
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonella Sgarbossa | -- | 3237 | 2023-12-12 14:12:29 | | | |
| 2 | Lindsay Dong | Meta information modification | 3237 | 2023-12-18 01:47:00 | | |
Video Upload Options
Photodynamic therapy (PDT), largely employed as a clinical treatment for several malignant pathologies, has also gained importance as a promising antimicrobial approach. Antimicrobial PDT (aPDT) relies on the application of a photosensitizer able to produce singlet oxygen (1O2) or other cytotoxic reactive oxygen species (ROS) upon exposure to appropriate light, which leads to cell death after the induced photodamage. Among different types of 2D nanomaterials with antimicrobial properties, phosphorene, the exfoliated form of black phosphorus (bP), has the unique property intrinsic photoactivity exploitable for photothermal therapy (PTT) as well as for PDT against pathogenic bacteria.
1. Introduction
2. Antimicrobial Phototherapy
2.1. Basic Mechanisms
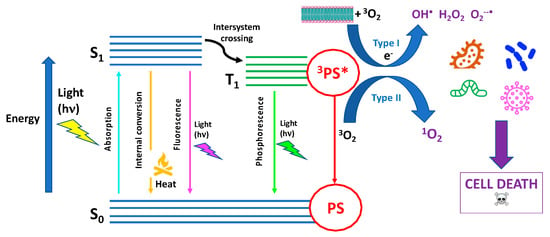
2.2. Antimicrobial PDT Activity
- -
-
Positive charge for high-affinity binding to negatively charged bacterial cell membranes;
- -
-
Low molecular weight or a structure that facilitates penetration through the biofilm matrix;
- -
-
High 1O2 quantum yield;
- -
-
High photostability;
- -
-
No dark toxicity and/or mutagenicity towards host eukaryotic cells in the “therapeutic window” where microorganisms can be killed without damaging the surrounding cells.
2.3. Antimicrobial PTT Activity
3. Structure and Properties of bP and bP-NPs
3.1. Physical, Mechanical, and Chemical Features of bP and Phosphorene (bP-NPs)
3.2. Preparation of bP and bP-NPs
4. Antimicrobial Photoactivity of bP
After its discovery, bP attracted the interest of researchers mainly due to its possible applications in optoelectronics, photonics, and advanced engineering, and only in the last few years has it also emerged as a possible new 2D material for biomedical applications. bP is highly biodegradable, biocompatible, and safe for use. These properties are essential for the use of bP in medicine [33][41][52][53][54][55][56][57][58]. In living organisms, phosphorus is a crucial element that constitutes approximately 1% of the total body weight. When it degrades, it transforms into harmless phosphate, which exhibits high biocompatibility and low cytotoxicity, preventing its in vivo accumulation. As a 2D material, it intrinsically has a large surface area, making it suitable for the absorption of drug molecules and making it easier to control the kinetics of release [59]. It also has a high modulus; thus, it can be used to improve the mechanical strength of biomedical implants.
4.1. Mechanisms of bP Photoactivity
4.2. Bare bP-NPs
4.3. bP-NPs-Based Hybrid Materials
5. Conclusions
References
- Shaw, Z.L.; Kuriakose, S.; Cheeseman, S.; Dickey, M.D.; Genzer, J.; Christofferson, A.J.; Crawford, R.J.; McConville, C.F.; Chapman, J.; Truong, V.K.; et al. Antipathogenic Properties and Applications of Low-Dimensional Materials. Nat. Commun. 2021, 12, 3897.
- Jayakumar, A.; Mathew, S.; Radoor, S.; Kim, J.T.; Rhim, J.-W.; Siengchin, S. Recent Advances in Two-Dimensional Nanomaterials: Properties, Antimicrobial, and Drug Delivery Application of Nanocomposites. Mater. Today Chem. 2023, 30, 101492.
- Zhang, C.; Wang, Y.; Ma, J.; Zhang, Q.; Wang, F.; Liu, X.; Xia, T. Black Phosphorus for Fighting Antibiotic-Resistant Bacteria: What Is Known and What Is Missing. Sci. Total Environ. 2020, 721, 137740.
- Favron, A.; Gaufrès, E.; Fossard, F.; Phaneuf-L’Heureux, A.-L.; Tang, N.Y.-W.; Lévesque, P.L.; Loiseau, A.; Leonelli, R.; Francoeur, S.; Martel, R. Photooxidation and Quantum Confinement Effects in Exfoliated Black Phosphorus. Nat. Mater. 2015, 14, 826–832.
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial Photodynamic Therapy—What We Know and What We Don’t. Crit. Rev. Microbiol. 2018, 44, 571–589.
- Maisch, T. Photoantimicrobials—An Update. Transl. Biophotonics 2020, 2, e201900033.
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332.
- Kashef, N.; Huang, Y.-Y.; Hamblin, M.R. Advances in Antimicrobial Photodynamic Inactivation at the Nanoscale. Nanophotonics 2017, 6, 853–879.
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial Photodynamic Therapy for Inactivation of Biofilms Formed by Oral Key Pathogens. Front. Microbiol. 2014, 5, 405.
- Hu, X.; Huang, Y.-Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299.
- Delcanale, P.; Abbruzzetti, S.; Viappiani, C. Photodynamic Treatment of Pathogens. Riv. Nuovo Cim. 2022, 45, 407–459.
- Pérez-Laguna, V.; Gilaberte, Y.; Millán-Lou, M.I.; Agut, M.; Nonell, S.; Rezusta, A.; Hamblin, M.R. A Combination of Photodynamic Therapy and Antimicrobial Compounds to Treat Skin and Mucosal Infections: A Systematic Review. Photochem. Photobiol. Sci. 2019, 18, 1020–1029.
- Nonell, S. Oxygen (and Lack Thereof) in Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2023, 41, 103440.
- Wong, W.F.; Melendez, A.J. INTRODUCTION. Clin. Exp. Pharma Physio 2006, 33, 480–481.
- Zhang, Y.; Zhu, Y.; Chen, J.; Wang, Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Hooper, D.C.; Hamblin, M.R.; Dai, T. Antimicrobial Blue Light Inactivation of Candida Albicans: In Vitro and In Vivo Studies. Virulence 2016, 7, 536–545.
- Wang, Y.; Wu, X.; Chen, J.; Amin, R.; Lu, M.; Bhayana, B.; Zhao, J.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; et al. Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and In Vivo Studies. J. Infect. Dis. 2016, 213, 1380–1387.
- Dai, T.; Gupta, A.; Huang, Y.-Y.; Yin, R.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Tegos, G.P.; Hamblin, M.R. Blue Light Rescues Mice from Potentially Fatal Pseudomonas Aeruginosa Burn Infection: Efficacy, Safety, and Mechanism of Action. Antimicrob. Agents Chemother. 2013, 57, 1238–1245.
- Hamblin, M.R.; Viveiros, J.; Yang, C.; Ahmadi, A.; Ganz, R.A.; Tolkoff, M.J. Helicobacter Pylori Accumulates Photoactive Porphyrins and Is Killed by Visible Light. Antimicrob. Agents Chemother. 2005, 49, 2822–2827.
- Morici, P.; Battisti, A.; Tortora, G.; Menciassi, A.; Checcucci, G.; Ghetti, F.; Sgarbossa, A. The In Vitro Photoinactivation of Helicobacter Pylori by a Novel LED-Based Device. Front. Microbiol. 2020, 11, 283.
- Battisti, A.; Morici, P.; Sgarbossa, A. Fluorescence Lifetime Imaging Microscopy of Porphyrins in Helicobacter Pylori Biofilms. Pharmaceutics 2021, 13, 1674.
- Schmid, J.; Hoenes, K.; Vatter, P.; Hessling, M. Antimicrobial Effect of Visible Light—Photoinactivation of Legionella Rubrilucens by Irradiation at 450, 470, and 620 Nm. Antibiotics 2019, 8, 187.
- Wang, Y.; Ferrer-Espada, R.; Baglo, Y.; Goh, X.S.; Held, K.D.; Grad, Y.H.; Gu, Y.; Gelfand, J.A.; Dai, T. Photoinactivation of Neisseria Gonorrhoeae: A Paradigm-Changing Approach for Combating Antibiotic-Resistant Gonococcal Infection. J. Infect. Dis. 2019, 220, 873–881.
- Fyrestam, J.; Bjurshammar, N.; Paulsson, E.; Johannsen, A.; Östman, C. Determination of Porphyrins in Oral Bacteria by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2015, 407, 7013–7023.
- Yoshida, A.; Sasaki, H.; Toyama, T.; Araki, M.; Fujioka, J.; Tsukiyama, K.; Hamada, N.; Yoshino, F. Antimicrobial Effect of Blue Light Using Porphyromonas Gingivalis Pigment. Sci. Rep. 2017, 7, 5225.
- Biener, G.; Masson-Meyers, D.S.; Bumah, V.V.; Hussey, G.; Stoneman, M.R.; Enwemeka, C.S.; Raicu, V. Blue/Violet Laser Inactivates Methicillin-Resistant Staphylococcus Aureus by Altering Its Transmembrane Potential. J. Photochem. Photobiol. B Biol. 2017, 170, 118–124.
- Alves, E.; Faustino, M.A.; Neves, M.G.; Cunha, A.; Tome, J.; Almeida, A. An Insight on Bacterial Cellular Targets of Photodynamic Inactivation. Future Med. Chem. 2014, 6, 141–164.
- Kaur, K.; Reddy, S.; Barathe, P.; Shriram, V.; Anand, U.; Proćków, J.; Kumar, V. Combating Drug-Resistant Bacteria Using Photothermally Active Nanomaterials: A Perspective Review. Front. Microbiol. 2021, 12, 747019.
- Ahamed, M.I.; Shakeel, N.; Anwar, N. Structure and Fundamental Properties of Black Phosphorus; Inamuddin, Boddula, R., Asiri, A.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 139–156.
- Fukuoka, S.; Taen, T.; Osada, T. Electronic Structure and the Properties of Phosphorene and Few-Layer Black Phosphorus. J. Phys. Soc. Jpn. 2015, 84, 121004.
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An Unexplored 2D Semiconductor with a High Hole Mobility. ACS Nano 2014, 8, 4033–4041.
- Du, H.; Lin, X.; Xu, Z.; Chu, D. Recent Developments in Black Phosphorus Transistors. J. Mater. Chem. C 2015, 3, 8760–8775.
- Kou, L.; Chen, C.; Smith, S.C. Phosphorene: Fabrication, Properties, and Applications. J. Phys. Chem. Lett. 2015, 6, 2794–2805.
- Pica, M.; D’Amato, R. Chemistry of Phosphorene: Synthesis, Functionalization and Biomedical Applications in an Update Review. Inorganics 2020, 8, 29.
- Cai, Y.; Zhang, G.; Zhang, Y.-W. Electronic Properties of Phosphorene/Graphene and Phosphorene/Hexagonal Boron Nitride Heterostructures. J. Phys. Chem. C 2015, 119, 13929–13936.
- Peruzzini, M.; Bini, R.; Bolognesi, M.; Caporali, M.; Ceppatelli, M.; Cicogna, F.; Coiai, S.; Heun, S.; Ienco, A.; Benito, I.I.; et al. A Perspective on Recent Advances in Phosphorene Functionalization and Its Applications in Devices. Eur. J. Inorg. Chem. 2019, 2019, 1476–1494.
- Carvalho, A.; Wang, M.; Zhu, X.; Rodin, A.S.; Su, H.; Castro Neto, A.H. Phosphorene: From Theory to Applications. Nat. Rev. Mater. 2016, 1, 16061.
- Zhang, Y.; Wang, J.; Liu, Q.; Gu, S.; Sun, Z.; Chu, P.K.; Yu, X. The Electrical, Thermal, and Thermoelectric Properties of Black Phosphorus. APL Mater. 2020, 8, 120903.
- Torbatian, Z.; Asgari, R. Optical Absorption Properties of Few-Layer Phosphorene. Phys. Rev. B 2018, 98, 205407.
- Molas, M.R.; Macewicz, Ł.; Wieloszyńska, A.; Jakóbczyk, P.; Wysmołek, A.; Bogdanowicz, R.; Jasinski, J.B. Photoluminescence as a Probe of Phosphorene Properties. npj 2d Mater. Appl. 2021, 5, 83.
- Jiang, J.-W.; Park, H.S. Negative Poisson’s Ratio in Single-Layer Black Phosphorus. Nat. Commun. 2014, 5, 4727.
- Chaudhary, V.; Neugebauer, P.; Mounkachi, O.; Lahbabi, S.; El Fatimy, A. Phosphorene—An Emerging Two-Dimensional Material: Recent Advances in Synthesis, Functionalization, and Applications. 2D Mater. 2022, 9, 032001.
- Van Druenen, M.; Davitt, F.; Collins, T.; Glynn, C.; O’Dwyer, C.; Holmes, J.D.; Collins, G. Evaluating the Surface Chemistry of Black Phosphorus during Ambient Degradation. Langmuir 2019, 35, 2172–2178.
- Van Druenen, M. Degradation of Black Phosphorus and Strategies to Enhance Its Ambient Lifetime. Adv. Mater. Inter. 2020, 7, 2001102.
- Abellán, G.; Wild, S.; Lloret, V.; Scheuschner, N.; Gillen, R.; Mundloch, U.; Maultzsch, J.; Varela, M.; Hauke, F.; Hirsch, A. Fundamental Insights into the Degradation and Stabilization of Thin Layer Black Phosphorus. J. Am. Chem. Soc. 2017, 139, 10432–10440.
- Zhang, T.; Wan, Y.; Xie, H.; Mu, Y.; Du, P.; Wang, D.; Wu, X.; Ji, H.; Wan, L. Degradation Chemistry and Stabilization of Exfoliated Few-Layer Black Phosphorus in Water. J. Am. Chem. Soc. 2018, 140, 7561–7567.
- Zhou, Q.; Chen, Q.; Tong, Y.; Wang, J. Light-Induced Ambient Degradation of Few-Layer Black Phosphorus: Mechanism and Protection. Angew. Chem. Int. Ed. 2016, 55, 11437–11441.
- Walia, S.; Sabri, Y.; Ahmed, T.; Field, M.R.; Ramanathan, R.; Arash, A.; Bhargava, S.K.; Sriram, S.; Bhaskaran, M.; Bansal, V.; et al. Defining the Role of Humidity in the Ambient Degradation of Few-Layer Black Phosphorus. 2D Mater. 2016, 4, 015025.
- Wu, S.; Hui, K.S.; Hui, K.N. 2D Black Phosphorus: From Preparation to Applications for Electrochemical Energy Storage. Adv. Sci. 2018, 5, 1700491.
- Smith, J.B.; Hagaman, D.; Ji, H.-F. Growth of 2D Black Phosphorus Film from Chemical Vapor Deposition. Nanotechnology 2016, 27, 215602.
- El Hammoumi, M.; Chaudhary, V.; Neugebauer, P.; El Fatimy, A. Chemical Vapor Deposition: A Potential Tool for Wafer Scale Growth of Two-Dimensional Layered Materials. J. Phys. D Appl. Phys. 2022, 55, 473001.
- Mukhopadhyay, T.K.; Datta, A. Disentangling the Liquid Phase Exfoliation of Two-Dimensional Materials: An “In Silico ” Perspective. Phys. Chem. Chem. Phys. 2020, 22, 22157–22179.
- Zhu, Y.; Xie, Z.; Li, J.; Liu, Y.; Li, C.; Liang, W.; Huang, W.; Kang, J.; Cheng, F.; Kang, L.; et al. From Phosphorus to Phosphorene: Applications in Disease Theranostics. Coord. Chem. Rev. 2021, 446, 214110.
- Qiu, M.; Ren, W.X.; Jeong, T.; Won, M.; Park, G.Y.; Sang, D.K.; Liu, L.-P.; Zhang, H.; Kim, J.S. Omnipotent Phosphorene: A next-Generation, Two-Dimensional Nanoplatform for Multidisciplinary Biomedical Applications. Chem. Soc. Rev. 2018, 47, 5588–5601.
- Tatullo, M.; Genovese, F.; Aiello, E.; Amantea, M.; Makeeva, I.; Zavan, B.; Rengo, S.; Fortunato, L. Phosphorene Is the New Graphene in Biomedical Applications. Materials 2019, 12, 2301.
- Akhtar, M.; Anderson, G.; Zhao, R.; Alruqi, A.; Mroczkowska, J.E.; Sumanasekera, G.; Jasinski, J.B. Recent Advances in Synthesis, Properties, and Applications of Phosphorene. npj 2d Mater. Appl. 2017, 1, 5.
- Liu, X.; Gaihre, B.; George, M.N.; Li, Y.; Tilton, M.; Yaszemski, M.J.; Lu, L. 2D Phosphorene Nanosheets, Quantum Dots, Nanoribbons: Synthesis and Biomedical Applications. Biomater. Sci. 2021, 9, 2768–2803.
- Kim, J.; Sando, S.; Cui, T. Biosensor Based on Layer by Layer Deposited Phosphorene Nanoparticles for Liver Cancer Detection. In Proceedings of the ASME 2017 International Mechanical Engineering Congress and Exposition, Tampa, FL, USA, 3–9 November 2017.
- Liu, H.; Mei, Y.; Zhao, Q.; Zhang, A.; Tang, L.; Gao, H.; Wang, W. Black Phosphorus, an Emerging Versatile Nanoplatform for Cancer Immunotherapy. Pharmaceutics 2021, 13, 1344.
- Wang, Z.; Liu, Z.; Su, C.; Yang, B.; Fei, X.; Li, Y.; Hou, Y.; Zhao, H.; Guo, Y.; Zhuang, Z.; et al. Biodegradable Black Phosphorus-Based Nanomaterials in Biomedicine: Theranostic Applications. CMC 2019, 26, 1788–1805.
- Wang, H.; Yang, X.; Shao, W.; Chen, S.; Xie, J.; Zhang, X.; Wang, J.; Xie, Y. Ultrathin Black Phosphorus Nanosheets for Efficient Singlet Oxygen Generation. J. Am. Chem. Soc. 2015, 137, 11376–11382.
- Sun, Z.; Zhang, Y.; Yu, H.; Yan, C.; Liu, Y.; Hong, S.; Tao, H.; Robertson, A.W.; Wang, Z.; Pádua, A.A.H. New Solvent-Stabilized Few-Layer Black Phosphorus for Antibacterial Applications. Nanoscale 2018, 10, 12543–12553.
- Shaw, Z.L.; Cheeseman, S.; Huang, L.Z.Y.; Penman, R.; Ahmed, T.; Bryant, S.J.; Bryant, G.; Christofferson, A.J.; Orrell-Trigg, R.; Dekiwadia, C.; et al. Illuminating the Biochemical Interaction of Antimicrobial Few-Layer Black Phosphorus with Microbial Cells Using Synchrotron Macro-ATR-FTIR. J. Mater. Chem. B 2022, 10, 7527–7539.
- Liu, W.; Dong, A.; Wang, B.; Zhang, H. Current Advances in Black Phosphorus-Based Drug Delivery Systems for Cancer Therapy. Adv. Sci. 2021, 8, 2003033.




