
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shadma Wahab | -- | 4141 | 2023-12-13 11:07:03 | | | |
| 2 | Lindsay Dong | Meta information modification | 4141 | 2023-12-19 04:35:58 | | |
Video Upload Options
Nigella sativa (NS), also known as black cumin, is one of the plants used in traditional medicine the most. Many studies on the NS have shown that their therapeutic properties are attributed to the seed, oil, and secondary metabolites. This plant has been studied extensively and has many medical uses, such as anti-inflammatory. NS or its phytochemical compounds, such as thymoquinone, can cause cell apoptosis via oxidative stress, block efflux pumps, enhance membrane permeability, and exert potent biocidal effects.
1. Introduction
People have used this plant for over a thousand years as a medicine. Evidence of the oldest cultivation of NS has been discovered by archaeologists, with dating indicating its presence as early as the 2nd century BCE [1]. Nigella is derived from the Latin word Niger, which means “black”. The word “nigellus” denotes a shade of black or dark, explicitly referring to the color of the seed coat. The cultivation of black cumin is seen in several regions throughout Asia, Africa, Europe, and the Americas. NS is growing wild in Turkey, Iraq, Iran, Syria, and Africa [2]. The species is also grown commercially in India [3] and other parts of Southern Asia, Pakistan [4], Iran [5], Western Asia [6], Iraq [6], Israel [7], Jordan [8], Syria [9], Lebanon [10], Turkey [11], Yemen [11], Sudan [12], Northern Africa [12], Egypt [6], Tunisia [6], Ethiopia and Eastern Africa [13]. The primary nations engaged in the production of the commodity are India, Bangladesh, Sri Lanka, Pakistan, Afghanistan, Iraq, Iran, Syria, Ethiopia, and Turkey. Black cumin’s natural average productivity has been reported to be 0.79 tons/ha. In 2018, the black cumin oil market was valued at over 15 million USD; substantial growth is anticipated by 2025 [14]. India cultivates this plant on a large scale due to its heavy consumption in medicine. Annual quantities of black cumin are estimated to be traded in India for more than 100 metric tons. According to Gashaw’s (2020) findings, the average yield in Ethiopia was recorded at 0.64 tons per hectare, a significantly lower figure compared to other nations known for their high agricultural productivity [13]. In 2015, Ermias et al. said the low production was because the cultivars were not very productive [15]. In 2008, Hammo said that it was because the farmers were not using good agronomic practices [16].
2. Pharmacological Activities of Nigella sativa
2.1. Antioxidant and Anti-Inflammatory
2.2. Immunomodulatory Effects of Nigella sativa
2.3. Anticancer Activity
2.4. Antidiabetic Activity
2.5. Cardiovascular Disease (CVD)
2.6. Neurological Disorder
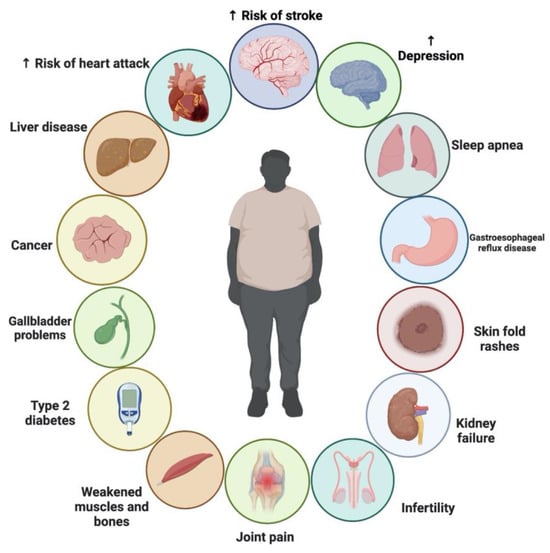
2.7. Obesity
2.8. The Influence of NS on Asthma Control
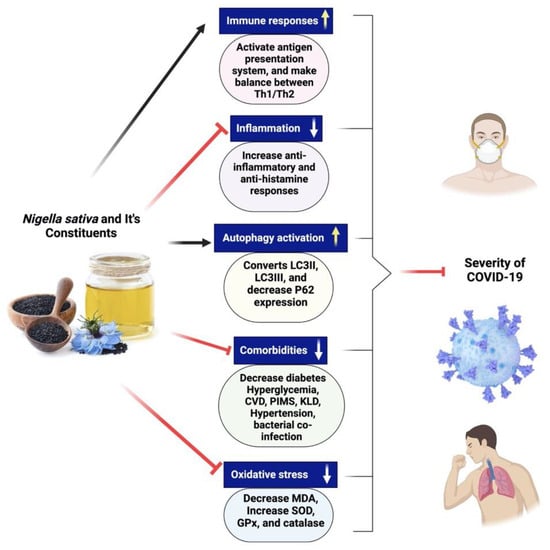
2.9. Nigella sativa for the Treatment of COVID-19
References
- Corneanu, C.G.; Corneanu, M. Considerations on Human Evolution and on Species Origin Centers. Oltenia Stud. Comunicări Științele Nat. 2011, 27, 210–217.
- Saad, B.; Said, O. Greco-Arab and Islamic Herbal Medicine: Traditional System, Ethics, Safety, Efficacy, and Regulatory Issues; Wiley: Hoboken, NJ, USA, 2011; ISBN 9780470474211.
- Ved, D.K.; Goraya, G.S. Demand and Supply of Medicinal Plants in India; NMPB: New Delhi India; FRLHT: Bangalore, India, 2007; pp. 1–18.
- Rabbani, M.A.; Ghafoor, A.; Masood, M.S. Narc-Kalonji: An Early Maturing and High Yielding Variety of Nigella sativa Released for Cultivation in Pakistan. Pakistan J. Bot. 2011, 43, 191–195.
- Koocheki, A.A. Indigenous Knowledge in Agriculture with Particular Reference to Saffron Production in Iran. Acta Hortic. 2004, 650, 175–182.
- Toma, C.C.; Simu, G.M.; Hanganu, D.; Olah, N.; Vata, F.M.G.; Hammami, C.; Hammami, M. Chemical Composition of the Tunisian Nigella sativa. Note II. Profile on Fatty Oil. Farmacia 2013, 61, 454–458.
- Botnick, I.; Xue, W.; Bar, E.; Ibdah, M.; Schwartz, A.; Joel, D.M.; Lev, E.; Fait, A.; Lewinsohn, E. Distribution of Primary and Specialized Metabolites in Nigella sativa Seeds, a Spice with Vast Traditional and Historical Uses. Molecules 2012, 17, 10159–10177.
- Burdock, G.A. Assessment of Black Cumin (Nigella sativa L.) as a Food Ingredient and Putative Therapeutic Agent. Regul. Toxicol. Pharmacol. 2022, 128, 105088.
- Hoppe, B. Handbuch des Arznei-und Gewürzpflanzenbaus; Verein für Arznei-und Gewürzpflanzen Saluplanta: Aschersleben, Germany, 2009.
- Paarakh, P.M. Nigella sativa Linn.—A Comprehensive Review. Indian J. Nat. Prod. Resour. 2010, 1, 409–429.
- Al-Naqe, G.N.; Ismail, M.M.; Al-Zuba, A.S.; Esa, N.M. Nutrients Composition and Minerals Content of Three Different Samples of Nigella sativa L. Cultivated in Yemen. Asian J. Biol. Sci. 2009, 2, 43–48.
- Teuscher, E.; Bauermann, U.; Werner, M.; Brinckmann, J.A.; Lindenmaier, M.P.; Duke, J.A. Book Review: Medicinal Spices: A Handbook of Culinary Herbs, Spices, Spice Mixtures and Their Essential Oils. Food Nutr. Bull. 2006, 27, 271.
- Gashaw, Z. Status of Black Cumin (Nigella sativa L.) Research and Production in Ethiopia; A Review. Int. J. For. Hortic. 2020, 6, 20–29.
- Kifelew, H.; Fikere, D.; Luleseged, T.; Bekele, D.; Mitiku, H. Wakjira Seed Spices Production Guideline; Ethiopian Institute of Agricultural Research: Addis Ababa, Ethiopia, 2017; pp. 1–36.
- Assefa, E. Adaptability Study of Black Cumin (Nigella sativa L.) Varieties in the Mid and High Land Areas of Kaffa Zone, South West Ethiopia. Agric. For. Fish. 2015, 4, 14.
- Hammo, Y.H. Effect of Very High Levels of Nitrogen and Phospours Fertilizers, Pinching, and Seed Rate Sowing on Growth, Seed Yield and Componentes of Nigella sativa L. 2—Seed Compenents. Mesopotamia J. Agric. 2008, 36, 2–11.
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559.
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A Review on Therapeutic Potential of Nigella sativa: A Miracle Herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352.
- Cheikh-Rouhou, S.; Besbes, S.; Hentati, B.; Blecker, C.; Deroanne, C.; Attia, H. Nigella sativa L.: Chemical Composition and Physicochemical Characteristics of Lipid Fraction. Food Chem. 2007, 101, 673–681.
- Nickavar, B.; Mojab, F.; Javidnia, K.; Roodgar Amoli, M.A. Chemical Composition of the Fixed and Volatile Oils of Nigella sativa L. from Iran. Zeitschrift Naturforsch.-Sect. C J. Biosci. 2003, 58, 629–631.
- Venkatachallam, S.K.T.; Pattekhan, H.; Divakar, S.; Kadimi, U.S. Chemical Composition of Nigella sativa L. Seed Extracts Obtained by Supercritical Carbon Dioxide. J. Food Sci. Technol. 2010, 47, 598–605.
- Jrah Harzallah, H.; Kouidhi, B.; Flamini, G.; Bakhrouf, A.; Mahjoub, T. Chemical Composition, Antimicrobial Potential against Cariogenic Bacteria and Cytotoxic Activity of Tunisian Nigella sativa Essential Oil and Thymoquinone. Food Chem. 2011, 129, 1469–1474.
- Ghedira, K. La Nigelle Cultivée: Nigella sativa L. (Ranunculaceae). Phytotherapie 2006, 4, 220–226.
- Houghton, P.J.; Zarka, R.; De Las Heras, B.; Hoult, J.R.S. Fixed Oil of Nigella sativa and Derived Thymoquinone Inhibit Eicosanoid Generation in Leukocytes and Membrane Lipid Peroxidation. Planta Med. 1995, 61, 33–36.
- El–Dakhakhny, M. Studies on the Chemical Constitution of Egyptian Nigella sativa L. Seeds. II 1) the Essential Oil. Planta Med. 1963, 11, 465–470.
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882.
- Burits, M.; Asres, K.; Bucar, F. The Antioxidant Activity of the Essential Oils of Artemisia Afra, Artemisia Abyssinica and Juniperus Procera. Phyther. Res. 2001, 15, 103–108.
- Begum, S.; Mannan, A. A Review on Nigella sativa: A Marvel Herb. J. Drug Deliv. Ther. 2020, 10, 213–219.
- Keyhanmanesh, R.; Bagban, H.; Nazemiyeh, H.; Bavil, F.M.; Alipour, M.R.; Ahmady, M. The Relaxant Effects of Different Methanolic Fractions of Nigella sativa on Guinea Pig Tracheal Chains. Iran. J. Basic Med. Sci. 2013, 16, 123–128.
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and Anti-Inflammatory Properties of Nigella sativa Oil in Human Pre-Adipocytes. Antioxidants 2019, 8, 51.
- Salem, M.L. Immunomodulatory and Therapeutic Properties of the Nigella sativa L. Seed. Int. Immunopharmacol. 2005, 5, 1749–1770.
- Khaldi, T.; Chekchaki, N.; Boumendjel, M.; Taibi, F.; Abdellaoui, M.; Messarah, M.; Boumendjel, A. Ameliorating Effects of Nigella sativa Oil on Aggravation of Inflammation, Oxidative Stress and Cytotoxicity Induced by Smokeless Tobacco Extract in an Allergic Asthma Model in Wistar Rats. Allergol. Immunopathol. 2018, 46, 472–481.
- Rizvi, A.; Mahdi, A.A.; Wahab, S.; Mishra, A. Protective Effects of Butea frondosa Leaves against Stress Induced Immune Impairment in Sprague Dawley Rats. Pak. J. Pharm. Sci. 2018, 31, 2457–2462.
- Bascones-Martinez, A.; Mattila, R.; Gomez-Font, R.; Meurman, J.H. Immunomodulatory Drugs: Oral and Systemic Adverse Effects. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e24–e31.
- Haq, A.; Abdullatif, M.; Lobo, P.I.; Khabar, K.S.A.; Sheth, K.V.; Al-Sedairy, S.T. Nigella sativa: Effect on Human Lymphocytes and Polymorphonuclear Leukocyte Phagocytic Activity. Immunopharmacology 1995, 30, 147–155.
- Taifa, S.; Muhee, A.; Bhat, R.A.; Nabi, S.U.I.; Roy, A.; Rather, G.A.; Khan, A.A.; Bashir, S.M.; Patwekar, M.; Wahab, S.; et al. Evaluation of Therapeutic Efficacy of Copper Nanoparticles in Staphylococcus Aureus—Induced Rat Mastitis Model. J. Nanomater. 2022, 2022, 7124114.
- Islam, S.N.; Begum, P.; Ahsan, T.; Huque, S.; Ahsan, M. Immunosuppressive and Cytotoxic Properties of Nigella sativa. Phyther. Res. 2004, 18, 395–398.
- Swamy, S.M.K.; Tan, B.K.H. Cytotoxic and Immunopotentiating Effects of Ethanolic Extract of Nigella sativa L. Seeds. J. Ethnopharmacol. 2000, 70, 1–7.
- Twilley, D.; Rademan, S.; Lall, N. A Review on Traditionally Used South African Medicinal Plants, Their Secondary Metabolites and Their Potential Development into Anticancer Agents. J. Ethnopharmacol. 2020, 261, 113101.
- Wahab, S.; Ahmad, I.; Irfan, S.; Baig, M.H.; Farouk, A.-E.; Dong, J.-J. Use of Natural Compounds as a Potential Therapeutic Agent Against COVID-19. Curr. Pharm. Des. 2021, 27, 1144–1152.
- Alshehri, S.A.; Wahab, S.; Abullais, S.S.; Das, G.; Hani, U.; Ahmad, W.; Amir, M.; Ahmad, A.; Kandasamy, G.; Vasudevan, R. Pharmacological Efficacy of Tamarix Aphylla: A Comprehensive Review. Plants 2022, 11, 118.
- Wahab, S.; Hussain, A. Cytokines as Targets for Immunomodulation. Int. J. Pharm. Pharm. Sci. 2013, 5, 60–64.
- Dhanasekaran, S. Phytochemical Characteristics of Aerial Part of Cissus quadrangularis (L) and Its in-Vitro Inhibitory Activity against Leukemic Cells and Antioxidant Properties. Saudi J. Biol. Sci. 2020, 27, 1302–1309.
- Jadhav, V.; Bhagare, A.; Wahab, S.; Lokhande, D.; Vaidya, C.; Dhayagude, A.; Khalid, M.; Aher, J.; Mezni, A.; Dutta, M. Green Synthesized Calcium Oxide Nanoparticles (CaO NPs) Using Leaves Aqueous Extract of Moringa Oleifera and Evaluation of Their Antibacterial Activities. J. Nanomater. 2022, 2022, 9047507.
- Hani, U.; Osmani, R.A.M.; Yasmin, S.; Gowda, B.H.J.; Ather, H.; Ansari, M.Y.; Siddiqua, A.; Ghazwani, M.; Fatease, A.A.; Alamri, A.H.; et al. Novel Drug Delivery Systems as an Emerging Platform for Stomach Cancer Therapy. Pharmaceutics 2022, 14, 1576.
- Shoaib, A.; Tabish, M.; Ali, S.; Arafah, A.; Wahab, S.; Almarshad, F.M.; Rashid, S.; Rehman, M.U. Dietary Phytochemicals in Cancer Signalling Pathways: Role of MiRNA Targeting. Curr. Med. Chem. 2021, 28, 8036–8067.
- Akhtar, M.F.; Saleem, A.; Rasul, A.; Faran Ashraf Baig, M.M.; Bin-Jumah, M.; Abdel Daim, M.M. Anticancer Natural Medicines: An Overview of Cell Signaling and Other Targets of Anticancer Phytochemicals. Eur. J. Pharmacol. 2020, 888, 173488.
- Palanisamy, N.; Manian, S. Protective Effects of Asparagusracemosus on Oxidative Damage in Isoniazid-Induced Hepatotoxic Rats: An in Vivo Study. Toxicol. Ind. Health 2012, 28, 238–244.
- Ahmad, I.; Irfan, S.; Abohashrh, M.; Wahab, S.; Abullais, S.S.; Javali, M.A.; Nisar, N.; Alam, M.M.; Srivastava, S.; Saleem, M.; et al. Inhibitory Effect of Nepeta Deflersiana on Climax Bacterial Community Isolated from the Oral Plaque of Patients with Periodontal Disease. Molecules 2021, 26, 202.
- Khalid, M.; Alqarni, M.H.; Alsayari, A.; Foudah, A.I.; Aljarba, T.M.; Mukim, M.; Alamri, M.A.; Abullais, S.S.; Wahab, S. Anti-Diabetic Activity of Bioactive Compound Extracted from Spondias Mangifera Fruit: In-Vitro and Molecular Docking Approaches. Plants 2022, 11, 562.
- Juárez, P. Plant-Derived Anticancer Agents: A Promising Treatment for Bone Metastasis. Bonekey Rep. 2014, 3, 599.
- Ghosheh, O.A.; Houdi, A.A.; Crooks, P.A. High Performance Liquid Chromatographic Analysis of the Pharmacologically Active Quinones and Related Compounds in the Oil of the Black Seed (Nigella sativa L.). J. Pharm. Biomed. Anal. 1999, 19, 757–762.
- Ali, B.H.; Blunden, G. Pharmacological and Toxicological Properties of Nigella sativa. Phyther. Res. 2003, 17, 299–305.
- Mehraj, T.; Elkanayati, R.M.; Farooq, I.; Mir, T.M. A Review of Nigella sativa and Its Active Principles as Anticancer Agents. In Black Seeds (Nigella sativa); Elsevier: Amsterdam, The Netherlands, 2022; pp. 91–118.
- Jia, S.S.; Xi, G.P.; Zhang, M.; Chen, Y.B.; Lei, B.; Dong, X.S.; Yang, Y.M. Induction of Apoptosis by D-Limonene Is Mediated by Inactivation of Akt in LS174T Human Colon Cancer Cells. Oncol. Rep. 2013, 29, 349–354.
- Swamy, S.M.K.; Huat, B.T.K. Intracellular Glutathione Depletion and Reactive Oxygen Species Generation Are Important in α-Hederin-Induced Apoptosis of P388 Cells. Mol. Cell. Biochem. 2003, 245, 127–139.
- Effenberger, K.; Breyer, S.; Schobert, R. Terpene Conjugates of the Nigella sativa Seed-Oil Constituent Thymoquinone with Enhanced Efficacy in Cancer Cells. Chem. Biodivers. 2010, 7, 129–139.
- Farah, I.O.; Begum, R.A. Effect of Nigella sativa (N. sativa L.) and Oxidative Stress on the Survival Pattern of MCF-7 Breast Cancer Cells. Biomed. Sci. Instrum. 2003, 39, 359–364.
- Gali-Muhtasib, H.; Diab-Assaf, M.; Boltze, C.; Al-Hmaira, J.; Hartig, R.; Roessner, A.; Schneider-Stock, R. Thymoquinone Extracted from Black Seed Triggers Apoptotic Cell Death in Human Colorectal Cancer Cells via a P53-Dependent Mechanism. Int. J. Oncol. 2004, 25, 857–866.
- Salim, E.I.; Fukushima, S. Chemopreventive Potential of Volatile Oil from Black Cumin (Nigella sativa L.) Seeds against Rat Colon Carcinogenesis. Nutr. Cancer 2003, 45, 195–202.
- Rooney, S.; Ryan, M.F. Modes of Action of Alpha-Hederin and Thymoquinone, Active Constituents of Nigella sativa, against HEp-2 Cancer Cells. Anticancer Res. 2005, 25, 4255–4259.
- Chehl, N.; Chipitsyna, G.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Anti-Inflammatory Effects of the Nigella sativa Seed Extract, Thymoquinone, in Pancreatic Cancer Cells. HPB 2009, 11, 373–381.
- Association, A.D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67.
- Kifle, Z.D.; Abdelwuhab, M.; Melak, A.D.; Genet, G.; Meseret, T.; Adugna, M. Pharmacological Evaluation of Medicinal Plants with Antidiabetic Activities in Ethiopia: A Review. Metab. Open 2022, 13, 100174.
- Patel, D.K.; Kumar, R.; Laloo, D.; Hemalatha, S. Diabetes Mellitus: An Overview on Its Pharmacological Aspects and Reported Medicinal Plants Having Antidiabetic Activity. Asian Pac. J. Trop. Biomed. 2012, 2, 411–420.
- Kumar, J.A.; Tiwari, A.K.; Ali, A.Z.; Madhusudhana, K.; Reddy, B.S.; Ramakrishna, S.; China Raju, B. New Antihyperglycemic, α-Glucosidase Inhibitory, and Cytotoxic Derivatives of Benzimidazoles. J. Enzyme Inhib. Med. Chem. 2010, 25, 80–86.
- Ahmad, W.; Amir, M.; Ahmad, A.; Ali, A.; Ali, A.; Wahab, S.; Barkat, H.A.; Ansari, M.A.; Sarafroz, M.; Ahmad, A.; et al. Aegle Marmelos Leaf Extract Phytochemical Analysis, Cytotoxicity, in Vitro Antioxidant and Antidiabetic Activities. Plants 2021, 10, 2573.
- Derosa, G.; Maffioli, P. α-Glucosidase Inhibitors and Their Use in Clinical Practice. Arch. Med. Sci. 2012, 8, 899–906.
- Asghari, B.; Salehi, P.; Farimani, M.M.; Ebrahimi, S.N. α-Glucosidase Inhibitors from Fruits of Rosa canina L. Rec. Nat. Prod. 2015, 9, 276–283.
- Mohebbati, R.; Abbasnezhad, A. Effects of Nigella sativa on Endothelial Dysfunction in Diabetes Mellitus: A Review. J. Ethnopharmacol. 2020, 252, 112585.
- Hamdan, A.; Idrus, R.H.; Mokhtar, M.H. Effects of Nigella sativa on Type-2 Diabetes Mellitus: A Systematic Review. Int. J. Environ. Res. Public Health 2019, 16, 4911.
- Khader, M.; Eckl, P.M. Thymoquinone: An Emerging Natural Drug with a Wide Range of Medical Applications. Iran. J. Basic Med. Sci. 2014, 17, 950–957.
- Alsayari, A.; Wahab, S. Genus Ziziphus for treating Chronic Inflammatory Diseases. Saudi J. Biol. Sci. 2021, 28, 6897–6914.
- Ahmad, S.; Beg, Z.H. Elucidation of Mechanisms of Actions of Thymoquinone-Enriched Methanolic and Volatile Oil Extracts from Nigella sativa against Cardiovascular Risk Parameters in Experimental Hyperlipidemia. Lipids Health Dis. 2013, 12, 86.
- Chen, L.H. Nutritional Aspects of Aging; CRC Press: Boca Raton, FL, USA, 2018; Volume 1, ISBN 9781351083584.
- Stehbens, W.E. An Appraisal of Cholesterol Feeding in Experimental Atherogenesis. Prog. Cardiovasc. Dis. 1986, 29, 107–128.
- Farkhondeh, T.; Samarghandian, S.; Azimin-Nezhad, M.; Samini, F. Effect of Chrysin on Nociception in Formalin Test and Serum Levels of Noradrenalin and Corticosterone in Rats. Int. J. Clin. Exp. Med. 2015, 8, 2465–2470.
- Samarghandian, S.; Azimi-Nezhad, M.; Borji, A.; Farkhondeh, T. Effect of Crocin on Aged Rat Kidney through Inhibition of Oxidative Stress and Proinflammatory State. Phyther. Res. 2016, 30, 1345–1353.
- Samarghandian, S.; Azimini-Nezhad, M.; Farkhondeh, T. The Effects of Zataria Multiflora on Blood Glucose, Lipid Profile and Oxidative Stress Parameters in Adult Mice During Exposure to Bisphenol A. Cardiovasc. Hematol. Disord. Targets 2016, 16, 41–46.
- Shafiq, H.; Ahmad, A.; Masud, T.; Kaleem, M. Cardio-Protective and Anti-Cancer Therapeutic Potential of Nigella sativa. Iran. J. Basic Med. Sci. 2014, 17, 967–980.
- Mansour, M.A.; Nagi, M.N.; El-Khatib, A.S.; Al-Bekairi, A.M. Effects of Thymoquinone on Antioxidant Enzyme Activities, Lipid Peroxidation and Dt-Diaphorase in Different Tissues of Mice: A Possible Mechanism of Action. Cell Biochem. Funct. 2002, 20, 143–151.
- Nagi, M.N.; Mansour, M.A. Protective Effect of Thymoquinone against Doxorubicin-Induced Cardiotoxicity in Rats: A Possible Mechanism of Protection. Pharmacol. Res. 2000, 41, 283–289.
- Khalife, K.H.; Lupidi, G. Reduction of Hypervalent States of Myoglobin and Hemoglobin to Their Ferrous Forms by Thymoquinone: The Role of GSH, NADH and NADPH. Biochim. Biophys. Acta-Gen. Subj. 2008, 1780, 627–637.
- Liu, H.; Liu, H.Y.; Jiang, Y.N.; Li, N. Protective Effect of Thymoquinone Improves Cardiovascular Function, and Attenuates Oxidative Stress, Inflammation and Apoptosis by Mediating the PI3K/Akt Pathway in Diabetic Rats. Mol. Med. Rep. 2016, 13, 2836–2842.
- Beheshti, F.; Hosseini, M.; Vafaee, F.; Shafei, M.N.; Soukhtanloo, M. Feeding of Nigella sativa during Neonatal and Juvenile Growth Improves Learning and Memory of Rats. J. Tradit. Complement. Med. 2016, 6, 146–152.
- Cascella, M.; Bimonte, S.; Barbieri, A.; Del Vecchio, V.; Muzio, M.R.; Vitale, A.; Benincasa, G.; Ferriello, A.B.; Azzariti, A.; Arra, C.; et al. Dissecting the Potential Roles of Nigella sativa and Its Constituent Thymoquinone on the Prevention and on the Progression of Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 1208–1218.
- Khan, M.I.; Ahmad, M.F.; Ahmad, I.; Ashfaq, F.; Wahab, S.; Alsayegh, A.A.; Kumar, S.; Hakeem, K.R. Arsenic Exposure through Dietary Intake and Associated Health Hazards in the Middle East. Nutrients 2022, 14, 2136.
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-Based Therapy for Alzheimer’s Disease: Challenges, Successes and Future. Signal Transduct. Target. Ther. 2023, 8, 248.
- Selkoe, D.J. Alzheimer’s Disease: Genes, Proteins, and Therapy. Physiol. Rev. 2001, 81, 741–766.
- Elibol, B.; Beker, M.; Terzioglu-Usak, S.; Dalli, T.; Kilic, U. Thymoquinone Administration Ameliorates Alzheimer’s Disease-like Phenotype by Promoting Cell Survival in the Hippocampus of Amyloid Beta1–42 Infused Rat Model. Phytomedicine 2020, 79, 153324.
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Soliman, K.F.A. Thymoquinone Increases the Expression of Neuroprotective Proteins While Decreasing the Expression of Pro-Inflammatory Cytokines and the Gene Expression NFκB Pathway Signaling Targets in LPS/IFNγ -Activated BV-2 Microglia Cells. J. Neuroimmunol. 2018, 320, 87–97.
- Alhibshi, A.H.; Odawara, A.; Suzuki, I. Neuroprotective Efficacy of Thymoquinone against Amyloid Beta-Induced Neurotoxicity in Human Induced Pluripotent Stem Cell-Derived Cholinergic Neurons. Biochem. Biophys. Rep. 2019, 17, 122–126.
- Poorgholam, P.; Yaghmaei, P.; Hajebrahimi, Z. Thymoquinone Recovers Learning Function in a Rat Model of Alzheimer’s Disease. Avicenna J. Phytomed. 2018, 8, 188–197.
- El-Naggar, T.; Gómez-Serranillos, M.P.; Palomino, O.M.; Arce, C.; Carretero, M.E. Nigella sativa L. Seed Extract Modulates the Neurotransmitter Amino Acids Release in Cultured Neurons in Vitro. J. Biomed. Biotechnol. 2010, 2010, 398312.
- Sandhua, K.S.; Cranab, A. Evaluation of Anti Parkinson’S Activity of Nigella sativa (Kalonji) Seeds in Chlorpromazineinduced Experimental Animal Model. Int. J. Pharm. Pharm. Sci. 2013, 5, 884–888.
- Hosseinzadeh, H.; Parvardeh, S.; Asl, M.N.; Sadeghnia, H.R.; Ziaee, T. Effect of Thymoquinone and Nigella sativa Seeds Oil on Lipid Peroxidation Level during Global Cerebral Ischemia-Reperfusion Injury in Rat Hippocampus. Phytomedicine 2007, 14, 621–627.
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P.H. Management of Obesity. Lancet 2016, 387, 1947–1956.
- WOF. One Billion People Globally Estimated to Be Living with Obesity by 2030; World Obesity Federation: London, UK, 2020.
- Jung, U.J.; Choi, M.S. Obesity and Its Metabolic Complications: The Role of Adipokines and the Relationship between Obesity, Inflammation, Insulin Resistance, Dyslipidemia and Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2014, 15, 6184–6223.
- Kushner, R.F. Weight Loss Strategies for Treatment of Obesity: Lifestyle Management and Pharmacotherapy. Prog. Cardiovasc. Dis. 2018, 61, 246–252.
- Hasani-Ranjbar, S.; Nayebi, N.; Larijani, B.; Abdollahi, M. A Systematic Review of the Efficacy and Safety of Herbal Medicines Used in the Treatment of Obesity. World J. Gastroenterol. 2009, 15, 3073–3085.
- Daryabeygi-Khotbehsara, R.; Golzarand, M.; Ghaffari, M.P.; Djafarian, K. Nigella sativa Improves Glucose Homeostasis and Serum Lipids in Type 2 Diabetes: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2017, 35, 6–13.
- Zaoui, A.; Cherrah, Y.; Mahassini, N.; Alaoui, K.; Amarouch, H.; Hassar, M. Acute and Chronic Toxicity of Nigella sativa Fixed Oil. Phytomedicine 2002, 9, 69–74.
- Le, P.M.; Benhaddou-Andaloussi, A.; Elimadi, A.; Settaf, A.; Cherrah, Y.; Haddad, P.S. The Petroleum Ether Extract of Nigella sativa Exerts Lipid-Lowering and Insulin-Sensitizing Actions in the Rat. J. Ethnopharmacol. 2004, 94, 251–259.
- Houcher, Z.; Boudiaf, K.; Benboubetra, M.; Houcher, B. Effects of Methanolic Extract and Commercial Oil of Nigella sativa L. on Blood Glucose and Antioxidant Capacity in Alloxan-Induced Diabetic Rats. Pteridines 2007, 18, 8–18.
- Heshmati, J.; Namazi, N.; Memarzadeh, M.R.; Taghizadeh, M.; Kolahdooz, F. Nigella sativa Oil Affects Glucose Metabolism and Lipid Concentrations in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Food Res. Int. 2015, 70, 87–93.
- Kaplan, S.A.; Meehan, A.G.; Shah, A. The Age Related Decrease in Testosterone Is Significantly Exacerbated in Obese Men With the Metabolic Syndrome. What Are the Implications for the Relatively High Incidence of Erectile Dysfunction Observed in These Men? J. Urol. 2006, 176, 1524–1528.
- Meddah, B.; Ducroc, R.; El Abbes Faouzi, M.; Eto, B.; Mahraoui, L.; Benhaddou-Andaloussi, A.; Martineau, L.C.; Cherrah, Y.; Haddad, P.S. Nigella sativa Inhibits Intestinal Glucose Absorption and Improves Glucose Tolerance in Rats. J. Ethnopharmacol. 2009, 121, 419–424.
- Hadi, S.; Daryabeygi-Khotbehsara, R.; Mirmiran, P.; McVicar, J.; Hadi, V.; Soleimani, D.; Askari, G. Effect of Nigella sativa Oil Extract on Cardiometabolic Risk Factors in Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phyther. Res. 2021, 35, 3747–3755.
- Badar, A.; Kaatabi, H.; Bamosa, A.; Al-Elq, A.; Abou-Hozaifa, B.; Lebda, F.; Alkhadra, A.; Al-Almaie, S. Effect of Nigella sativa Supplementation over a One-Year Period on Lipid Levels, Blood Pressure and Heart Rate in Type-2 Diabetic Patients Receiving Oral Hypoglycemic Agents: Nonrandomized Clinical Trial. Ann. Saudi Med. 2017, 37, 56–63.
- Najmi, A.; Nasiruddin, M.; Khan, R.A.; Haque, S.F. Indigenous Herbal Product Nigella sativa Proved Effective as an Antihypertensive in Metabolic Syndrome. Asian J. Pharm. Clin. Res. 2013, 6, 61–64.
- Ibrahim, R.M.; Hamdan, N.S.; Mahmud, R.; Imam, M.U.; Saini, S.M.; Rashid, S.N.A.; Abd Ghafar, S.A.; Latiff, L.A.; Ismail, M. A Randomised Controlled Trial on Hypolipidemic Effects of Nigella sativa Seeds Powder in Menopausal Women. J. Transl. Med. 2014, 12, 82.
- Hosseini, M.S.; Mirkarimi, S.A.; Amini, M.; Mohtashami, R.; Kianbakht, S.; Fallah Huseini, H. Effects of Nigella sativa L. Seed Oil in Type II Diabetic Patients: A Randomized, Double-Blind, Placebo—Controlled Clinical Trial. J. Med. Plants 2013, 12, 93–99.
- Farzaneh, E.; Nia, F.R.; Mehrtash, M.; Mirmoeini, F.S.; Jalilvand, M. The Effects of 8-Week Nigella sativa Supplementation and Aerobic Training on Lipid Profile and VO2 Max in Sedentary Overweight Females. Int. J. Prev. Med. 2014, 5, 210–216.
- Sethi, G.S.; Dharwal, V.; Naura, A.S. Immunological Basis of Oxidative Stress-Induced Lung Inflammation in Asthma and COPD. In Oxidative Stress in Lung Diseases: Volume 1; Springer: Singapore, 2019; Volume 1, pp. 192–223. ISBN 9789811384134.
- Wahab, S.; Ahmad, I.; Irfan, S.; Siddiqua, A.; Usmani, S.; Ahmad, M.P. Pharmacological Efficacy and Safety of Glycyrrhiza Glabra in the Treatment of Respiratory Tract Infections. Mini-Rev. Med. Chem. 2021, 22, 1476–1494.
- Lambrecht, B.N.; Hammad, H.; Fahy, J.V. The Cytokines of Asthma. Immunity 2019, 50, 975–991.
- Boulet, L.P. Airway Remodeling in Asthma: Update on Mechanisms and Therapeutic Approaches. Curr. Opin. Pulm. Med. 2018, 24, 56–62.
- Vuolo, F.; Abreu, S.C.; Michels, M.; Xisto, D.G.; Blanco, N.G.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Reis, C.; Bahl, M.; et al. Cannabidiol Reduces Airway Inflammation and Fibrosis in Experimental Allergic Asthma. Eur. J. Pharmacol. 2019, 843, 251–259.
- Chu, E.K.; Drazen, J.M. Asthma One Hundred Years of Treatment and Onward. Am. J. Respir. Crit. Care Med. 2005, 171, 1202–1208.
- Ahmad, M.D.F.; Wahab, S.; Ali Ahmad, F.; Intakhab Alam, M.; Ather, H.; Siddiqua, A.; Amir Ashraf, S.; Abu Shaphe, M.; Idreesh Khan, M.; Ali Beg, R. A Novel Perspective Approach to Explore Pros and Cons of Face Mask in Prevention the Spread of SARS-CoV-2 and Other Pathogens. Saudi Pharm. J. 2021, 29, 121–133.
- Wahab, S.; Annadurai, S.; Abullais, S.S.; Das, G.; Ahmad, W.; Ahmad, M.F.; Kandasamy, G.; Vasudevan, R.; Ali, M.S.; Amir, M. Glycyrrhiza Glabra (Licorice): A Comprehensive Review on Its Phytochemistry, Biological Activities, Clinical Evidence and Toxicology. Plants 2021, 10, 2751.
- Ahmad, M.F.; Ahmad, F.A.; Ashraf, S.A.; Saad, H.H.; Wahab, S.; Khan, M.I.; Ali, M.; Mohan, S.; Hakeem, K.R.; Athar, M.T. An Updated Knowledge of Black Seed (Nigella sativa Linn.): Review of Phytochemical Constituents and Pharmacological Properties. J. Herb. Med. 2021, 25, 100404.
- Balaha, M.F.; Tanaka, H.; Yamashita, H.; Abdel Rahman, M.N.; Inagaki, N. Oral Nigella sativa Oil Ameliorates Ovalbumin-Induced Bronchial Asthma in Mice. Int. Immunopharmacol. 2012, 14, 224–231.
- Noorbakhsh, M.F.; Shaterzadeh-Yazdi, H.; Hayati, F.; Samarghandian, S.; Farkhondeh, T. Protective Effects of Thymoquinon on Pulmonary Disorders in Experimental Studies. Tanaffos 2018, 17, 211–222.
- Saadat, S.; Aslani, M.R.; Ghorani, V.; Keyhanmanesh, R.; Boskabady, M.H. The Effects of Nigella sativa on Respiratory, Allergic and Immunologic Disorders, Evidence from Experimental and Clinical Studies, a Comprehensive and Updated Review. Phyther. Res. 2021, 35, 2968–2996.
- Koshak, A.; Koshak, E.; Heinrich, M. Medicinal Benefits of Nigella sativa in Bronchial Asthma: A Literature Review. Saudi Pharm. J. 2017, 25, 1130–1136.
- Boskabady, M.H.; Ghasemzadeh Rahbardar, M.; Nemati, H.; Esmaeilzadeh, M. Inhibitory Effect of Crocus sativus (Saffron) on Histamine (H1) Receptors of Guinea Pig Tracheal Chains. Pharmazie 2010, 65, 300–305.
- Boskabady, M.H.; Shahabi, M. Bronchodilatory and Anticholinergic Effects of Nigella sativa on Isolated Guinea Pig Tracheal Chains. Iran. J. Med. Sci. 1997, 22, 127–133.
- Gilani, A.H.; Aziz, N.; Khurram, I.M.; Chaudhary, K.S.; Iqbal, A. Bronchodilator, Spasmolytic and Calcium Antagonist Activities of Nigella sativa Seeds (Kalonji): A Traditional Herbal Product with Multiple Medicinal Uses. J. Pak. Med. Assoc. 2001, 51, 115–120.
- Boskabady, M.H.; Mohsenpoor, N.; Takaloo, L. Antiasthmatic Effect of Nigella sativa in Airways of Asthmatic Patients. Phytomedicine 2010, 17, 707–713.
- Boskabady, M.H.; Javan, H.; Sajady, M.; Rakhshandeh, H. The Possible Prophylactic Effect of Nigella sativa Seed Extract in Asthmatic Patients (Fundamental and Clinical Pharmacology (2007) 21, 5, (559–566)). Fundam. Clin. Pharmacol. 2008, 22, 105.
- Kalus, U.; Pruss, A.; Bystron, J.; Jurecka, M.; Smekalova, A.; Lichius, J.J.; Kiesewetter, H. Effect of Nigella sativa (Black Seed) on Subjective Feeling in Patients with Allergic Diseases. Phyther. Res. 2003, 17, 1209–1214.
- Ciesielska-Figlon, K.; Wojciechowicz, K.; Wardowska, A.; Lisowska, K.A. The Immunomodulatory Effect of Nigella sativa. Antioxidants 2023, 12, 1340.
- Shahzad, M.; Yang, X.; Raza Asim, M.B.; Sun, Q.; Han, Y.; Zhang, F.; Cao, Y.; Lu, S. Black Seed Oil Ameliorates Allergic Airway Inflammation by Inhibiting T-Cell Proliferation in Rats. Pulm. Pharmacol. Ther. 2009, 22, 37–43.
- Almaghasla, D.; Alsayari, A.; Wahab, S.; Motaal, A.A. Knowledge, Attitudes and Practices with Regard to Prophetic Medicine during the COVID-19 Pandemic in Saudi Arabia. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 352–358.
- Abullais, S.S.; Arora, S.; Wahab, S.; Grover, V.; Alshahrani, M.Y.; Shamsudeen, S.M.; Mohammed Asif, S.; Faragalla, A.I.; Elagib, M.F. Convalescent Plasma Therapy against COVID-19: An Update on the Changing Facets of the Ongoing Pandemic. Curr. Pharm. Biotechnol. 2023, 24, 1515–1523.
- Shoaib, S.; Ansari, M.A.; Kandasamy, G.; Vasudevan, R.; Hani, U.; Chauhan, W.; Alhumaidi, M.S.; Altammar, K.A.; Azmi, S.; Ahmad, W.; et al. An Attention towards the Prophylactic and Therapeutic Options of Phytochemicals for SARS-CoV-2: A Molecular Insight. Molecules 2023, 28, 795.
- Niknam, Z.; Jafari, A.; Golchin, A.; Danesh Pouya, F.; Nemati, M.; Rezaei-Tavirani, M.; Rasmi, Y. Potential Therapeutic Options for COVID-19: An Update on Current Evidence. Eur. J. Med. Res. 2022, 27, 6.
- Vijayvargiya, P.; Esquer Garrigos, Z.; Castillo Almeida, N.E.; Gurram, P.R.; Stevens, R.W.; Razonable, R.R. Treatment Considerations for COVID-19: A Critical Review of the Evidence (or Lack Thereof). Mayo Clin. Proc. 2020, 95, 1454–1466.
- Silveira, D.; Prieto-Garcia, J.M.; Boylan, F.; Estrada, O.; Fonseca-Bazzo, Y.M.; Jamal, C.M.; Magalhães, P.O.; Pereira, E.O.; Tomczyk, M.; Heinrich, M. COVID-19: Is There Evidence for the Use of Herbal Medicines as Adjuvant Symptomatic Therapy? Front. Pharmacol. 2020, 11, 1479.
- Khazdair, M.R.; Ghafari, S.; Sadeghi, M. Possible Therapeutic Effects of Nigella sativa and Its Thymoquinone on COVID-19. Pharm. Biol. 2021, 59, 696–703.
- Shirvani, H.; Rostamkhani, F.; Arabzadeh, E.; Mohammadi, F.; Mohammadi, F. Potential Role of Nigella sativa Supplementation with Physical Activity in Prophylaxis and Treatment of COVID-19: A Contemporary Review. Sport Sci. Health 2021, 17, 849–854.
- Koshak, A.; Wei, L.; Koshak, E.; Wali, S.; Alamoudi, O.; Demerdash, A.; Qutub, M.; Pushparaj, P.N.; Heinrich, M. Nigella sativa Supplementation Improves Asthma Control and Biomarkers: A Randomized, Double-Blind, Placebo-Controlled Trial. Phyther. Res. 2017, 31, 403–409.
- Barakat, E.M.F.; El Wakeel, L.M.; Hagag, R.S. Effects of Nigella sativa on Outcome of Hepatitis C in Egypt. World J. Gastroenterol. 2013, 19, 2529–2536.
- Onifade, A.A.; Jewell, A.P.; Ajadi, T.A.; Rahamon, S.K.; Ogunrin, O.O. Effectiveness of a Herbal Remedy in Six HIV Patients in Nigeria. J. Herb. Med. 2013, 3, 99–103.
- Onifade, A.A.; Jewell, A.P.; Adedeji, W.A. Nigella sativa Concoction Induced Sustained Seroreversion in HIV Patient. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 332–335.
- Salem, M.L.; Hossain, M.S. Protective Effect of Black Seed Oil from Nigella sativa against Murine Cytomegalovirus Infection. Int. J. Immunopharmacol. 2000, 22, 729–740.
- Oyero, O.G.; Toyama, M.; Mitsuhiro, N.; Onifade, A.A.; Hidaka, A.; Okamoto, M.; Baba, M. Selective Inhibition of Hepatitis c Virus Replication by Alpha-Zam, a Nigella sativa Seed Formulation. African J. Tradit. Complement. Altern. Med. 2016, 13, 144–148.
- Dorra, N.; El-Berrawy, M.; Sallam, S.; Mahmoud, R. Evaluation of Antiviral and Antioxidant Activity of Selected Herbal Extracts. J. High Inst. Public Health 2019, 49, 36–40.
- Ulasli, M.; Gurses, S.A.; Bayraktar, R.; Yumrutas, O.; Oztuzcu, S.; Igci, M.; Igci, Y.Z.; Cakmak, E.A.; Arslan, A. The Effects of Nigella sativa (Ns), Anthemis hyalina (Ah) and Citrus sinensis (Cs) Extracts on the Replication of Coronavirus and the Expression of TRP Genes Family. Mol. Biol. Rep. 2014, 41, 1703–1711.




