
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Itzae Adonai Gutiérrez Hurtado | -- | 2918 | 2023-12-13 02:28:00 | | | |
| 2 | Peter Tang | + 1 word(s) | 2919 | 2023-12-13 03:44:59 | | | | |
| 3 | Peter Tang | Meta information modification | 2919 | 2024-01-05 03:04:17 | | |
Video Upload Options
Microorganisms have a close relationship with humans, whether it is commensal, symbiotic, or pathogenic. It has been documented that microorganisms may influence the response to drug therapy. Pharmacomicrobiomics is an emerging field that focuses on the study of how variations in the microbiome affect the disposition, action, and toxicity of drugs. Two additional sciences have been added to complement pharmacomicrobiomics, namely toxicomicrobiomics, which explores how the microbiome influences drug metabolism and toxicity, and pharmacoecology, which refers to modifications in the microbiome as a result of drug administration. Additionally, the concept of "drug-infection interaction" is included to describe the influence of pathogenic microorganisms on drug response. This entry analyzes in detail each of these concepts.
1. Introduction
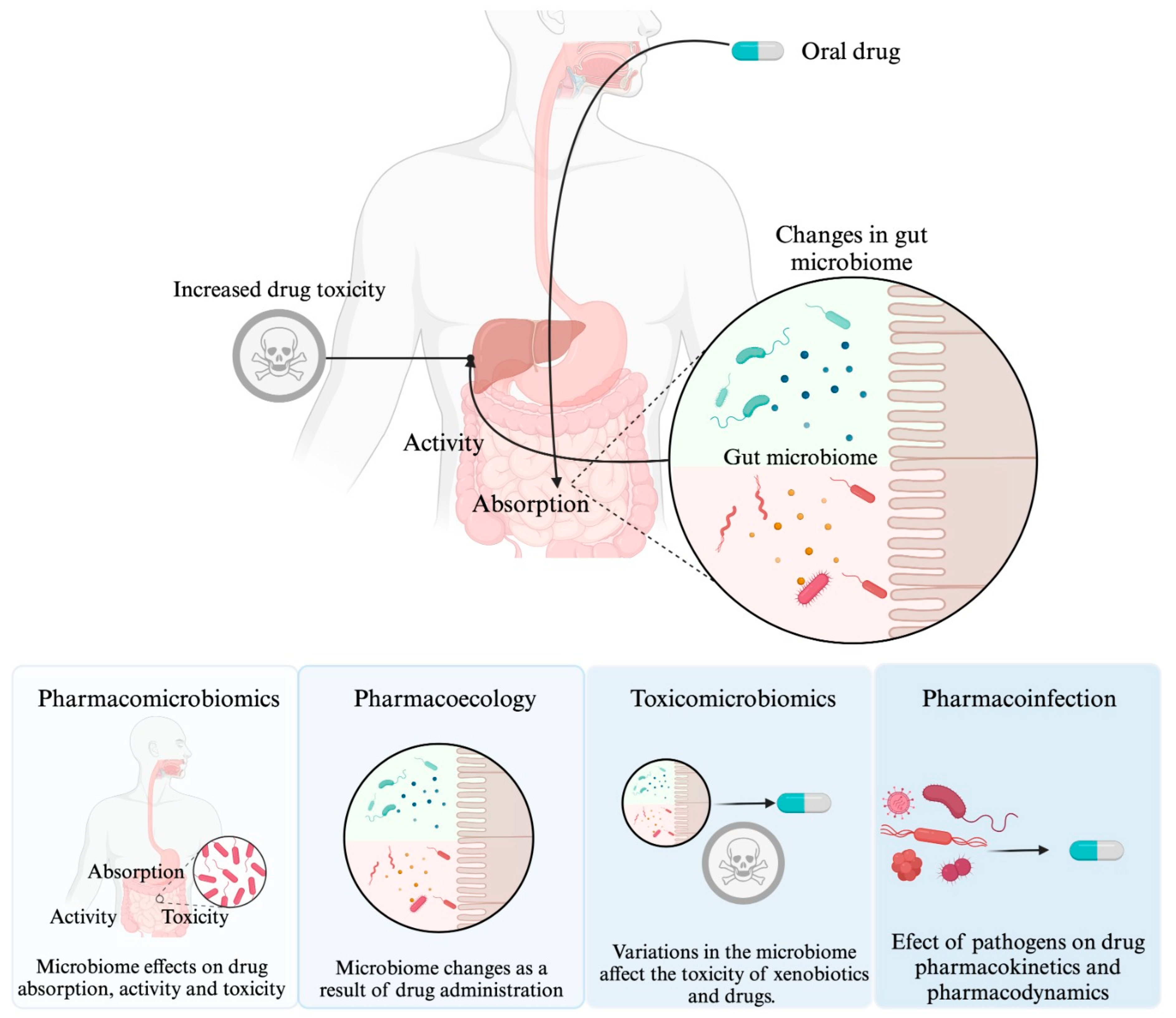
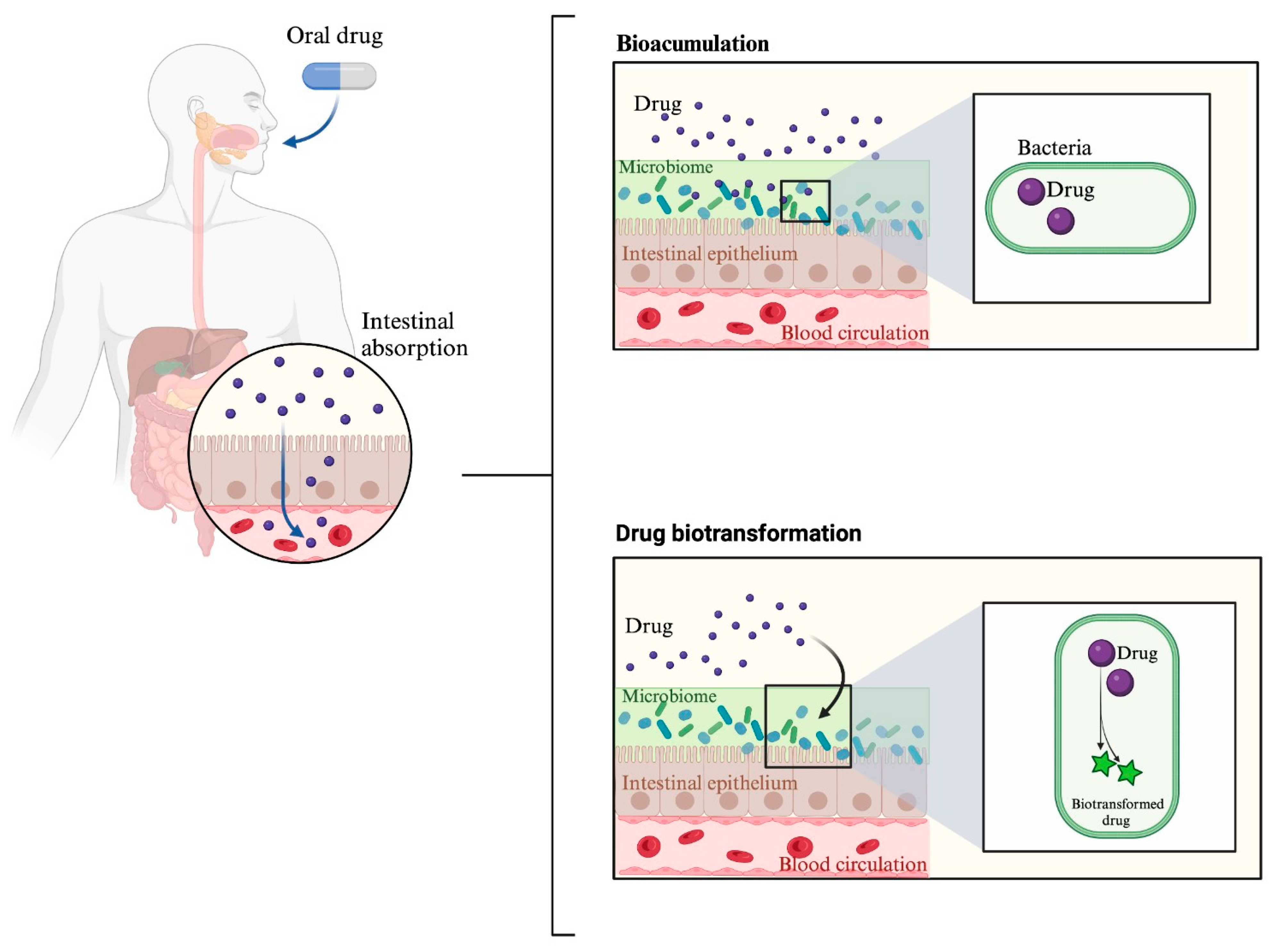
2. Effect of the Microbiome on Drug Response: “Pharmacomicrobiomics”
|
Drug Involved |
Affected Process |
Associated Microorganisms |
Clinical Effect |
Condition for Which Studied |
Organism in Which the Study Was Conducted |
|---|---|---|---|---|---|
|
Chemotherapy and/or immunotherapy |
Not described |
L. mucosae and L. salivarius |
Favorable response |
Metastatic/unresectable HER2-negative gastric/gastroesophageal junction adenocarcinoma |
Humans [26] |
|
FOLFOX regimen |
Drug metabolism |
Akkermansia muciniphila |
Better therapeutic effect |
Colon Cancer |
Mice [27] |
|
Hydrochlorothiazide |
Bioaccumulation |
Gram-negative enterobacteriaceae |
Impair glucose tolerance |
Metabolic control |
Mice [16] |
|
Mycophenolate mofetil |
Drug metabolism |
Bacteroides vulgatus, Bacteroides stercoris and Bacteroides thetaiotaomicron |
Graft-versus-host disease risk reduction |
Transplantation |
Humans [28] |
|
Simvastatin |
Drug metabolism and bioaccumulation |
Probiotic bacteria |
Alteration of simvastatin bioavailability and therapeutic effect |
Metabolic control |
in vitro [29] |
|
Statins |
Drug metabolism |
Bacteroides |
Intense statin responses |
Metabolic control |
Humans [30] |
3. Effect of an Infection on Drug Response
3.1. Difference between Pharmacomicrobiomics and Drug–Infection Interaction
3.2. Inflammation as a Result of Infection Modifies Drug Response
3.3. Other Mechanisms by Which Infections May Affect Drug Response
3.3.1. Alterations in Gastrointestinal Motility and Drug Absorption
3.3.2. Pharmacological Effect Mimicry
3.3.3. Unknown Mechanisms: The Case of Helicobacter pylori and Levodopa
References
- Patel, R.I.; Beckett, R.D. Evaluation of Resources for Analyzing Drug Interactions. J. Med. Libr. Assoc. 2016, 104, 290–295.
- Zeitlinger, M. Drug Interactions. In Clinical Pharmacology: Current Topics and Case Studies; Springer International Publishing: Cham, Switzerland, 2016; pp. 265–292.
- Rai, G.S.; Rozario, C.J. Mechanisms of Drug Interactions II: Pharmacokinetics and Pharmacodynamics. Anaesth. Intensive Care Med. 2023, 24, 217–220.
- Pichini, S.; Di Trana, A.; García-Algar, O.; Busardò, F.P. Editorial: Drug-Drug Interactions in Pharmacology. Front. Pharmacol. 2023, 14, 1155738.
- Heirali, A.; Moossavi, S.; Arrieta, M.C.; Coburn, B. Principles and Terminology for Host–Microbiome–Drug Interactions. Open Forum Infect. Dis. 2023, 10, ofad195.
- Attia, H.; ElBanna, S.A.; Khattab, R.A.; Farag, M.A.; Yassin, A.S.; Aziz, R.K. Integrating Microbiome Analysis, Metabolomics, Bioinformatics, and Histopathology to Elucidate the Protective Effects of Pomegranate Juice against Benzo-Alpha-Pyrene-Induced Colon Pathologies. Int. J. Mol. Sci. 2023, 24, 10691.
- Aziz, R.K.; Hegazy, S.M.; Yasser, R.; Rizkallah, M.R.; ElRakaiby, M.T. Drug Pharmacomicrobiomics and Toxicomicrobiomics: From Scattered Reports to Systematic Studies of Drug–Microbiome Interactions. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 1043–1055.
- Hannachi, N.; Camoin-Jau, L. Drug Response Diversity: A Hidden Bacterium? J. Pers. Med. 2021, 11, 345.
- Perez, N.B.; Dorsen, C.; Squires, A. Dysbiosis of the Gut Microbiome: A Concept Analysis. J. Holist. Nurs. 2020, 38, 223–232.
- Thomson, P.D.; Smith, D.J. What Is Infection? Am. J. Surg. 1994, 167, S7–S11.
- Rizkallah, M.; Saad, R.; Aziz, R. The Human Microbiome Project, Personalized Medicine and the Birth of Pharmacomicrobiomics. Curr. Pharmacogenom. Person. Med. 2010, 8, 182–193.
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411.
- Peretti, S.; Torracchi, S.; Russo, E.; Bonomi, F.; Fiorentini, E.; El Aoufy, K.; Bruni, C.; Lepri, G.; Orlandi, M.; Chimenti, M.S.; et al. The Yin-Yang Pharmacomicrobiomics on Treatment Response in Inflammatory Arthritides: A Narrative Review. Genes 2022, 14, 89.
- Conti, G.; D’Amico, F.; Fabbrini, M.; Brigidi, P.; Barone, M.; Turroni, S. Pharmacomicrobiomics in Anticancer Therapies: Why the Gut Microbiota Should Be Pointed Out. Genes 2022, 14, 55.
- Klünemann, M.; Andrejev, S.; Blasche, S.; Mateus, A.; Phapale, P.; Devendran, S.; Vappiani, J.; Simon, B.; Scott, T.A.; Kafkia, E.; et al. Bioaccumulation of Therapeutic Drugs by Human Gut Bacteria. Nature 2021, 597, 533–538.
- Luo, J.-Q.; Ren, H.; Chen, M.-Y.; Zhao, Q.; Yang, N.; Liu, Q.; Gao, Y.-C.; Zhou, H.-H.; Huang, W.-H.; Zhang, W. Hydrochlorothiazide-Induced Glucose Metabolism Disorder Is Mediated by the Gut Microbiota via LPS-TLR4-Related Macrophage Polarization. iScience 2023, 26, 107130.
- de la Cuesta-Zuluaga, J.; Mueller, N.T.; Corrales-Agudelo, V.; Velásquez-Mejía, E.P.; Carmona, J.A.; Abad, J.M.; Escobar, J.S. Metformin Is Associated with Higher Relative Abundance of Mucin-Degrading Akkermansia muciniphila and Several Short-Chain Fatty Acid–Producing Microbiota in the Gut. Diabetes Care 2017, 40, 54–62.
- Choi, U.; Lee, C.-R. Distinct Roles of Outer Membrane Porins in Antibiotic Resistance and Membrane Integrity in Escherichia coli. Front. Microbiol. 2019, 10, 952.
- Scheline, R.R. Drug Metabolism by Intestinal Microorganisms. J. Pharm. Sci. 1968, 57, 2021–2037.
- Jourova, L.; Anzenbacher, P.; Anzenbacherova, E. Human Gut Microbiota Plays a Role in the Metabolism of Drugs. Biomed. Pap. 2016, 160, 317–326.
- Dempsey, J.L.; Cui, J.Y. Microbiome Is a Functional Modifier of P450 Drug Metabolism. Curr. Pharmacol. Rep. 2019, 5, 481–490.
- Collins, S.L.; Patterson, A.D. The Gut Microbiome: An Orchestrator of Xenobiotic Metabolism. Acta Pharm. Sin. B 2020, 10, 19–32.
- Nelson, D.R. Cytochrome P450 Diversity in the Tree of Life. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2018, 1866, 141–154.
- Murphy, C.D. Drug Metabolism in Microorganisms. Biotechnol. Lett. 2015, 37, 19–28.
- Jeffreys, L.N.; Poddar, H.; Golovanova, M.; Levy, C.W.; Girvan, H.M.; McLean, K.J.; Voice, M.W.; Leys, D.; Munro, A.W. Novel Insights into P450 BM3 Interactions with FDA-Approved Antifungal Azole Drugs. Sci. Rep. 2019, 9, 1577.
- Han, Z.; Cheng, S.; Dai, D.; Kou, Y.; Zhang, X.; Li, F.; Yin, X.; Ji, J.; Zhang, Z.; Wang, X.; et al. The Gut Microbiome Affects Response of Treatments in HER2-negative Advanced Gastric Cancer. Clin. Transl. Med. 2023, 13, e1312.
- Hou, X.; Zhang, P.; Du, H.; Chu, W.; Sun, R.; Qin, S.; Tian, Y.; Zhang, Z.; Xu, F. Akkermansia muciniphila Potentiates the Antitumor Efficacy of FOLFOX in Colon Cancer. Front. Pharmacol. 2021, 12, 725583.
- Saqr, A.; Carlson, B.; Staley, C.; Rashidi, A.; Al-Kofahi, M.; Kaiser, T.; Holtan, S.; MacMillan, M.; Young, J.-A.; El Jurdi, N.; et al. Reduced Enterohepatic Recirculation of Mycophenolate and Lower Blood Concentrations Are Associated with the Stool Bacterial Microbiome after Hematopoietic Cell Transplantation. Transplant. Cell Ther. 2022, 28, 372.e1–372.e9.
- Đanić, M.; Pavlović, N.; Lazarević, S.; Stanimirov, B.; Vukmirović, S.; Al-Salami, H.; Mooranian, A.; Mikov, M. Bioaccumulation and Biotransformation of Simvastatin in Probiotic Bacteria: A Step towards Better Understanding of Drug-Bile Acids-Microbiome Interactions. Front. Pharmacol. 2023, 14, 1111115.
- Wilmanski, T.; Kornilov, S.A.; Diener, C.; Conomos, M.P.; Lovejoy, J.C.; Sebastiani, P.; Orwoll, E.S.; Hood, L.; Price, N.D.; Rappaport, N.; et al. Heterogeneity in Statin Responses Explained by Variation in the Human Gut Microbiome. Med 2022, 3, 388–405.e6.
- Turjeman, S.; Koren, O. Using the Microbiome in Clinical Practice. Microb. Biotechnol. 2022, 15, 129–134.
- Leardini, D.; Venturelli, F.; Baccelli, F.; Cerasi, S.; Muratore, E.; Brigidi, P.; Pession, A.; Prete, A.; Masetti, R. Pharmacomicrobiomics in Pediatric Oncology: The Complex Interplay between Commonly Used Drugs and Gut Microbiome. Int. J. Mol. Sci. 2022, 23, 15387.
- Fierer, J.; Looney, D.; Pechère, J.-C. Nature and Pathogenicity of Micro-Organisms. In Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2017; pp. 4–25.e1.
- Abdallah, Y.E.H.; Chahal, S.; Jamali, F.; Mahmoud, S.H. Drug-Disease Interaction: Clinical Consequences of Inflammation on Drugs Action and Disposition. J. Pharm. Pharm. Sci. 2023, 26, 11137.
- Renton, K.W.; Knickle, L.C. Regulation of Hepatic Cytochrome P-450 during Infectious Disease. Can. J. Physiol. Pharmacol. 1990, 68, 777–781.
- Lenoir, C.; Rollason, V.; Desmeules, J.A.; Samer, C.F. Influence of Inflammation on Cytochromes P450 Activity in Adults: A Systematic Review of the Literature. Front. Pharmacol. 2021, 12, 733935.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218.
- Morgan, E.T. Regulation of Cytochromes P450 During Inflammation and Infection. Drug Metab. Rev. 1997, 29, 1129–1188.
- de Jong, L.M.; Jiskoot, W.; Swen, J.J.; Manson, M.L. Distinct Effects of Inflammation on Cytochrome P450 Regulation and Drug Metabolism: Lessons from Experimental Models and a Potential Role for Pharmacogenetics. Genes 2020, 11, 1509.
- Sudsakorn, S.; Bahadduri, P.; Fretland, J.; Lu, C. 2020 FDA Drug-Drug Interaction Guidance: A Comparison Analysis and Action Plan by Pharmaceutical Industrial Scientists. Curr. Drug Metab. 2020, 21, 403–426.
- Fischer, L.; Lucendo-Villarin, B.; Hay, D.C.; O’Farrelly, C. Human PSC-Derived Hepatocytes Express Low Levels of Viral Pathogen Recognition Receptors, but Are Capable of Mounting an Effective Innate Immune Response. Int. J. Mol. Sci. 2020, 21, 3831.
- Lenoir, C.; Terrier, J.; Gloor, Y.; Curtin, F.; Rollason, V.; Desmeules, J.A.; Daali, Y.; Reny, J.; Samer, C.F. Impact of SARS-CoV-2 Infection (COVID-19) on Cytochromes P450 Activity Assessed by the Geneva Cocktail. Clin. Pharmacol. Ther. 2021, 110, 1358–1367.
- Morgan, E. Impact of Infectious and Inflammatory Disease on Cytochrome P450–Mediated Drug Metabolism and Pharmacokinetics. Clin. Pharmacol. Ther. 2009, 85, 434–438.
- Zoulikha, M.; Huang, F.; Wu, Z.; He, W. COVID-19 Inflammation and Implications in Drug Delivery. J. Control. Release 2022, 346, 260–274.
- Kumar, D.; Trivedi, N. Disease-Drug and Drug-Drug Interaction in COVID-19: Risk and Assessment. Biomed. Pharmacother. 2021, 139, 111642.
- Deshpande, K.; Lange, K.R.; Stone, W.B.; Yohn, C.; Schlesinger, N.; Kagan, L.; Auguste, A.J.; Firestein, B.L.; Brunetti, L. The Influence of SARS-CoV-2 Infection on Expression of Drug-Metabolizing Enzymes and Transporters in a HACE2 Murine Model. Pharmacol. Res. Perspect. 2023, 11, e01071.
- Le Carpentier, E.C.; Canet, E.; Masson, D.; Martin, M.; Deslandes, G.; Gaultier, A.; Dailly, É.; Bellouard, R.; Gregoire, M. Impact of Inflammation on Midazolam Metabolism in Severe COVID-19 Patients. Clin. Pharmacol. Ther. 2022, 112, 1033–1039.
- Lê, M.P.; Jaquet, P.; Patrier, J.; Wicky, P.-H.; Le Hingrat, Q.; Veyrier, M.; Kauv, J.; Sonneville, R.; Visseaux, B.; Laouénan, C.; et al. Pharmacokinetics of Lopinavir/Ritonavir Oral Solution to Treat COVID-19 in Mechanically Ventilated ICU Patients. J. Antimicrob. Chemother. 2020, 75, 2657–2660.
- Gregoire, M.; Le Turnier, P.; Gaborit, B.J.; Veyrac, G.; Lecomte, R.; Boutoille, D.; Canet, E.; Imbert, B.-M.; Bellouard, R.; Raffi, F. Lopinavir Pharmacokinetics in COVID-19 Patients. J. Antimicrob. Chemother. 2020, 75, 2702–2704.
- Knöchel, C.; Hefner, G.; Stiehl, T.; Schmidbauer, W. Elevated Clozapine Blood Concentrations After Second COVID-19 Vaccination with Spikevax (COVID-19 Vaccine Moderna). J. Clin. Psychopharmacol. 2022, 42, 317–320.
- Thompson, D.; Delorme, C.M.; White, R.F.; Honer, W.G. Elevated Clozapine Levels and Toxic Effects after SARS-CoV-2 Vaccination. J. Psychiatry Neurosci. 2021, 46, E210–E211.
- Jones, A.E.; Brown, K.C.; Werner, R.E.; Gotzkowsky, K.; Gaedigk, A.; Blake, M.; Hein, D.W.; van der Horst, C.; Kashuba, A.D.M. Variability in Drug Metabolizing Enzyme Activity in HIV-Infected Patients. Eur. J. Clin. Pharmacol. 2010, 66, 475–485.
- Jetter, A.; Fätkenheuer, G.; Frank, D.; Klaassen, T.; Seeringer, A.; Doroshyenko, O.; Kirchheiner, J.; Hein, W.; Schömig, E.; Fuhr, U.; et al. Do Activities of Cytochrome P450 (CYP)3A, CYP2D6 and P-Glycoprotein Differ between Healthy Volunteers and HIV-Infected Patients? Antivir. Ther. 2010, 15, 975–983.
- Obradovic, B.; Roberts, O.; Owen, A.; Milosevic, I.; Milic, N.; Ranin, J.; Dragovic, G. Expression of CYP2B6 Enzyme in Human Liver Tissue of HIV and HCV Patients. Medicina 2023, 59, 1207.
- Venuto, C.S.; Lim, J.; Messing, S.; Hunt, P.W.; McComsey, G.A.; Morse, G.D. Inflammation Investigated as a Source of Pharmacokinetic Variability of Atazanavir in AIDS Clinical Trials Group Protocol A5224s. Antivir. Ther. 2018, 23, 345–351.
- Parsons, R.L. Drug Absorption in Gastrointestinal Disease with Particular Reference to Malabsorption Syndromes. Clin. Pharmacokinet. 1977, 2, 45–60.
- Hatton, G.B.; Madla, C.M.; Rabbie, S.C.; Basit, A.W. All Disease Begins in the Gut: Influence of Gastrointestinal Disorders and Surgery on Oral Drug Performance. Int. J. Pharm. 2018, 548, 408–422.
- Hoque, K.M.; Chakraborty, S.; Sheikh, I.A.; Woodward, O.M. New Advances in the Pathophysiology of Intestinal Ion Transport and Barrier Function in Diarrhea and the Impact on Therapy. Expert. Rev. Anti-Infect. Ther. 2012, 10, 687–699.
- Lamberti, L.M.; Fischer Walker, C.L.; Black, R.E. Systematic Review of Diarrhea Duration and Severity in Children and Adults in Low- and Middle-Income Countries. BMC Public Health 2012, 12, 276.
- Sapuła, M.; Suchacz, M.; Kozłowska, J.; Cybula, A.; Siwak, E.; Krankowska, D.; Wiercińska-Drapało, A. Adenovirus 36 Infection in People Living with HIV—An Epidemiological Study of Seroprevalence and Associations with Cardiovascular Risk Factors. Viruses 2022, 14, 1639.
- Márquez, V.; Ballesteros, G.; Dobner, T.; González, R.A. Adipocyte Commitment of 3T3-L1 Cells Is Required to Support Human Adenovirus 36 Productive Replication Concurrent with Altered Lipid and Glucose Metabolism. Front. Cell Infect. Microbiol. 2022, 12, 1016200.
- Arnold, S.V.; Inzucchi, S.E.; Echouffo-Tcheugui, J.B.; Tang, F.; Lam, C.S.P.; Sperling, L.S.; Kosiborod, M. Understanding Contemporary Use of Thiazolidinediones. Circ. Heart Fail. 2019, 12, e005855.
- Pasarica, M.; Mashtalir, N.; McAllister, E.J.; Kilroy, G.E.; Koska, J.; Permana, P.; de Courten, B.; Yu, M.; Ravussin, E.; Gimble, J.M.; et al. Adipogenic Human Adenovirus Ad-36 Induces Commitment, Differentiation, and Lipid Accumulation in Human Adipose-Derived Stem Cells. Stem Cells 2008, 26, 969–978.
- Tapia-Rivera, J.C.; Mendoza-Jaramillo, H.E.; González-Villaseñor, C.O.; Ramirez-Flores, M.; Aguilar-Velazquez, J.A.; López-Quintero, A.; Pérez-Guerrero, E.E.; Vargas-Rodriguez, M.d.l.Á.; Gutiérrez-Hurtado, I.A.; Martínez-López, E. Effect of Human Adenovirus 36 on Response to Metformin Monotherapy in Obese Mexican Patients with Type 2 Diabetes: A Prospective Cohort Study. Viruses 2023, 15, 1514.
- Leta, V.; Klingelhoefer, L.; Longardner, K.; Campagnolo, M.; Levent, H.Ç.; Aureli, F.; Metta, V.; Bhidayasiri, R.; Chung-Faye, G.; Falup-Pecurariu, C.; et al. Gastrointestinal Barriers to Levodopa Transport and Absorption in Parkinson’s Disease. Eur. J. Neurol. 2023, 30, 1465–1480.
- Zhong, R.; Chen, Q.; Zhang, X.; Li, M.; Lin, W. Helicobacter Pylori Infection Is Associated with a Poor Response to Levodopa in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Neurol. 2022, 269, 703–711.
- Lolekha, P.; Sriphanom, T.; Vilaichone, R.-K. Helicobacter Pylori Eradication Improves Motor Fluctuations in Advanced Parkinson’s Disease Patients: A Prospective Cohort Study (HP-PD Trial). PLoS ONE 2021, 16, e0251042.




