Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ruiyang Zhang | -- | 2705 | 2023-12-12 09:28:18 | | | |
| 2 | Rita Xu | + 713 word(s) | 3418 | 2023-12-12 09:42:36 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, R.; Tang, C.; Ni, W.; Yuan, J.; Zhou, Y.; Liu, X. High-Purity Quartz Processing Technology. Encyclopedia. Available online: https://encyclopedia.pub/entry/52609 (accessed on 07 February 2026).
Zhang R, Tang C, Ni W, Yuan J, Zhou Y, Liu X. High-Purity Quartz Processing Technology. Encyclopedia. Available at: https://encyclopedia.pub/entry/52609. Accessed February 07, 2026.
Zhang, Ruiyang, Chunhua Tang, Wen Ni, Jing Yuan, Yu Zhou, Xiaolong Liu. "High-Purity Quartz Processing Technology" Encyclopedia, https://encyclopedia.pub/entry/52609 (accessed February 07, 2026).
Zhang, R., Tang, C., Ni, W., Yuan, J., Zhou, Y., & Liu, X. (2023, December 12). High-Purity Quartz Processing Technology. In Encyclopedia. https://encyclopedia.pub/entry/52609
Zhang, Ruiyang, et al. "High-Purity Quartz Processing Technology." Encyclopedia. Web. 12 December, 2023.
Copy Citation
Quartz deposits are widely dispersed in nature, but the presence of ore bodies capable of yielding high-purity quartz is exceedingly rare. As a result, the effective purification and processing of high-purity quartz from natural siliceous materials has emerged as a prominent area of research within the non-metallic mineral processing field.
high-purity quartz
mineralogy
purification
impurity
1. Introduction
High-purity quartz is a mineral obtained from natural siliceous materials after a series of physical and chemical purification processes. It exhibits specific particle size specifications and typically boasts an SiO2 content exceeding 99.9%. Due to its remarkable physical and chemical properties, including high-temperature resistance, low thermal expansion coefficient, exceptional transparency, effective electrical insulation, and resistance to corrosion, it has wide-ranging applications in high-tech sectors such as electronics, intelligent manufacturing equipment, advanced inorganic non-metal materials, high-performance fibers and products, solar energy, and more [1]. As early as 2018, China’s National Bureau of Statistics recognized the strategic importance of high-purity quartz mineral products in its ‘Classification of Strategic Emerging Industries’ [2].
Currently, countries worldwide have recognized high-purity quartz as a strategically significant resource. High-purity quartz products are categorized into four grades based on SiO2 purity: ultra-high purity (ω(SiO2) ≥ 99.998% or 4N8), high-purity (ω(SiO2) ≥ 99.995% or 4N5), medium-purity (ω(SiO2) ≥ 99.99% or 4N), and standard-purity (ω(SiO2) ≥ 99.9% or 3N). Abroad, extensive production of 4N grade high-purity quartz is already established, with these deposits primarily located in regions including North Carolina in the United States, Nordland County in Norway, Khanty-Mansi Autonomous Okrug in Russia, Queensland in Australia, and the Nuadibú Bay Province in Mauritania. Moreover, the purification and processing technology for 5N grade high-purity quartz has also reached a high level of maturity. For example, Unimin Corp in the United States can achieve impurity levels as low as 8 μg/g in their products [3].
China indeed possesses abundant reserves of quartz mineral resources; however, the presence of ore bodies capable of producing high-purity quartz is exceedingly rare. Domestically, the production of high-purity quartz from natural crystal sources, exemplified by Jiangsu Pacific Quartz Co., Ltd., has been limited, with an annual capacity not surpassing 20,000 tons. Furthermore, much of this output is directed towards the preparation of medium-purity or standard-purity quartz products. As the resources of natural crystal-grade quartz gradually deplete, a looming shortage of high-quality quartz raw materials becomes evident. Concurrently, the outdated state of high-purity quartz preparation technology further exacerbates the severe mismatch between supply and demand. In 2020, China imported a staggering 144,500 tons of high-purity quartz, representing over 70% of the global import total. In 2022, the price of high-purity polycrystalline silicon surged from RMB 110,000 per ton in 2021 to RMB 270,000 per ton [4]. Consequently, the effective purification of quartz from natural siliceous materials has emerged as a central focus of current research.
Quartz, one of the Earth’s most abundant minerals, is widespread in igneous, metamorphic, and sedimentary rocks. Under diverse geological conditions, it gives rise to various types of ore deposits, including natural crystal, quartz sandstone, quartzite, vein quartz, powder quartz, quartz sand, and granitic quartz. Quartz ores typically contain a multitude of impurity elements, associated/coexisting minerals, fluid inclusions, mineral inclusions, lattice impurities and hydroxyl impurities. These complexities make the purification process intricate, involving various complex steps such as pre-treatment, physical separation, chemical treatment, and advanced processing [5].
Pan et al. reviewed the distribution of high-purity quartz resources and their applications, summarized the various types of quartz ores and the characteristics of impurities, and elucidated the mechanisms behind different purification techniques [6]. Lin et al. provided an overview of quality assessment techniques and separation methods for high-grade quartz, with a focus on summarizing the thermal phase transitions of mineral inclusions, fluid inclusions, and trace elements within lattice structures [7]. Lin et al. also reviewed the current status, progress, and mechanisms of hydrometallurgy in the quartz purification process, including bioleaching, high-temperature leaching and high-pressure leaching [8]. It is worth noting that the formation of high-grade natural quartz deposits is constrained to specific geological environments. Consequently, from a mineralogical perspective, the purification potential of quartz is intrinsically linked to its ore deposit origin, ore characteristics, and mineral properties.
2. The Characteristics of Quartz Mineral Resources
2.1. The Reserves of Quartz Mineral Resources in China
As of 2022, the confirmed reserves of quartz mineral resources in China are depicted in Figure 1, with data sourced from the Ministry of Natural Resources of the People’s Republic of China. From Figure 1, it can be observed that China possesses abundant siliceous mineral resources, with total reserves of quartzite and vein quartz reaching 1364.18 million tons and 82.1 million tons, respectively. In contrast, the reserves of powder quartz and crystal resources are relatively limited, standing at only 5.64 million tons and 4.19 million tons, respectively.
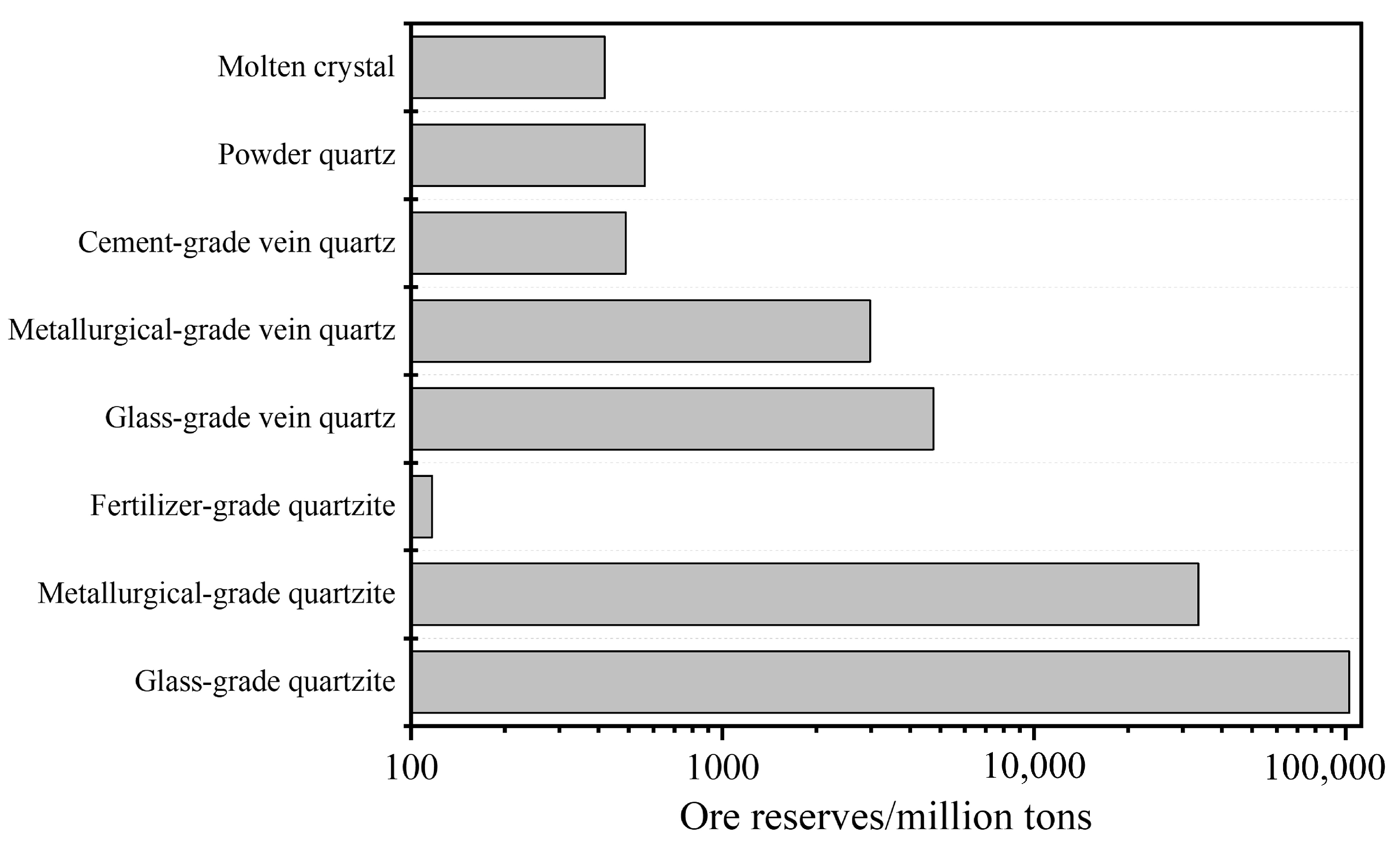
Figure 1. Statistics of confirmed quartz mineral resource reserves in China (as of 2022).
Taking Jiangxi Province in China as an example, the main mineral categories that have been explicitly categorized include natural crystal, powder quartz, quartzite, sandstone, quartz sand, and vein quartz. As of 2020, the mineral resource reserves in Jiangxi Province are depicted in Figure 2, with data sourced from the Digital Remote Sensing Center of the Jiangxi Geological Survey Institute.

Figure 2. (a) The reserves of siliceous raw materials and (b) the number of deposits (with bold numerals) and their proportions (within parentheses) in Jiangxi Province, China.
From Figure 2, it can be observed that in terms of resource reserves, sandstone, quartzite, and quartz sand have the highest reserves, collectively accounting for approximately 90% of the total resource reserves. Vein quartz and powder quartz have relatively lower proportions, standing at only 5.79% and 4.23%, respectively. The confirmed resource quantity of natural crystal is extremely scarce, accounting for 1.7 × 10−7 of the totals. In terms of the number of deposits, vein quartz holds the highest proportion, followed by quartzite and sandstone, accounting for 22% and 15%, respectively. Powder quartz and quartz sand exhibit smaller shares, while natural crystal has the least number of deposits, approximately 1%. These results indicate that the reserves of natural crystal are exceedingly low, with most of them lacking scale, resulting in a relatively limited exploration prospect.
2.2. Geological Origins of Quartz Deposits
Quartz minerals have a broad natural distribution, and the attributes of quartz deposits are shaped by geological factors. Currently, there are seven main industrial types of quartz deposits, namely natural crystal, quartz sandstone, quartzite, vein quartz, powder quartz, quartz sand and granite quartz. The primary silica raw material types in China, along with their fundamental characteristics, are detailed in Table 1 [9][10].
Table 1. The types and basic characteristics of siliceous raw material deposits in China.
| Ore Type | Ore Genesis | Deposit Scale | Mineralogical Features | Application Areas | Resource Distribution/Extraction Status |
|---|---|---|---|---|---|
| Natural Crystal | Slow growth in crystal caves | Small | High purity, high transparency | Optical instruments, electronics | Low resource reserves, difficult to mine |
| Vein Quartz | Hydrothermal filling along fault fractures | Medium/small | Well-crystallized | Glass, metallurgy | Many mining locations, low reserves |
| Quartz Sandstone | Lacustrine deposition | Large/medium | Comprising intricate cementing materials | Glass, ceramics, building materials | Concentrated in southern China, easy to mine |
| Quartz Sand | Alluvial deposition | Large/medium | Lack of natural grain shape | Glass, construction, casting molds | Large reserves, favorable mining |
| Powder Quartz | Weathering residual | Large/medium | Extremely fine particle size | Metallurgy, glass, cement, ceramics | Found in Jiangxi, Guizhou, Hunan |
| Quartzite | Sedimentary deposits altered by metamorphism | Large/very large | Blocky structure | Refractories, silicon alloys, glass | Large reserves |
| Granite Quartz | Slow crystallization in deep magma | Large/very large | Large grains, higher impurity | Underdeveloped utilization | Widely distributed |
Natural crystals are favored for their high purity and transparency, making them the primary choice for producing high-purity quartz. However, their resource reserves are extremely limited, and mining conditions are unfavorable. Additionally, high-quality quartz resources of this kind are gradually depleting.
Vein quartz is characterized by its single mineral composition and high quartz content, with numerous mining locations. However, it generally has relatively small reserves and is challenging to mine. The primary reason for the mining difficulties is the irregular vein-like nature of vein quartz deposits.
Quartz sandstone typically consists of more than 95% quartz and siliceous clasts, with gangue minerals often including clay minerals like feldspar, kaolin, illite, and montmorillonite, and a low proportion of heavy minerals. Quartz sand primarily originates from modern lacustrine deposits in lakes and alluvial sediments along riverbanks. Due to its fine particle size, producing high-purity quartz sand at suitable grain sizes is challenging, resulting in limited reports of its use for high-end quartz products, both domestically and internationally. Taking Thrakon, a Greek company, as an example, they leverage the low impurity content of the Alada River quartz sand. After undergoing cleaning and drying processes, this sand serves as the primary component in construction mortar [11].
Quartzite is a metamorphic rock formed from siliceous rocks through regional metamorphism or contact metamorphism induced by hydrothermal activity. It contains not only minerals like feldspar, mica, and clay minerals but also trace amounts of minerals such as tourmaline, hematite, and zircon. Natural powder quartz is a sedimentary weathering-type deposit formed through the weathering and disintegration of siliceous parent rocks. It is characterized by a high SiO2 content but possesses an extremely fine grain size, with particle diameters typically falling within the range of 5–50 μm.
Granite quartz typically exhibits a relatively low SiO2 content, where feldspar, mica, and kaolin collectively account for up to 80% of the total ore. Currently, the utilization of quartz in granite remains exceedingly limited. It is worth noting that some research suggests a direct proportionality between the concentration of impurities in quartz and its crystallization temperature. Quartz crystallized from granite melts with higher weathering levels tends to contain fewer structural impurities, as it forms at lower temperatures [12]. In comparison to other siliceous raw materials found in granite, coarse-grained pegmatite quartz offers greater potential for purification. Presently, only a select few mineral deposit types worldwide, including certain granitic pegmatites and hydrothermal quartz veins, have the capacity to produce high-purity quartz [13].
2.3. Impurities in Quartz Ores
The presence and quantity of impurities directly determine the ease or difficulty of quartz purification. Research indicates that siliceous raw materials contain various impurity minerals and elements, with the modes of occurrence and distribution patterns of these impurities being associated with the ore-forming processes [14]. For example, impurity distribution in layered hydrothermal vein quartz is typically highly uneven, while structural impurities in igneous and metamorphic quartz tend to have a more uniform distribution. In addition, Larsen et al. have identified a functional relationship between the concentration of structural impurities during quartz growth and the total impurity concentration in pegmatitic melts [14].
2.3.1. Impurity Elements
To examine the content and distribution of impurity elements within various types of quartz ores, five representative samples were selected, including vein quartz, powder quartz, quartzite, pegmatite quartz (referred to as pegmatite), and pegmatitic granite (referred to as granite) from Jiangxi Province. Based on the elements detected using X-ray fluorescence spectroscopy (XRF) of the original ores, chemical full-element analysis of the five types of ores was conducted using ICP-OES, ICP-MS, and high-frequency infrared carbon-sulfur analysis (HCS801A D548), as illustrated in Figure 3.
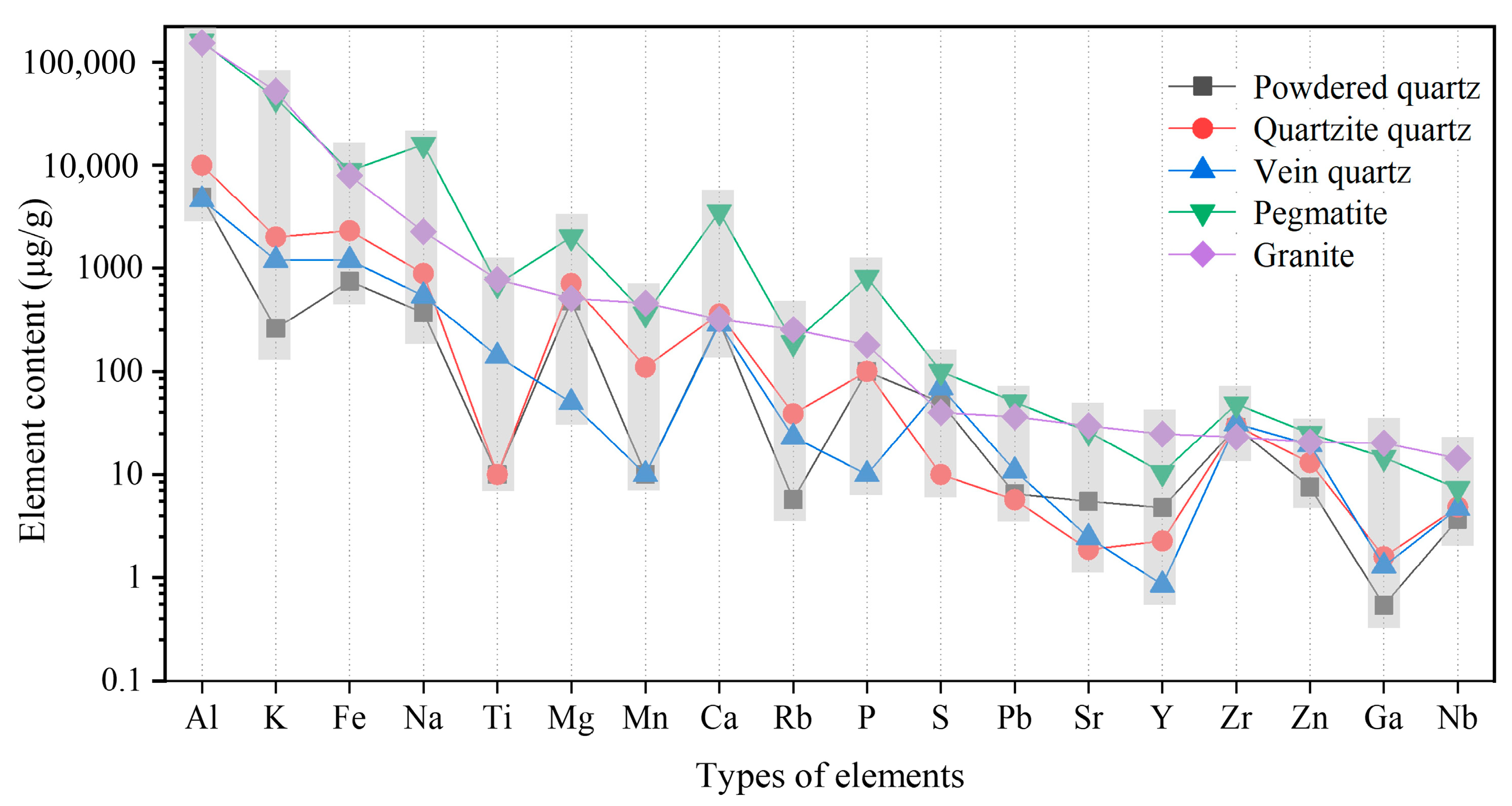
Figure 3. Comparison of impurity element content in different types of quartz ore.
Among the five selected types of quartz ores, the SiO2 content ranks from highest to lowest as follows: powder quartz > vein quartz > quartzite > granite > pegmatite, with respective contents of 99.08%, 98.83%, 98.14%, 74.10%, and 73.46%. As depicted in Figure 3, the primary impurity elements in these quartz samples include Al, Fe, K, and Na, with trace amounts of Ti, Mg, Mn, Ca, and S. Additionally, microelements such as Pb, Sr, Y, Zr, Zn, Ga, and Nb are present. Notably, the Rb content is relatively high in all quartz types except for powder quartz, reaching a maximum of 189 μg/g. The distribution characteristics of these trace elements can provide valuable insights for the exploration of high-purity quartz deposits.
Upon analyzing the specific values of Al and K element contents in Figure 3, it is evident that there is a positive correlation between the Al and K contents in the five different quartz types, indicating that the impurity minerals in the original ores belong to the same class of aluminosilicate minerals. The Al content is also positively correlated with Fe, suggesting the presence of iron minerals or iron elements within the aluminosilicate minerals. Furthermore, Al dominates the high-purity quartz lattice, with lattice Al accounting for an average of 70% of the total metal impurities in high-purity quartz. The higher the purity, the greater this proportion, reaching a maximum of 91%. This is a primary indicator for assessing product quality and purification efficiency.
2.3.2. Coexisting Gangue Minerals
In quartz ores, coexisting gangue minerals can generally be divided into two categories: (i) loosely associated minerals, that often exhibit relatively intact mineral crystals and no chemical bonding with quartz crystals, such as feldspar, mica, garnet, zircon and ilmenite; (ii) the second category consists of companion mineral fragments that chemically and physically bond to the surface of quartz crystals, primarily comprising iron-bearing minerals and aluminum-bearing minerals, such as iron pyrite and iron olivine [15].
Four types of raw ores, including quartzite, vein quartz, pegmatite, and granite, were crushed, ground, and screened to particle sizes of 0.1–0.2 mm. Subsequently, a magnetic separation process (with a magnetic field intensity of 1.2T) was applied to remove iron-bearing minerals. The pre-processed products were then subjected to mineral composition analysis using a BGRIMM Process Mineralogy Analyzer (BPMA, BGRIMM Technology Group, Beijing, China), which includes a TESCAN VEGA scanning electron microscope, a Bruker QUANTAX 200 dual-probe spectrometer, and automated analysis software for mineralogical processing (BMPA V2.0) [16]. The results are presented in Figure 4.
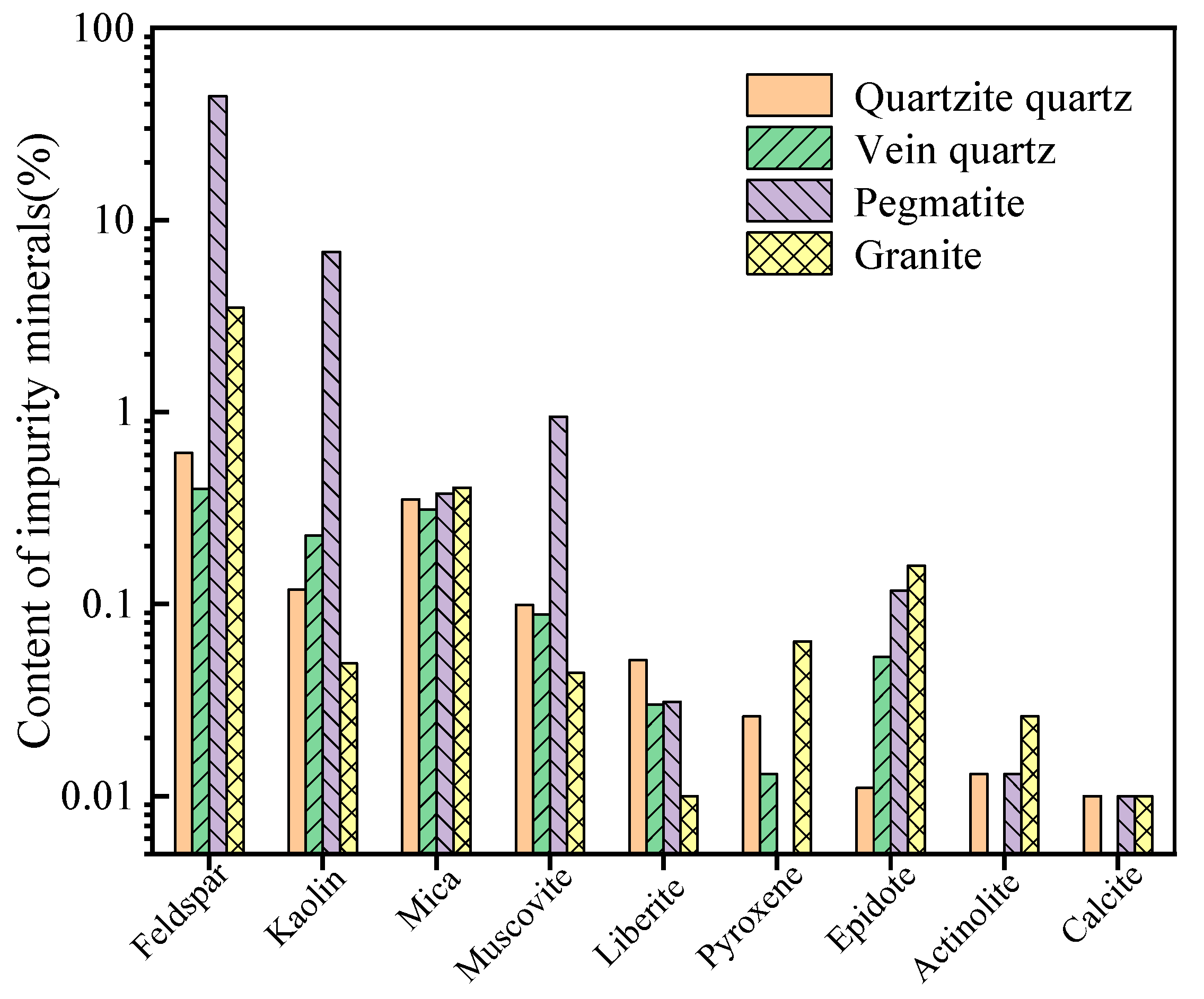
Figure 4. Comparison of impurity minerals content in different types of quartz ore.
As observed in Figure 4, the main impurity minerals include feldspar, amphibole, kaolin, and muscovite. Additionally, there are trace impurity minerals like liberite and epidote. It should be noted that the specific types of feldspar impurity minerals encompass potassium feldspar, sodium feldspar, albite, anorthoclase, and microcline. Similarly, the amphibole impurity minerals encompass riebeckite, blue amphibole, tremolite, and grunerite. The presence of minerals like actinolite, diopside, and augite, as well as calcite and rhodonite among trace impurities, depends on the type of ore.
2.3.3. Fluid Inclusions
Fluid inclusions are widely present in minerals, and their types and abundance depend on the petrogenetic environment and crystallographic variations of quartz minerals. Common types of fluid inclusions include gas phase, liquid phase, gas–liquid two-phase, and solid–liquid–gas three-phase inclusions, as illustrated in Figure 5 [17][18][19]. These fluid inclusions contain a significant quantity of impurities such as Na, K, Cl, and Ca, serving as the primary source of alkali metal impurities, while small molecular impurities are mainly found in gas and liquid phases. Fluid inclusions exhibit characteristics of fine-grain embedding, complex structural arrangements, diverse phase compositions, and high impurity element content. Therefore, the removal of impurity components constitutes crucial steps in most quartz raw material purification techniques. Schmidt-Mumm et al. suggest that the bursting of fluid inclusions can be categorized into the following processes: the expansion of micro-cracks, rupture of grain boundaries, trans-granular rupture, intragranular rupture, as well as the bursting of fluid inclusions [20]. It should be noted that the smaller the grain size of inclusions, the more challenging their removal becomes. For instance, a study conducted by Gaweł et al. indicated that −4 μm fluid inclusions within quartz crystals remain stable even at temperatures as high as 800 °C [21].

Figure 5. Photomicrograph of fluid inclusions in quartz. (a) Gas–liquid two-phase inclusions; (b) solid–liquid–gas three-phase inclusions.
2.3.4. Mineral Inclusions
Mineral inclusions refer to minerals that coexist with quartz during the quartz crystallization process, either within the crystal lattice, along crystal fractures, or on crystal surfaces. They can also form during the recrystallization of quartz minerals under metamorphic conditions. These inclusions primarily comprise micron-scale particles ranging from 1 to 10 μm, sub-micron particles in the range of 0.1 to 1 μm, impurities at the nanometer scale, and atomic clusters smaller than 1 nm [22]. Presently, research has predominantly focused on blue igneous rock quartz; however, the prevalence of sub-micron and nanometer-scale solid inclusions in natural quartz remains uncertain [23][24]. Taking granite quartz as an example, the morphology of micron-scale mineral inclusions can be observed under a microscopy (ZEISS Axioscope 5), as illustrated in Figure 6.

Figure 6. Micron-scale mineral inclusions in granite quartz. (a) Coarse-grained feldspar mineral inclusions (with a red circle) observed in plane-polarized light; (b) fine-grained mica mineral inclusions (with a red circle) observed in crossed-polarized light.
Given that mineral inclusions are predominantly situated within quartz mineral particles, their extraction poses a significant challenge. A primary contributing factor to this challenge is the strict particle size requirements in the production of high-end, high-purity quartz. For instance, products intended for photovoltaic materials demand a particle size range of 0.1 to 0.6 mm, making it unfeasible to overgrind the ore in an attempt to expose mineral inclusions. Therefore, further development of removal processes for sub-micron and nanometer-scale solid inclusions within quartz is of utmost importance.
2.4. Structural Impurities in Quartz Minerals
2.4.1. Lattice Impurities
Structural impurities in quartz lattice can be primarily categorized into two major classes: (i) lattice substitution impurities, such as Al3+, Fe3+, B3+, Ti4+, and P5+ atoms that act as isomorphic substitutes for the Si4+ atoms within the silica tetrahedra; (ii) charge-compensating impurities. Due to internal charge imbalances in the lattice, ions such as Li+, K+, Na+, H+, and Fe2+ enter the lattice to form interstitial atoms, residing within the voids of the silica tetrahedral framework [6]. Furthermore, although germanium is naturally scarce; the common occurrence is the isovalent substitution of Ge4+ for Si4+ [25]. These impurities are also prone to diffusing both out of and into the quartz crystal. The specific combinations are illustrated in Figure 7 [26]. It is crucial to recognize that, owing to disparities in charges and ionic radii, only a restricted number of ions can effectively substitute for Si4+ in the crystal lattice or be integrated into interstitial positions. As for the majority of other trace impurity elements (e.g., Ca, Mg, Mn, Sr, Ga, Zr, and Rb), they tend to exhibit a higher concentration within fluid inclusions [25].
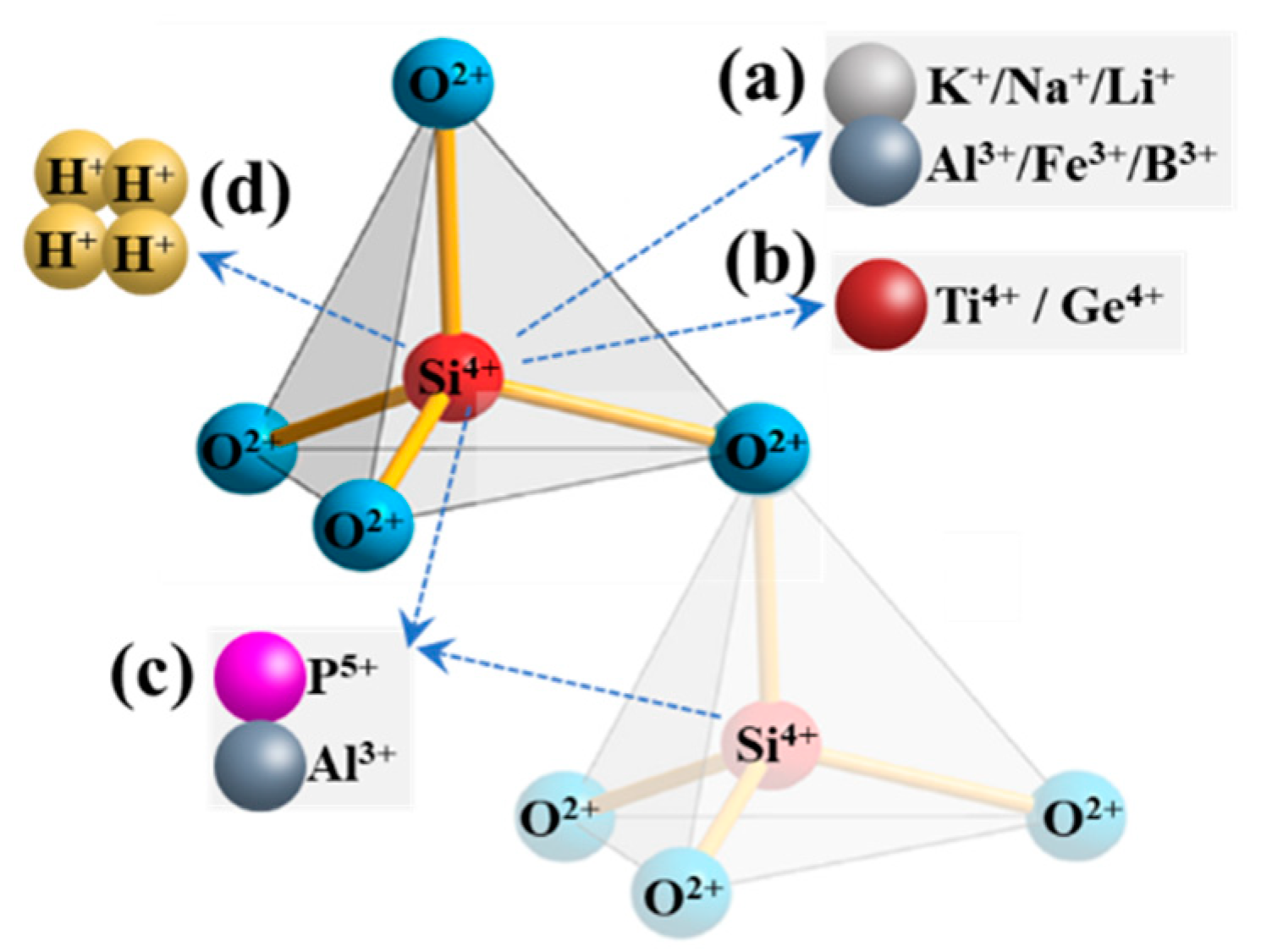
Figure 7. Diagram illustrating the forms of structural impurities in a quartz lattice. (a) Trivalent cation M3+ substituting Si4+; (b) tetravalent cation M4+ substituting Si4+; (c) different ion pairings replacing Si4+; (d) H+ substituting Si4+.
Unlike most other minerals, impurities in quartz crystals tend not to be uniformly distributed throughout the crystal. Within just a few micrometers, impurity concentrations can vary by an order of magnitude. Generally, in accordance with the principle of charge balance, the content of monovalent cations is similar to the content of trivalent cations. Recently, Götze et al. summarized impurity elements distributions in quartz of different origins: (i) Li+ appears to be the dominant charge-compensating ion in igneous and pegmatite, preferentially bonding with structural channels to balance the charge of Al; (ii) the highest Ti contents are typically observed in volcanic and igneous rocks; (iii) the distribution of P is unstable, with slight enrichment observed in granite and pegmatite; (iv) Fe is predominantly present in the marginal zones or damaged areas of quartz crystals [25]. Due to the inherent difficulties in treating lattice impurities using conventional refining techniques, impurity removal processes are both intricate and costly. Therefore, quartz deposits with lower lattice impurity content hold greater potential for producing high-purity quartz.
2.4.2. Hydroxyl Impurities
Hydroxyl impurities are also the target for removal in the production process of ultra-high purity quartz. This is because, although hydrogen is not typically considered an impurity in natural quartz, it can combine with hydroxyl groups to form molecular water (approximately 10 μg/g) [27]. This, in turn, reduces the fusion temperature of quartz and impacts its optical properties. The distribution of hydroxyl groups in quartz is generally non-uniform, with the highest concentrations commonly found at the crystal center. Additionally, hydroxyl groups may be present at crystal interfaces, defects, or within inclusions [27].
Biró et al. conducted an analysis of hydroxyl concentrations in quartz phenocrysts from several rhyolites using non-polarized micro-FTIR and SEM cathodoluminescence imaging. Their results indicated that the hydroxyl content in volcanic quartz (approximately 2.8 μg/g) was lower than that in granite, metamorphic rock, and hydrothermal quartz [28]. Gaweł et al. demonstrated that when heated to temperatures above 600 °C, liquid water at grain boundaries was removed. Further heating beyond 900 °C resulted in the vanishing of water existing in sealed fluid inclusions [29]; however, eliminating the remaining isolated surface -OH groups is quite challenging. Recently, Stalder conducted a comprehensive review on the incorporation of OH point defects in quartz, assessing their relevance to natural samples and geological processes. The study revealed that OH incorporation was influenced by the availability of trace metals (such as Li and Al) and protons. Furthermore, it was observed that pressure negatively affected the formation of OH defects, suggesting that the purest quartz formations likely occurred in the deep crust, close to the quartz/coesite transition [27]. In summary, when producing high-purity quartz, it is advisable to prioritize raw materials with low levels of structural and inclusion impurities.
References
- Zhang, L.; Lin, X. Research on preparation of High-purified quartz micropowder and quartz glass sand from quartz rock. Non-Met. Mines 2005, 28, 37–39.
- The National Bureau of Statistics. Strategic Emerging Industries Classification; National Bureau of Statistics Order No. 23; The National Bureau of Statistics: Beijing, China, 2018.
- Wang, J. Global high purity quartz deposits: Resources distribution and exploitation status. Acta Petrol. Et Mineral. 2021, 1, 131–141.
- China Industrial Research Network. Research Report on Comprehensive Investigation and Future Trends Analysis of China’s High-Purity Quartz Sand Industry from 2020 to 2026; China Industrial Research Network: Shanghai, China, 2020.
- Götze, J. Chemistry, textures and physical properties of quartz-geological interpretation and technical application. Mineral. Mag. 2009, 73, 645–671.
- Pan, X.; Li, S.; Li, Y.; Guo, P.; Zhao, X.; Cai, Y. Resource, characteristic, purification and application of quartz: A review. Miner. Eng. 2022, 183, 107600.
- Lin, M.; Liu, Z.; Wei, Y.; Liu, B.; Meng, Y.; Qiu, H.; Li, Y. A critical review on the mineralogy and processing for high-grade quartz. Min. Metall. Explor. 2020, 37, 1627–1639.
- Lin, M.; Lei, S.; Pei, Z.; Liu, Y.; Xia, Z.; Xie, F. Application of hydrometallurgy techniques in quartz processing and purification: A review. Metall. Res. Technol. 2018, 115, 303.
- Li, P. Geological characteristics of the non-metallic mineral resource: Powdered quartz deposits. Reg. Gov. 2019, 277, 83–85.
- Ma, C.; Feng, A.; Liu, C.; Shao, W.; Zhao, P. Mineralogical Characteristics and Progress in Processing Technology of Raw Materials of High Purity Quartz. Conserv. Util. Miner. Resour. 2019, 39, 48–57.
- Vatalis, K.I.; Charalambides, G.; Benetis, N.P. Market of high purity quartz innovative applications. Procedia Econ. Financ. 2015, 24, 734–742.
- London, D. Ore-forming processes within granitic pegmatites. Ore Geol. Rev. 2018, 101, 349–383.
- Müller, A.; Ihlen, P.M.; Wanvik, J.E.; Flem, B. High-purity quartz mineralisation in kyanite quartzites, Norway. Miner. Depos. 2007, 42, 523–535.
- Larsen, R.B.; Polvé, M.; Juve, G. Granite pegmatite quartz from Evje-Iveland: Trace element chemistry and implications for the formation of high-purity quartz. Nor. Geol. Unders. 2000, 436, 57–66.
- Götze, J.; Möckel, R.; Pan, Y. Mineralogy, Geochemistry and Genesis of Agate—A Review. Minerals 2020, 10, 1037.
- Wang, M.; Cai, L.; Wen, J.; Li, W.; Yang, X.; Yang, H. The Prospect of Recovering Vanadium, Nickel, and Molybdenum from Stone Coal by Using Combined Beneficiation and Metallurgy Technology Based on Mineralogy Features. Minerals 2022, 13, 21.
- Kendrick, M.A.; Burnard, P. Noble Gases and Halogens in Fluid Inclusions: A Journey Through the Earth’s Crust. In The Noble Gases as Geochemical Tracers. Advances in Isotope Geochemistry; Burnard, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2013.
- Zhu, Z.; Yin, W.; Wang, D.; Sun, H.; Chen, K.; Yang, B. The role of surface roughness in the wettability and floatability of quartz particles. Appl. Surf. Sci. 2020, 527, 146799.
- Cai, W.; Liu, X.; Zhang, Z.; Gao, J.; Lei, M.; Cui, Q.; Ma, M.; Li, Y.; Song, Y. Genesis of the Yi’nan Tongjing Gold–Copper Skarn Deposit, Luxi District, North China Craton: Evidence from Fluid Inclusions and H–O Isotopes. Minerals 2023, 13, 1348.
- Schmidt-Mumm, A. Low frequency acoustic emission from quartz upon heating from 90 to 610 °C. Phys. Chem. Miner. 1991, 17, 545–553.
- Gaweł, B.A.; Ulvensøen, A.; Łukaszuk, K.; Muggerud, A.M.F.; Erbe, A. In situ high temperature spectroscopic study of liquid inclusions and hydroxyl groups in high purity natural quartz. Miner. Eng. 2021, 174, 107238.
- Müller, A.; Koch-Müller, M. Hydrogen speciation and trace element contents of igneous, hydrothermal and metamorphic quartz from Norway. Mineral. Mag. 2009, 73, 569–583.
- Yang, B.; Fu, Y.; Yin, W.; Sheng, Q.; Zhu, Z.; Yin, X. Selective collection performance of an efficient quartz collector and its response to flotation separation of malachite from quartz. Miner. Eng. 2021, 172, 107174.
- Müller, A.; Lennox, P.; Trzebski, R. Cathodoluminescence and micro-structural evidence for crystallisation and deformation processes of granites in the Eastern Lachlan Fold Belt (SE Australia). Contrib. Mineral. Petrol. 2002, 143, 510–524.
- Götze, J.; Pan, Y.; Müller, A. Mineralogy and mineral chemistry of quartz: A review. Mineral. Mag. 2021, 85, 639–664.
- Li, Y.; Ma, Q.; Xia, Z.; Li, W.; Lei, S. Influences of Na2CO3 roasting and H3PO4 hot-pressure leaching on the purification of vein quartz to achieve high-purity quartz. Hydrometallurgy 2023, 218, 106065.
- Stalder, R. OH point defects in quartz—A review. Eur. J. Mineral. 2021, 33, 145–163.
- Biró, T.; Kovács, I.J.; Király, E.; Falus, G.; Karátson, D.; Bendő, Z.; Sándorné, J.K. Concentration of hydroxyl defects in quartz from various rhyolitic ignimbrite horizons: Results from unpolarized micro-FTIR analyses on unoriented phenocryst fragments. Eur. J. Mineral. 2016, 28, 313–327.
- Gaweł, B.A.; Ulvensøen, A.; Łukaszuk, K.; Arstad, B.; Muggerud, A.M.F.; Erbe, A. Structural evolution of water and hydroxyl groups during thermal, mechanical and chemical treatment of high purity natural quartz. RSC Adv. 2020, 10, 29018–29030.
More
Information
Subjects:
Mining & Mineral Processing
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
3.7K
Revisions:
2 times
(View History)
Update Date:
12 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




