition by regulating secreted frizzled-related protein 4 and transmembrane-4 L six family member 1 in oral submucous fibrosis. Oncol. Rep. 2015, 33, 2753–2760.
- Li, X.; Chen, W.; Gao, Y.; Song, J.; Gu, Y.; Zhang, J.; Cheng, X.; Ai, Y. Fat mass and obesity-associated protein regulates arecoline-exposed oral cancer immune response through programmed cell death-ligand 1. Cancer Sci. 2022, 113, 2962–2973.
- Chuerduangphui, J.; Ekalaksananan, T.; Heawchaiyaphum, C.; Vatanasapt, P.; Pientong, C. Peroxiredoxin 2 is highly expressed in human oral squamous cell carcinoma cells and is upregulated by human papillomavirus oncoproteins and arecoline, promoting proliferation. PLoS ONE 2020, 15, e0242465.
- Chen, Q.; Jiao, J.; Wang, Y.; Mai, Z.; Ren, J.; He, S.; Li, X.; Chen, Z. Egr-1 mediates low-dose arecoline induced human oral mucosa fibroblast proliferation via transactivation of Wnt5a expression. BMC Mol. Cell Biol. 2020, 21, 80.
- Shiah, S.G.; Hsiao, J.R.; Chang, W.M.; Chen, Y.W.; Jin, Y.T.; Wong, T.Y.; Huang, J.S.; Tsai, S.T.; Hsu, Y.M.; Chou, S.T.; et al. Downregulated miR329 and miR410 promote the proliferation and invasion of oral squamous cell carcinoma by targeting Wnt-7b. Cancer Res. 2014, 74, 7560–7572.
- Shiah, S.G.; Hsiao, J.R.; Chang, H.J.; Hsu, Y.M.; Wu, G.H.; Peng, H.Y.; Chou, S.T.; Kuo, C.C.; Chang, J.Y. MiR-30a and miR-379 modulate retinoic acid pathway by targeting DNA methyltransferase 3B in oral cancer. J. Biomed. Sci. 2020, 27, 46.
- Adhikari, B.R.; Yoshida, K.; Paudel, D.; Morikawa, T.; Uehara, O.; Sato, J.; Muthumala, M.; Amaratunga, P.; Arakawa, T.; Chiba, I.; et al. Aberrant expression of DUSP4 is a specific phenomenon in betel quid-related oral cancer. Med. Mol. Morphol. 2021, 54, 79–86.
- Zheng, L.; Guan, Z.J.; Pan, W.T.; Du, T.F.; Zhai, Y.J.; Guo, J. Tanshinone Suppresses Arecoline-Induced Epithelial-Mesenchymal Transition in Oral Submucous Fibrosis by Epigenetically Reactivating the p53 Pathway. Oncol. Res. 2018, 26, 483–494.
- Chang, M.C.; Wu, H.L.; Lee, J.J.; Lee, P.H.; Chang, H.H.; Hahn, L.J.; Lin, B.R.; Chen, Y.J.; Jeng, J.H. The induction of prostaglandin E2 production, interleukin-6 production, cell cycle arrest, and cytotoxicity in primary oral keratinocytes and KB cancer cells by areca nut ingredients is differentially regulated by MEK/ERK activation. J. Biol. Chem. 2004, 279, 50676–50683.
- Wang, Y.C.; Tsai, Y.S.; Huang, J.L.; Lee, K.W.; Kuo, C.C.; Wang, C.S.; Huang, A.M.; Chang, J.Y.; Jong, Y.J.; Lin, C.S. Arecoline arrests cells at prometaphase by deregulating mitotic spindle assembly and spindle assembly checkpoint: Implication for carcinogenesis. Oral Oncol. 2010, 46, 255–262.
- Tsai, Y.S.; Lee, K.W.; Huang, J.L.; Liu, Y.S.; Juo, S.H.; Kuo, W.R.; Chang, J.G.; Lin, C.S.; Jong, Y.J. Arecoline, a major alkaloid of areca nut, inhibits p53, represses DNA repair, and triggers DNA damage response in human epithelial cells. Toxicology 2008, 249, 230–237.
- Ho, T.J.; Chiang, C.P.; Hong, C.Y.; Kok, S.H.; Kuo, Y.S.; Yen-Ping Kuo, M. Induction of the c-jun protooncogene expression by areca nut extract and arecoline on oral mucosal fibroblasts. Oral Oncol. 2000, 36, 432–436.
- Li, M.; Gao, F.; Zhou, Z.S.; Zhang, H.M.; Zhang, R.; Wu, Y.F.; Bai, M.H.; Li, J.J.; Lin, S.R.; Peng, J.Y. Arecoline inhibits epithelial cell viability by upregulating the apoptosis pathway: Implication for oral submucous fibrosis. Oncol. Rep. 2014, 31, 2422–2428.
- Jeng, J.H.; Kuo, M.L.; Hahn, L.J.; Kuo, M.Y. Genotoxic and non-genotoxic effects of betel quid ingredients on oral mucosal fibroblasts in vitro. J. Dent. Res. 1994, 73, 1043–1049.
- Zhou, Z.S.; Li, M.; Gao, F.; Peng, J.Y.; Xiao, H.B.; Dai, L.X.; Lin, S.R.; Zhang, R.; Jin, L.Y. Arecoline suppresses HaCaT cell proliferation through cell cycle regulatory molecules. Oncol. Rep. 2013, 29, 2438–2444.
- Chen, P.H.; Lee, K.W.; Hsu, C.C.; Chen, J.Y.; Wang, Y.H.; Chen, K.K.; Wang, H.M.; Huang, H.W.; Huang, B. Expression of a splice variant of CYP26B1 in betel quid-related oral cancer. Sci. World J. 2014, 2014, 810561.
- Chang, M.C.; Chan, C.P.; Wang, W.T.; Chang, B.E.; Lee, J.J.; Tseng, S.K.; Yeung, S.Y.; Hahn, L.J.; Jeng, J.H. Toxicity of areca nut ingredients: Activation of CHK1/CHK2, induction of cell cycle arrest, and regulation of MMP-9 and TIMPs production in SAS epithelial cells. Head. Neck 2013, 35, 1295–1302.
- Ji, W.T.; Yang, S.R.; Chen, J.Y.; Cheng, Y.P.; Lee, Y.R.; Chiang, M.K.; Chen, H.R. Arecoline downregulates levels of p21 and p27 through the reactive oxygen species/mTOR complex 1 pathway and may contribute to oral squamous cell carcinoma. Cancer Sci. 2012, 103, 1221–1229.
- Yang, J.S.; Chen, M.K.; Yang, S.F.; Chang, Y.C.; Su, S.C.; Chiou, H.L.; Chien, M.H.; Lin, C.W. Increased expression of carbonic anhydrase IX in oral submucous fibrosis and oral squamous cell carcinoma. Clin. Chem. Lab. Med. 2014, 52, 1367–1377.
- Rehman, A.; Ali, S.; Lone, M.A.; Atif, M.; Hassona, Y.; Prime, S.S.; Pitiyage, G.N.; James, E.L.; Parkinson, E.K. Areca nut alkaloids induce irreparable DNA damage and senescence in fibroblasts and may create a favourable environment for tumour progression. J. Oral Pathol. Med. 2016, 45, 365–372.
- Shieh, D.H.; Chiang, L.C.; Shieh, T.Y. Augmented mRNA expression of tissue inhibitor of metalloproteinase-1 in buccal mucosal fibroblasts by arecoline and safrole as a possible pathogenesis for oral submucous fibrosis. Oral Oncol. 2003, 39, 728–735.
- Li, X.; Ling, T.Y.; Gao, Y.J.; Tang, D.S.; Li, W.H. Arecoline and oral keratinocytes may affect the collagen metabolism of fibroblasts. J. Oral Pathol. Med. 2009, 38, 422–426.
- Hu, X.; Wang, W.; Hu, Y.; Chen, W.; Wang, C.; Yang, L.; Mao, T.; Xia, K.; Min, A.; Xiong, H.; et al. Overexpression of DEC1 in the epithelium of OSF promotes mesenchymal transition via activating FAK/Akt signal axis. J. Oral Pathol. Med. 2022, 51, 780–790.
- Chiang, C.H.; Wu, C.C.; Lee, L.Y.; Li, Y.C.; Liu, H.P.; Hsu, C.W.; Lu, Y.C.; Chang, J.T.; Cheng, A.J. Proteomics Analysis Reveals Involvement of Krt17 in Areca Nut-Induced Oral Carcinogenesis. J. Proteome Res. 2016, 15, 2981–2997.
- Xie, C.; Li, Z.; Hua, Y.; Sun, S.; Zhong, L.; Chen, Q.; Feng, H.; Ji, N.; Li, T.; Zhou, X.; et al. Identification of a BRAF/PA28γ/MEK1 signaling axis and its role in epithelial-mesenchymal transition in oral submucous fibrosis. Cell Death Dis. 2022, 13, 701.
- Lee, S.S.; Tsai, C.H.; Yu, C.C.; Chang, Y.C. Elevated snail expression mediates tumor progression in areca quid chewing-associated oral squamous cell carcinoma via reactive oxygen species. PLoS ONE 2013, 8, e67985.
- Chang, Y.C.; Tsai, C.H.; Lai, Y.L.; Yu, C.C.; Chi, W.Y.; Li, J.J.; Chang, W.W. Arecoline-induced myofibroblast transdifferentiation from human buccal mucosal fibroblasts is mediated by ZEB1. J. Cell. Mol. Med. 2014, 18, 698–708.
- Ho, C.M.; Hu, F.W.; Lee, S.S.; Shieh, T.M.; Yu, C.H.; Lin, S.S.; Yu, C.C. ZEB1 as an indicator of tumor recurrence for areca quid chewing-associated oral squamous cell carcinomas. J. Oral Pathol. Med. 2015, 44, 693–698.
- Ren, H.; He, G.; Lu, Z.; He, Q.; Li, S.; Huang, Z.; Chen, Z.; Cao, C.; Wang, A. Arecoline induces epithelial-mesenchymal transformation and promotes metastasis of oral cancer by SAA1 expression. Cancer Sci. 2021, 112, 2173–2184.
- Fang, C.Y.; Hsia, S.M.; Hsieh, P.L.; Liao, Y.W.; Peng, C.Y.; Wu, C.Z.; Lin, K.C.; Tsai, L.L.; Yu, C.C. Slug mediates myofibroblastic differentiation to promote fibrogenesis in buccal mucosa. J. Cell Physiol. 2019, 234, 6721–6730.
- Lee, S.S.; Tseng, L.H.; Li, Y.C.; Tsai, C.H.; Chang, Y.C. Heat shock protein 47 expression in oral squamous cell carcinomas and upregulated by arecoline in human oral epithelial cells. J. Oral Pathol. Med. 2011, 40, 390–396.
- Lee, C.H.; Liu, S.Y.; Lin, M.H.; Chiang, W.F.; Chen, T.C.; Huang, W.T.; Chou, D.S.; Chiu, C.T.; Liu, Y.C. Upregulation of matrix metalloproteinase-1 (MMP-1) expression in oral carcinomas of betel quid (BQ) users: Roles of BQ ingredients in the acceleration of tumour cell motility through MMP-1. Arch. Oral Biol. 2008, 53, 810–818.
- Yu, C.C.; Tsai, C.H.; Hsu, H.I.; Chang, Y.C. Elevation of S100A4 expression in buccal mucosal fibroblasts by arecoline: Involvement in the pathogenesis of oral submucous fibrosis. PLoS ONE 2013, 8, e55122.
- Yang, S.F.; Hsieh, Y.S.; Tsai, C.H.; Chou, M.Y.; Chang, Y.C. The upregulation of type I plasminogen activator inhibitor in oral submucous fibrosis. Oral Oncol. 2003, 39, 367–372.
- Chen, S.C.; Liu, C.M.; Hsieh, P.L.; Liao, Y.W.; Lin, Y.J.; Yu, C.C.; Yu, C.H. E3 ligase carboxyl-terminus of Hsp70-interacting protein (CHIP) suppresses fibrotic properties in oral mucosa. J. Formos. Med. Assoc. 2020, 119, 595–600.
- Chang, N.W.; Pei, R.J.; Tseng, H.C.; Yeh, K.T.; Chan, H.C.; Lee, M.R.; Lin, C.; Hsieh, W.T.; Kao, M.C.; Tsai, M.H.; et al. Co-treating with arecoline and 4-nitroquinoline 1-oxide to establish a mouse model mimicking oral tumorigenesis. Chem. Biol. Interact. 2010, 183, 231–237.
- Chang, M.C.; Lin, L.D.; Wu, H.L.; Ho, Y.S.; Hsien, H.C.; Wang, T.M.; Jeng, P.Y.; Cheng, R.H.; Hahn, L.J.; Jeng, J.H. Areca nut-induced buccal mucosa fibroblast contraction and its signaling: A potential role in oral submucous fibrosis--a precancer condition. Carcinogenesis 2013, 34, 1096–1104.
- Shih, Y.H.; Chiu, K.C.; Wang, T.H.; Lan, W.C.; Tsai, B.H.; Wu, L.J.; Hsia, S.M.; Shieh, T.M. Effects of melatonin to arecoline-induced reactive oxygen species production and DNA damage in oral squamous cell carcinoma. J. Formos. Med. Assoc. 2021, 120 Pt 3, 668–678.
- Deng, Y.T.; Chang, J.Z.; Yeh, C.C.; Cheng, S.J.; Kuo, M.Y. Arecoline stimulated Cyr61 production in human gingival epithelial cells: Inhibition by lovastatin. Oral Oncol. 2011, 47, 256–261.
- Lee, S.S.; Tsai, C.H.; Yang, S.F.; Ho, Y.C.; Chang, Y.C. Hypoxia inducible factor-1α expression in areca quid chewing-associated oral squamous cell carcinomas. Oral Dis. 2010, 16, 696–701.
- Lee, S.S.; Tsai, C.H.; Ho, Y.C.; Yu, C.C.; Chang, Y.C. Heat shock protein 27 expression in areca quid chewing-associated oral squamous cell carcinomas. Oral Dis. 2012, 18, 713–719.
- Lee, S.S.; Yang, S.F.; Tsai, C.H.; Chou, M.C.; Chou, M.Y.; Chang, Y.C. Upregulation of heme oxygenase-1 expression in areca-quid-chewing-associated oral squamous cell carcinoma. J. Formos. Med. Assoc. 2008, 107, 355–363.
- Tsai, C.H.; Yang, S.F.; Lee, S.S.; Chang, Y.C. Augmented heme oxygenase-1 expression in areca quid chewing-associated oral submucous fibrosis. Oral Dis. 2009, 15, 281–286.
- Moutasim, K.A.; Jenei, V.; Sapienza, K.; Marsh, D.; Weinreb, P.H.; Violette, S.M.; Lewis, M.P.; Marshall, J.F.; Fortune, F.; Tilakaratne, W.M.; et al. Betel-derived alkaloid up-regulates keratinocyte alphavbeta6 integrin expression and promotes oral submucous fibrosis. J. Pathol. 2011, 223, 366–377.
- Li, X.; Gao, Y.; Chen, W.; Gu, Y.; Song, J.; Zhang, J.; Ai, Y. N6-methyladenosine modification contributes to arecoline-mediated oral submucosal fibrosis. J. Oral Pathol. Med. 2022, 51, 474–482.
- Chuerduangphui, J.; Ekalaksananan, T.; Chaiyarit, P.; Patarapadungkit, N.; Chotiyano, A.; Kongyingyoes, B.; Promthet, S.; Pientong, C. Effects of arecoline on proliferation of oral squamous cell carcinoma cells by dysregulating c-Myc and miR-22, directly targeting oncostatin M. PLoS ONE 2018, 13, e0192009.
- Jeng, J.H.; Wang, Y.J.; Chiang, B.L.; Lee, P.H.; Chan, C.P.; Ho, Y.S.; Wang, T.M.; Lee, J.J.; Hahn, L.J.; Chang, M.C. Roles of keratinocyte inflammation in oral cancer: Regulating the prostaglandin E2, interleukin-6 and TNF-alpha production of oral epithelial cells by areca nut extract and arecoline. Carcinogenesis 2003, 24, 1301–1315.
- Hsu, H.J.; Chang, K.L.; Yang, Y.H.; Shieh, T.Y. The effects of arecoline on the release of cytokines using cultured peripheral blood mononuclear cells from patients with oral mucous diseases. Kaohsiung J. Med. Sci. 2001, 17, 175–182.
- Fang, C.Y.; Yu, C.C.; Liao, Y.W.; Hsieh, P.L.; Ohiro, Y.; Chu, P.M.; Huang, Y.C.; Yu, C.H.; Tsai, L.L. miR-10b regulated by Twist maintains myofibroblasts activities in oral submucous fibrosis. J. Formos. Med. Assoc. 2020, 119, 1167–1173.
- Chen, Y.F.; Yang, C.C.; Kao, S.Y.; Liu, C.J.; Lin, S.C.; Chang, K.W. MicroRNA-211 Enhances the Oncogenicity of Carcinogen-Induced Oral Carcinoma by Repressing TCF12 and Increasing Antioxidant Activity. Cancer Res. 2016, 76, 4872–4886.
- Wang, T.Y.; Peng, C.Y.; Lee, S.S.; Chou, M.Y.; Yu, C.C.; Chang, Y.C. Acquisition cancer stemness, mesenchymal transdifferentiation, and chemoresistance properties by chronic exposure of oral epithelial cells to arecoline. Oncotarget 2016, 7, 84072–84081.
- Zhang, Y.; Wang, X.; Han, S.; Wang, Y.; Liu, R.; Meng, F.; Su, Z.; Huo, F. Suppression of miR-886-3p mediated by arecoline (ARE) contributes to the progression of oral squamous cell carcinoma. J. Investig. Med. 2021, 69, 377–381.
- Tsai, Y.S.; Lin, C.S.; Chiang, S.L.; Lee, C.H.; Lee, K.W.; Ko, Y.C. Areca nut induces miR-23a and inhibits repair of DNA double-strand breaks by targeting FANCG. Toxicol. Sci. 2011, 123, 480–490.
- Lu, M.Y.; Yu, C.C.; Chen, P.Y.; Hsieh, P.L.; Peng, C.Y.; Liao, Y.W.; Yu, C.H.; Lin, K.H. miR-200c inhibits the arecoline-associated myofibroblastic transdifferentiation in buccal mucosal fibroblasts. J. Formos. Med. Assoc. 2018, 117, 791–797.
- Yang, S.-H.; Lee, T.-Y.; Ho, C.A.; Yang, C.-Y.; Huang, W.-Y.; Lin, Y.-C.; Nieh, S.; Lin, Y.-S.; Chen, S.-F.; Lin, F.-H. Exposure to nicotine-derived nitrosamine ketone and arecoline synergistically facilitates tumor aggressiveness via overexpression of epidermal growth factor receptor and its downstream signaling in head and neck squamous cell carcinoma. PLoS ONE 2018, 13, e0201267.
- Horenstein, N.A.; Quadri, M.; Stokes, C.; Shoaib, M.; Papke, R.L. Cracking the betel nut: Cholinergic activity of areca alkaloids and related compounds. Nicotine Tob. Res. 2019, 21, 805–812.
- Papke, R.L.; Horenstein, N.A.; Stokes, C. Nicotinic activity of arecoline, the psychoactive element of" Betel Nuts", suggests a basis for habitual use and anti-inflammatory activity. PLoS ONE 2015, 10, e0140907.
- Siregar, P.; Audira, G.; Feng, L.-Y.; Lee, J.-H.; Santoso, F.; Yu, W.-H.; Lai, Y.-H.; Li, J.-H.; Lin, Y.-T.; Chen, J.-R. Pharmaceutical assessment suggests locomotion hyperactivity in zebrafish triggered by arecoline might be associated with multiple muscarinic acetylcholine receptors activation. Toxins 2021, 13, 259.
- Arredondo, J.; Hall, L.; Ndoye, A.; Chernyavsky, A.; Jolkovsky, D.; Grando, S. Muscarinic acetylcholine receptors regulating cell cycle progression are expressed in human gingival keratinocytes. J. Periodontal Res. 2003, 38, 79–89.
- Foulad, D.P.; Cirillo, N.; Grando, S.A. The Role of Non-Neuronal Acetylcholine in the Autoimmune Blistering Disease Pemphigus Vulgaris. Biology 2023, 12, 354.
- Ortiz, A.; Grando, S.A. Smoking and the skin. Int. J. Dermatol. 2012, 51, 250–262.
- Grando, S.A. Connections of nicotine to cancer. Nat. Rev. Cancer 2014, 14, 419–429.
- Shah, N.; Khurana, S.; Cheng, K.; Raufman, J.-P. Muscarinic receptors and ligands in cancer. Am. J. Physiol.-Cell Physiol. 2009, 296, C221–C232.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
569
Revisions:
2 times
(View History)
Update Date:
19 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




