Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Toshiro Ito | -- | 2717 | 2023-12-12 07:42:20 | | | |
| 2 | Peter Tang | + 1 word(s) | 2718 | 2023-12-12 08:43:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Wang, X.; Tan, N.W.K.; Chung, F.Y.; Yamaguchi, N.; Gan, E.; Ito, T. Transcriptional Regulators of Plant Adaptation to Heat Stress. Encyclopedia. Available online: https://encyclopedia.pub/entry/52602 (accessed on 07 February 2026).
Wang X, Tan NWK, Chung FY, Yamaguchi N, Gan E, Ito T. Transcriptional Regulators of Plant Adaptation to Heat Stress. Encyclopedia. Available at: https://encyclopedia.pub/entry/52602. Accessed February 07, 2026.
Wang, Xuejing, Nicholas Wui Kiat Tan, Fong Yi Chung, Nobutoshi Yamaguchi, Eng-Seng Gan, Toshiro Ito. "Transcriptional Regulators of Plant Adaptation to Heat Stress" Encyclopedia, https://encyclopedia.pub/entry/52602 (accessed February 07, 2026).
Wang, X., Tan, N.W.K., Chung, F.Y., Yamaguchi, N., Gan, E., & Ito, T. (2023, December 12). Transcriptional Regulators of Plant Adaptation to Heat Stress. In Encyclopedia. https://encyclopedia.pub/entry/52602
Wang, Xuejing, et al. "Transcriptional Regulators of Plant Adaptation to Heat Stress." Encyclopedia. Web. 12 December, 2023.
Copy Citation
Heat stress (HS) is becoming an increasingly large problem for food security as global warming progresses. As sessile species, plants have evolved different mechanisms to cope with the disruption of cellular homeostasis, which can impede plant growth and development.
heat stress
transcription factor
epigenetics
histone modification
protein homeostasis
ROS homeostasis
1. Introduction
1.1. Global Warming and Its Impact on Plants
Climate change describes the gradual shift in average temperature and weather patterns over time, which is occurring worldwide. Such a phenomenon is primarily attributed to human activities, with burning fossil fuels and deforestation being the top two causal factors contributing to greenhouse gas emissions. The severity of heat stress due to global warming is expected to bring about catastrophic disruptions to the world food supply, with tropical regions experiencing the most significant rise in temperature over time [1]. Climate change is also predicted to impact agricultural productivity, including staple crops such as maize and wheat [2]. Climate models forecast that up to ten times the number of crops will be damaged by the end of the century due to rising global temperatures [3].
Elevated temperature causes heat stress (HS), an abiotic stress to plant growth and development, thereby influencing crop yield. In addition to perturbation of plant growth and development, HS impacts biological processes and metabolic pathways, including enzymatic activity, protein folding, and lipid oxidation.
1.2. Thermotolerance in Plants
Despite the detrimental effects of HS, most plants are capable of adapting to moderate heat. According to the HS temperature regimes and developmental stages of the plants examined, there are various types of thermotolerance in plants that can be attained through inherent resistance and acquired thermotolerance (AT) [4][5]. There are typically three categories of organismal thermotolerance in response to various HS events in Arabidopsis, comprising basal thermotolerance to HS at temperatures ranging from 40 to 45 °C, AT following a short period of nonlethal temperature or priming HS, and thermotolerance to prolonged exposure to moderately high temperatures between 30 and 38 °C [4]. A series of molecular and cellular responses is triggered upon sensing heat stimulus. Mechanistic differences as well as overlapping but distinct molecular and cellular responses exist among these three modes of thermotolerance [4]. Crosstalk among transcription factors (TFs), such as heat shock factors or heat stress transcription factors (HSFs), regulatory RNAs, and epigenetic regulators, induces physiological responses during HS [5].
Generally, plants adapt to HS via HSF- and heat shock protein (HSP)-mediated heat stress responses (HSRs). Understanding the underlying mechanisms of plant cell activities in response to HS, especially transcriptional regulation activity, is essential for safeguarding crop resilience and the development of heat-tolerant crop varieties.
2. Transcriptional and Epigenetic Regulation for Adaptation to HS
2.1. Plant Heat Stress Transcription Factors: Classes A, B, and C
Transcriptional regulation of genes involved in plant heat stress responses is guarded by HSFs (Figure 1). Plant HSFs, consisting of Classes A, B and C, share evolutionarily conserved core transcription regulators. The diversity and functions of HSFs were reviewed by Koskull-Doring et al. [6]. The functional domains of HSFs consist of a DNA-binding domain at the N-terminal, followed by several hydrophobic amino acid residues essential for HSF oligomerization, basic amino acid residues for nuclear localization, the nuclear export signal (NES) at the C-terminus and short peptide motifs for transcriptional activation of HSFs. Shuttling of HSFs between the cytoplasm and the nucleus is regulated by the nuclear localization signal and NES at the C-terminus of class A HSFs. Activation motifs or aromatic and hydrophobic amino acid residues (AHAs) located proximal to the NES are crucial for transcriptional activation via interaction with other transcriptional elements. Transcriptional, posttranscriptional, and protein modifications give rise to the structural and functional diversification of HSFs [7].
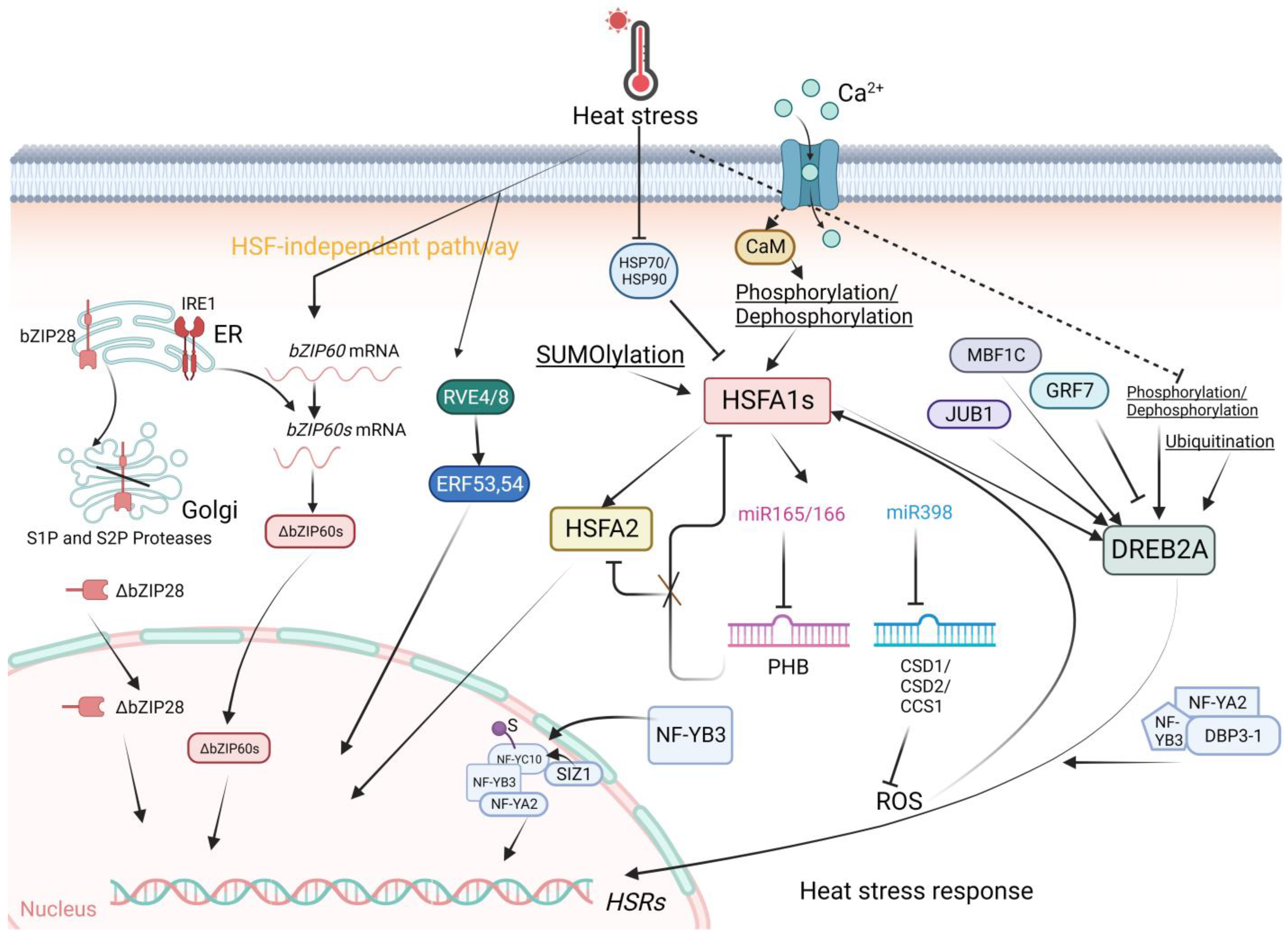
Figure 1. The Heat Stress Response in Arabidopsis. HSFA1s function as central regulators orchestrating plant responses to heat stress (HS). HSFA1 expression is induced by heat, and HSFA1 activity is precisely modulated by various factors. Upon heat stress, HSFA1 is released from HSP70 and HSP90, leading to HSFA1 activation. In addition, the increases of cytoplasmic Ca2+ levels mediated by the Ca2+ channels, CNGCs, triggered by HS may be important for HSFA1 activation. Post-translational modifications, such as phosphorylation/SUMOylation/Ubiquitination regulate the activity of HSFA1 and DREB2A. HSFA2 as a target of HSFA1s is an important regulator of the expression of HSR genes through sustained H3K4 methylation. The miR398 inhibits the expression of ROS scavenger genes CSD1, CSD2, and CCS1, thereby promoting ROS accumulation, which subsequently activates HSFA1s. The miR165/166–PHB module regulates thermotolerance through at least two pathways: one involving direct transcriptional regulation of HSFs, with PHB modulating HSR transcription in an HSFA1-dependent manner; and another HSFA1-independent pathway, where PHB directly regulates the transcription of heat-inducible HSFA2. Additionally, PHB physically interacts with HSFA1s, influencing their transcriptional function. DREB2A is positively regulated by MBF1C and JUB1 in response to HS, whereas it is negatively regulated by GRF7 under normal conditions. In addition, DREB2A activity is enhanced by the NF-YA2/NFYB3/DBP3-1 complex. During HS, SIZ1 facilitates the SUMOylation of NF-YC10. The SUMO conjugation on NF-YC10 enhances its association with NF-YB3 via a SUMO-SIM interaction and improves the nuclear translocation of NFYB3. In the nucleus, the NF-YC10–NF-YB3 dimer binds to NF-YA2 to form an NF-YC trimeric complex to promote the transcription of HS-responsive genes. HS triggers the ER-localized transcription factors bZIP60 and bZIP28 to translocate into the nucleus and activate HSR expression. The circadian clock proteins RVE4 and RVE8 also can induce HSR gene expression in an HSFA1-independent way. Created with BioRender.com; accessed on 31 July 2023.
At normal temperatures, HSPs expressed at basal levels bind to HSFs to prevent them from activating the expression of heat-responsive genes and increasing the transcription of various HSP-encoding genes [8]. When stimulated by heat stress, denatured and misfolded proteins are sensed by HSPs. Misfolded proteins bind to HSPs, thereby releasing HSFs for activation of heat stress responses. Transcriptional profiling of HSFs revealed their critical roles in the upregulation of other stress-inducible genes under various biotic/abiotic (pathogen, wound, cold, drought, heavy metal, light, pH, oxidative, salinity) stresses and developmental/physiological (flowering, apoptosis, circadian rhythm) conditions. The overlapping of different stress response profiles suggests intricate crosstalk in multiple networks and pathways. In-depth reviews of the structure and function of HSFs have been conducted [9][10].
The class A members of HSFs mainly function to activate HSRs [11][12]. In primary HSRs, HSFA1s (including HSFA1a, HSFA1b, HSFA1d, and HSFA1e), HSFA2, and HSFA3 play pivotal roles as class A members [13]. HSFA1s serve as the most important transcriptional activators of HSR gene expression [14][15]. HSFA1s function as master regulators of HS-regulated gene expression in plants because they can further bind to other TFs [16][17]. For instance, under ambient temperature, HSP70 and HSP90 repress HSFA1 activity through direct protein interactions. The interaction negatively regulates HSFA1 activity by repressing transcription activation and nuclear localization [18]. Under HS, direct protein interactions activate a series of downstream target genes, including DEHYDRATION-RESPONSIVE ELEMENT BINDING 2A (DREB2A), HSFA2, HSFA7A, HSFA7B, and MBF1C, releasing these TFs from the cytoplasm to nuclei for transcription activation and expression of heat-induced genes [18]. HSFB1, HSFB2A, and HSFB2B can also be induced by HSFA1. Notably, HSFB1 and HSFB2B function as transcriptional repressors. Negative regulation of HSFA2 and HSFA7A expression acts as a modulator of the action of class A HSFs [19]. Interestingly, a recent study reported that by investigating genome-wide chromatin changes related to the transcriptional reprogramming response to HS in tomato, HSFA1a-mediated chromatin reorganization likely drives the expression of HS-responsive genes [20]. Heat stress induces rapid changes in chromatin architecture, leading to the transient formation of promoter enhancer contacts, thereby inducing HSRs [20]. In addition, the activity of HSFA1 is tightly controlled by protein modification. This explains why the impact of the overexpression of HSFA1 on the upregulation of HS-inducible genes is relatively limited [18][21] compared with the overexpression of other HS-inducible HSFAs, such as HSFA2 and HSFA3 [13][18][22][23]. Posttranslational regulation, such as phosphorylation and SUMOylation, plays a major role in the regulation of HSFA1 activity.
To date, the understanding of class C HSFs remains unclear. The highly conserved DNA-binding domains of class C HSFs imply strong evolutionary pressure for functional conservation. In contrast, variabilities in the OD and AHA domains encourage functional divergence of HSFs. For instance, the expanded HSFC family observed in monocots is hypothesized to carry out regulatory activities in response to stressors, developmental processes, and fine-tuning of gene expression [24].
2.2. Other Transcriptional Regulators
Other TF families that also play roles in the HS response include the DREB2A, DREB2C, MULTIPROTEIN BRIDGING FACTOR 1C (MBF1C), NAM/ATAF1/2/CUC2 (NAC), WRKY, basic REGION/LEUCINE ZIPPER (bZIP), and MYB families (Figure 1). Increasing amounts of data are indicating that the AP2/ERF family DREB2A is another important regulator under HS [25]. As part of HSFA1 activation, the expression of DREB2A is stimulated [13]. Then, DREB2A directly activates HSFA3 expression which functions as a heat-induced gene in acquiring thermotolerance and/or heat stress resistance. Moreover, VASCULAR PLANT ONE-ZINC-FINGER PROTEIN (VOZ1) from the NAC family inhibits the activity of DREB2C, thereby abolishing the downstream activities of HSFA3 [26]. Interestingly, DREB2A functions in the crosstalk between heat and drought stress signaling, indicating its specific roles in abiotic stress. DREB2A can be induced by JUNGBRUNNEN 1 (JUB1) and MBF1C in addition to HSFA1s under heat stress [27][28][29]. In contrast, the expression of DREB2A is repressed by GROWTH-REGULATING FACTOR 7 (GRF7) under nonstress conditions [30]. Posttranslational regulation processes, such as phosphorylation and SUMOylation, also play important roles in the regulation of DREB2A activity [31].
NUCLEAR FACTOR-Y (NF-Y) family members are central regulators of HS-responsive transcription in plant cells as well [32]. A recent study showed that the SUMO E3 ligase SAP AND MIZ1 DOMAIN-CONTAINING LIGASE1 (SIZ1) interacts with NF-YC10 and enhances its SUMOylation during HS. The SUMOylation of NF-YC10 facilitates its interaction with, and the nuclear translocation of, NF-YB3. After translocation to the nucleus, the NF-YC10/NF-YB3 dimer binds to NF-YA2 to form an NF-YC trimeric complex to promote the transcription of HS-responsive genes, thereby improving heat tolerance in plants [32]. Moreover, a previous study reported that NF-YB3 translocates to the nucleus and couples with DNA POLYMERASE II SUBUNIT B3-1 (DPB3-1) to form a trimer with NF-YA2, thereby promoting DREB2A activity under heat stress [33][34].
2.3. Epigenetic Regulation in the HSRs
Multilayered regulatory systems under HS include epigenetic regulation [31]. Epigenetic regulation—involving DNA methylation, histone modification, and chromatin remodeling—is stimulated upon HS. The molecular mechanisms underlying AT have been studied and are mainly related to epigenetic regulation, especially histone modification, which modulates transcriptional activity through either “open” or “closed” chromatin configurations [35]. Furthermore, histone modifications function in AT by exploiting the involvement of TFs.
Multiple studies have indicated that HSFA2 is a master regulator of AT [36][37] (Figure 2). The maintenance of HSP gene expression by HSFA2 prolongs AT. One recent study showed that HSFA2 recruits histone methyltransferases to memory loci and leads to deposition of di- and trimethylation of histone H3 lysine 4 (H3K4me2/3). This kind of histone modification induces sustained expression of various types of heat memory genes, which are specifically required for AT in Arabidopsis [38]. After a nonlethal priming HS, H3K4me2/3 hallmarks the memory loci as recently transcribed loci, enabling these memory loci to be rapidly or more strongly re-induced (i.e., refer to a kind of transcriptional memory) by subsequent lethal HS [38]. Furthermore, it has been reported that HSFA2 can assemble into a heteromeric HSF complex alongside HSFA3/FORGETTER3 (FGT3), which subsequently triggers the expression of memory genes, thereby orchestrating AT [37]. However, it is unclear what distinguishes memory from nonmemory genes. A recent study identified the global target genes of these two key memory HSFs, HSFA2 and HSFA3, using time course Chromatin Immunoprecipitation-sequencing (ChIP-seq), and HSFA2 and HSFA3 showed nearly identical binding patterns [38]. In vitro and in vivo binding strength is highly correlated, indicating the importance of DNA sequence elements. In particular, genes with transcriptional memory are strongly enriched for a tripartite heat shock element (HSE) and are hallmarked by three features: low expression levels under normal conditions, accessible chromatin environment, and heat stress-induced enrichment of H3K4 trimethylation [39].
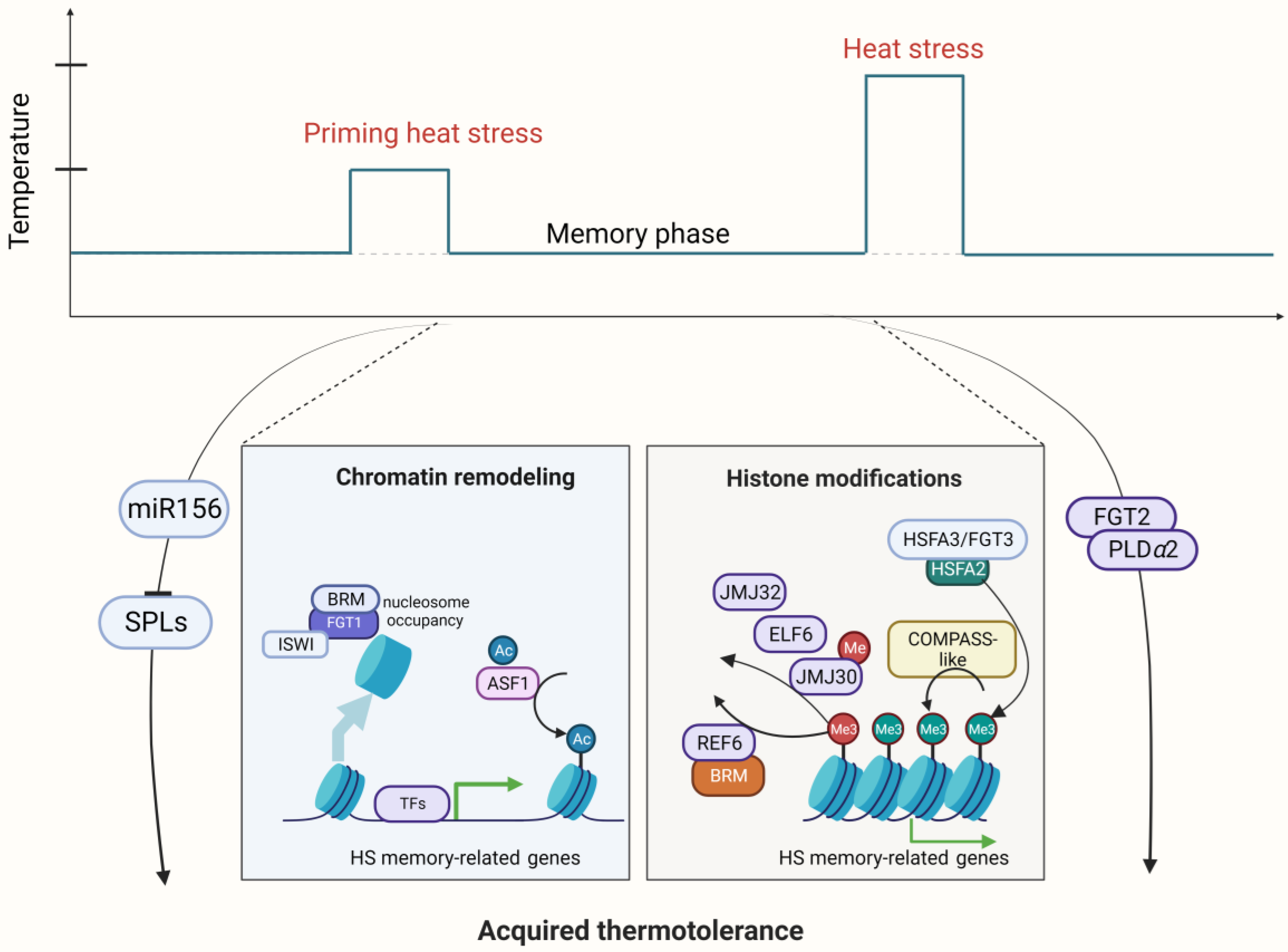
Figure 2. Schematic representation of regulators involved in Acquired thermotolerance (AT) in Arabidopsis. A mild heat stress (HS) can act as a priming cue and trigger enhanced tolerance to HS in the primed state. This primed state is maintained over time in a memory phase so that primed plants that encounter a second severe stress event are able to survive in contrast to nonprimed plants. Priming HS activates the expression of HSFA2 through HSFA1s, which are released from the HSP chaperone under priming HS and enter the nucleus. HSFA2 forms a heteromeric transcription factor complex with HSFA3, which activates the expression of HS memory genes or heat stress response (HSR) genes. Histone modifications play a critical role to regulate HS memory genes, including H3K27 demethylation by REF6, ELF6, JMJ30, and JMJ32; H3K4 trimethylation by COMPASS-like; and nucleosome positioning by the ATP-dependent chromatin remodeler complex consisting of FGT1, BRM, and ISWI. In addition, the FGT2/phosphatase-PLDa2/phospholipase complex and the miR156-SPL module are involved in the regulation of thermomemory genes in Arabidopsis. Created with BioRender.com; accessed on 31 July 2023.
Nucleosome dynamics through chromatin remodeling are also related to AT. The helicase FGT1 associates with the ATP-dependent chromatin remodelers SWI/SNF (BRM) and Imitation Switch (ISWI) (CHROMATIN-REMODELING PROTEIN 11 (CHR11) and CHR17) to reduce nucleosome abundance at memory loci in the phase of memory after priming HS [40]. The removal of nucleosomes facilitates the sustained activation of memory genes and thus confers AT in Arabidopsis [40].
In summary, the chromatin-based histone modification for AT regulation primarily encompasses HSFA2-mediated H3K4 hyper-trimethylation, JMJ demethylase-dependent H3K27 demethylation, ASF1-mediated H3K56ac, and FGT1-BRM/CHR11/CHR17-dependent nucleosome positioning, and these mechanisms collectively contribute to the modulation of AT in land plants [41]. The effect of AT is usually erased in several days. The precise mechanism underlying the deposition and removal of these histone-modifying proteins in the process of AT remains to be understood.
2.4. Regulatory RNA (microRNA, siRNA, lncRNA, and circRNA)
Much involvement of regulatory RNA has been documented when plants have been subjected to heat stimulus (Figure 1). The regulatory activities of TFs or genes are attributed to noncoding RNAs, including microRNAs, small interfering RNAs (siRNAs), long noncoding RNAs (lncRNAs), and circular RNAs (circRNAs). MicroRNAs are small noncoding RNAs that regulate target messenger RNA (mRNA) through degradation or translational repression of mRNA. Gene expression is negatively regulated, thereby inhibiting translation. miR398 acts downstream of HSFA7b and HSFA1s during HS [42].
As mentioned above, ONSEN responds to HS as a target of HSFA1. Moreover, a siRNA-mediated pathway regulates ONSEN activity. Inhibitory action by siRNA on ONSEN yields upregulation of heat-induced gene expression. Apart from interaction with ONSEN, HSFA1s induce thermotolerance by binding at promoters, triggering transcriptional activation of the HEAT-INDUCED TAS1 TARGET 1 (HTT1) and HTT2 genes. In addition to the influence of HSFA1s, the expression of HTT1 and HTT2 genes is also garnered by trans-acting siRNA (TAS1). The natural antisense transcript siRNA (nat-siRNA) contributes to heat resistance by negatively regulating HTT1 and HTT2. CircRNAs are single-stranded RNAs that join head-to-tail in a circular form. They are reported to play a role in the regulation of the plant HS response, synergistic with plant hormone signal transduction [43].
3. Other Cell Activities for Adaptation to HS
Exposure of plant cells to high temperatures results in cellular damage and can even lead to cell death. The damage can be ascribed to the action of misfolded proteins and reactive oxygen species (ROS), which accumulate during abiotic stresses such as heat stress. To protect cells, protein homeostasis and ROS homeostasis are essential. Thus, the researchers briefly review the recent study below about how plants maintain protein homeostasis and ROS homeostasis to cope with HS downstream of transcriptional regulation (Figure 3).
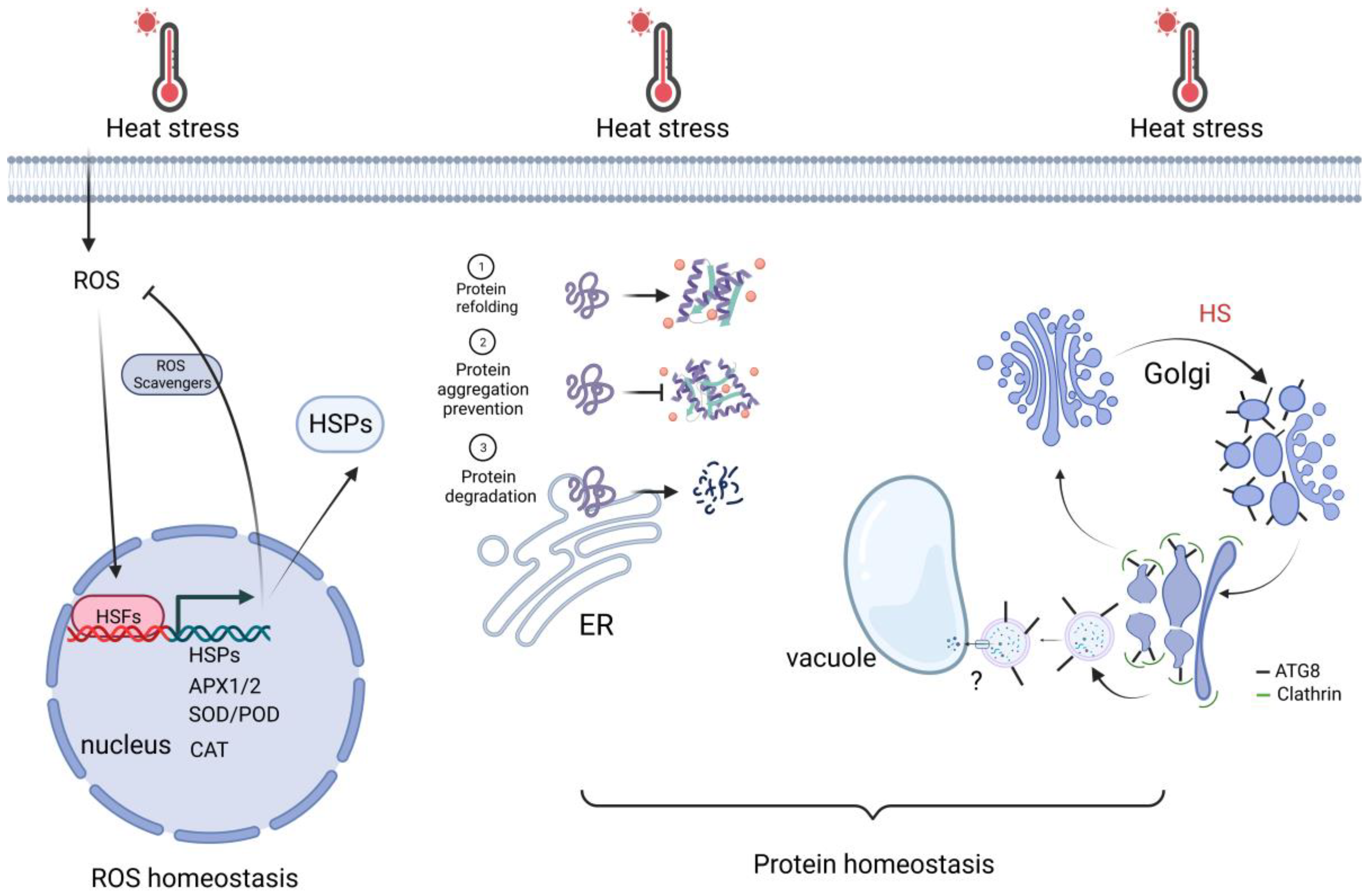
Figure 3. Cell activities for keeping ROS and protein homeostasis. Upon heat stress, nuclear redox oxidation is sensed by HSFs. HSFs activate HSR genes, which are important for ROS scavenging and protein homeostasis. In addition, autophagy-related (ATG) protein ATG8 is rapidly translocated to the sites of swelling Golgi bodies. It recruits the clathrin component CLC2 to mediate the vesicle budding, which fuses with the vacuole. It facilitates the reassembly of the Golgi apparatus, thereby increasing thermotolerance. Created with BioRender.com; accessed on 31 July 2023.
References
- Corlett, R.T. Climate change in the tropics: The end of the world as we know it? Biol. Conserv. 2012, 151, 22–25.
- Tao, F.L.; Zhang, S.A.; Zhu, Z. Changes in rice disasters across China in recent decades and the meteorological and agronomic causes. Reg. Environ. Chang. 2012, 13, 743–759.
- Miller, S.; Chua, K.; Coggins, J.; Mohtadi, H. Heat Waves, Climate Change, and Economic Output. J. Eur. Econ. Assoc. 2021, 19, 2658–2694.
- Yeha, C.H.; Kaplinsky, N.J.; Hu, C.; Charng, Y.Y. Some like it hot, some like it warm: Phenotyping to explore thermotolerance diversity. Plant Sci. 2012, 195, 10–23.
- Chen, Z.L.; Galli, M.; Gallavotti, A. Mechanisms of temperature-regulated growth and thermotolerance in crop species. Curr. Opin. Plant Biol. 2022, 65, 102134.
- Koskull-Doring, P.; Scharf, K.; Nover, L. The diversity of plant heat stress transcription factors. Trends Plant Sci. 2007, 12, 452–457.
- Guerra, D.; Crosatti, C.; Khoshro, H.H.; Mastrangelo, A.M.; Mica, E.; Mazzucotelli, E. Posttranscriptional and post-translational regulations of drought and heat response in plants: A spider’s web of mechanisms. Front. Plant Sci. 2015, 6, 57.
- Zhang, H.M.; Zhu, J.H.; Gong, Z.Z.; Zhu, J.K. Abiotic stress responses in plants. Nature 2022, 23, 104–119.
- Guo, M.; Liu, J.; Ma, X.; Luo, D.; Gong, Z.; Lu, M. The Plant Heat Stress Transcription Factors (HSFs): Structure, Regulation, and Function in Response to Abiotic Stresses. Front. Plant Sci. 2016, 7, 114.
- El-Shershaby, A.; Ullrich, S.; Simm, S.; Scharf, K.D.; Schleiff, E.; Fragkostefanakis, S. Functional diversification of tomato HsfA1 factors is based on DNA binding domain properties. Gene 2019, 714, 143985.
- Nover, L.; Bharti, K.; Döring, P.; Mishra, S.K.; Ganguli, A.; Scharf, K.D. Arabidopsis and the heat stress transcription factor world: How many heat stress transcription factors do we need? Cell Stress Chaperones 2001, 6, 77–189.
- Scharf, K.-D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. Biophys. Acta 2012, 1819, 104–119.
- Yoshida, T.; Ohama, N.; Nakajima, J.; Kidokoro, S.; Mizoi, J.; Nakashima, K.; Maruyama, K.; Kim, J.-M.; Seki, M.; Todaka, D.; et al. Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol. Genet. Genom. 2011, 286, 321–332.
- Mishra, S.K.; Tripp, J.; Winkelhaus, S.; Tschiersch, B.; Theres, K.; Nover, L.; Scharf, K. In the complex family of heat stress transcription factors, HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev. 2002, 16, 1555–1567.
- Albertos, P.; Dündar, G.; Schenk, P.; Carrera, S.; Cavelius, P.; Sieberer, T.; Poppenberger, B. Transcription factor BES1 interacts with HSFA1 to promote heat stress resistance of plants. EMBO J. 2022, 41, e108664.
- Liu, H.C.; Liao, H.T.; Charng, Y.Y. The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ. 2011, 34, 738–751.
- Castellanos, R.U.; Friedrich, T.; Petrovic, N.; Altmann, S.; Brzezinka, K.; Gorka, M.; Graf, A.; Bäurle, I. FORGETTER2 protein phosphatase and phospholipase D modulate heat stress memory in Arabidopsis. Plant J. 2020, 104, 7–17.
- Ohama, N.; Kusakab, K.; Mizoi, J.; Zha, H.; Kidokoro, S.; Koizumi, S.; Takahashi, F.; Ishida, T.; Yanagisawa, S.; Kazuo, S. The Transcriptional Cascade in the Heat Stress Response of Arabidopsis Is Strictly Regulated at the Level of Transcription Factor Expression. Plant Cell 2016, 28, 181–201.
- Lin, K.F.; Tsai, M.Y.; Lu, C.A.; Wu, S.J.; Yeh, C.H. The roles of Arabidopsis HSFA2, HSFA4a, and HSFA7a in the heat shock response and cytosolic protein response. Bot. Stud. 2018, 59, 15.
- Huang, Y.; An, J.; Sircar, S.; Bergis, C.; Lopes, C.D.; He, X.; Costa, B.D.; Tan, F.Q.; Bazin, J.; Antunez-Sanchez, J.; et al. HSFA1a modulates plant heat stress responses and alters the 3D chromatin organization of enhancer-promoter interactions. Nat. Commun. 2023, 14, 469.
- Prändl, R.; Hinderhofer, K.; Eggers-Schumacher, G.; Schöffl, F. HSF3, a new heat shock factor from Arabidopsis thaliana, derepresses the heat shock response and confers thermotolerance when overexpressed in transgenic plants. Mol. Gen. Genet. 1998, 258, 269–278.
- Yoshida, T.; Sakuma, Y.; Todaka, D.; Maruyama, K.; Qin, F.; Mizoi, J.; Kidokoro, S.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis heat-shock transcription factor HsfA3 in the transcriptional cascade downstream of the DREB2A stress-regulatory system. Biochem. Biophys. Res. Commun. 2008, 368, 515–521.
- Nishizawa, A.; Yabuta, Y.; Yoshida, E.; Maruta, T.; Yoshimura, K.; Shigeoka, S. Arabidopsis heat shock transcription factor A2 as a key regulator in response to several types of environmental stress. Plant J. 2006, 48, 535–547.
- Wang, X.M.; Shi, X.; Chen, S.Y.; Ma, C.; Xu, S.B. Evolutionary Origin, Gradual Accumulation and Functional Divergence of Heat Shock Factor Gene Family with Plant Evolution. Front. Plant Sci. 2018, 9, 71.
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406.
- Song, C.; Lee, J.; Kim, T.; Hong, J.C.; Lim, C.O. VOZ1, a transcriptional repressor of DREB2C, mediates heat stress responses in Arabidopsis. Planta 2018, 247, 1439–1448.
- Sakuma, Y.; Maruyama, K.; Qin, F.; Osakabe, Y.; Shinozaki, K.; Yamaguchi Shinozaki, K. Dual function of an Arabidopsis transcription factor DREB2A in water-stress-responsive and heatstress-responsive gene expression. Proc. Natl. Acad. Sci. USA 2006, 103, 18822–18827.
- Suzuki, N.; Sejima, H.; Tam, R.; Schlauch, K.; Mittler, R. Identification of the MBF1 heat-response regulon of Arabidopsis thaliana. Plant J. 2011, 66, 844–851.
- Wu, A.H.; Allu, A.D.; Garapati, P.; Siddiqui, H.; Dortay, H.; Zanor, M.I.; Asensi-Fabado, M.A.; Munne-Bosch, S.; Antonio, C.; Tohge, T.; et al. JUNGBRUNNEN1, a reactive oxygen speciesresponsive NAC transcription factor, regulates longevity in Arabidopsis. Plant Cell 2012, 24, 482–506.
- Kim, J.S.; Mizoi, J.; Kidokoro, S.; Maruyama, K.; Nakajima, J.; Nakashima, K.; Mitsuda, N.; Takiguchi, Y.; Ohme-Takagi, M.; Kondou, Y.; et al. Arabidopsis GROWTHREGULATING FACTOR7 functions as a transcriptional repressor of abscisic acid- and osmotic stress-responsive genes, including DREB2A. Plant Cell 2012, 24, 3393–3405.
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Network of Plant Heat Stress Response. Trends Plant Sci. 2017, 22, 53–65.
- Huang, J.W.; Huang, J.J.; Feng, Q.Y.; Shi, Y.Q.; Wang, F.G.; Zheng, K.Y.; Huang, Q.Z.; Jiang, J.M.; Luo, S.Y.; Xie, Y.; et al. SUMOylation facilitates the assembly of a Nuclear Factor-Y complex to enhance thermotolerance in Arabidopsis. J. Integr. Plant Biol. 2022, 65, 692–702.
- Sato, H.; Mizoi, J.; Tanaka, H.; Maruyama, K.; Qin, F.; Osakabe, Y.; Morimoto, K.; Ohori, T.; Kusakabe, K.; Nagata, M.; et al. Arabidopsis DPB3-1, a DREB2A interactor, specifically enhances heat stress-induced gene expression by forming a heat stressspecific transcriptional complex with NF-Y subunits. Plant Cell 2014, 26, 4954–4973.
- Sato, H.; Todaka, D.; Kudo, M.; Mizoi, J.; Kidokoro, S.; Zhao, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K. The Arabidopsis transcriptional regulator DPB3-1 enhances heat stress tolerance without growth retardation in rice. Plant Biotechnol. J. 2016, 14, 1756–1767.
- Shekhawat, K.; Almeida-Trapp, M.; García-Ramírez, G.X.; Hirt, H. Beat the heat: Plant- and microbe-mediated strategies for crop thermotolerance. Trends Plant Sci. 2022, 27, 802–813.
- Charng, Y.Y.; Liu, H.C.; Liu, N.Y.; Chi, W.T.; Wang, C.N.; Chang, S.H.; Wang, T.T. A Heat-Inducible Transcription Factor, HsfA2, Is Required for Extension of Acquired Thermotolerance in Arabidopsis. Plant Physiol. 2007, 143, 251–262.
- Friedrich, T.; Oberkofler, V.; Trindade, I.; Altmann, S.; Brzezinka, K.; Lämke, J.; Gorka, M.; Kappel, C.; Sokolowska, E.; Skirycz, A.; et al. Heteromeric HSFA2/HSFA3 complexes drive transcriptional memory after heat stress in Arabidopsis. Nat. Commun. 2021, 12, 3426.
- Lämke, J.; Brzezinka, K.; Altmann, S.; Bäurle, I. A hit-and-run heat shock factor governs sustained histone methylation and transcriptional stress memory. EMBO J. 2016, 35, 162–175.
- Kappel, C.; Friedrich, T.; Oberkofler, V.; Jiang, L.; Crawford, T.; Lenhard, M.; Bäurle, I. Genomic and epigenomic determinants of heat stress-induced transcriptional memory in Arabidopsis. Genome Biol. 2023, 24, 129.
- Brzezinka, K.; Altmann, S.; Czesnick, H.; Nicolas, P.; Gorka, M.; Benke, E.; Kabelitz, T.; Jahne, F.; Graf, A.; Kappel, C.; et al. Arabidopsis FORGETTER1 mediates stress-induced chromatin memory through nucleosome remodeling. eLife 2016, 5, e17061.
- Gao, Z.X.; Zhou, Y.; He, Y.H. Molecular epigenetic mechanisms for the memory of temperature stresses in plants. J. Genet. Genom. 2022, 49, 991–1001.
- Guan, Q.; Lu, X.; Zeng, H.; Zhang, Y.; Zhu, J. Heat stress induction of miR398 triggers a regulatory loop that is critical for thermotolerance in Arabidopsis. Plant J. 2013, 74, 840–851.
- Zhang, J.J.; Liu, R.Q.; Zhu, Y.F.; Gong, J.X.; Yin, S.W.; Sun, P.S.; Feng, H.; Wang, Q.; Zhao, S.J.; Wang, Z.Y.; et al. Identification and Characterization of circRNAs Responsive to Methyl Jasmonate in Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 792.
More
Information
Subjects:
Plant Sciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
842
Revisions:
2 times
(View History)
Update Date:
12 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




