You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joana M. Gil-Mohapel | -- | 2601 | 2023-12-11 19:58:56 | | | |
| 2 | Camila Xu | Meta information modification | 2601 | 2023-12-12 03:13:41 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Souza, P.B.D.; De Araujo Borba, L.; Castro De Jesus, L.; Valverde, A.P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Major Depressive Disorder and Gut Microbiota. Encyclopedia. Available online: https://encyclopedia.pub/entry/52593 (accessed on 23 December 2025).
Souza PBD, De Araujo Borba L, Castro De Jesus L, Valverde AP, Gil-Mohapel J, Rodrigues ALS. Major Depressive Disorder and Gut Microbiota. Encyclopedia. Available at: https://encyclopedia.pub/entry/52593. Accessed December 23, 2025.
Souza, Pedro Borges De, Laura De Araujo Borba, Louise Castro De Jesus, Ana Paula Valverde, Joana Gil-Mohapel, Ana Lúcia S. Rodrigues. "Major Depressive Disorder and Gut Microbiota" Encyclopedia, https://encyclopedia.pub/entry/52593 (accessed December 23, 2025).
Souza, P.B.D., De Araujo Borba, L., Castro De Jesus, L., Valverde, A.P., Gil-Mohapel, J., & Rodrigues, A.L.S. (2023, December 11). Major Depressive Disorder and Gut Microbiota. In Encyclopedia. https://encyclopedia.pub/entry/52593
Souza, Pedro Borges De, et al. "Major Depressive Disorder and Gut Microbiota." Encyclopedia. Web. 11 December, 2023.
Copy Citation
Major depressive disorder (MDD) is a multifactorial psychiatric disorder characterized by at least one depressive episode for a minimum of 2 weeks. The gut is the biggest digestive, immune and endocrine organ of the human body and can sometimes be referred to as the second brain. The gut is a microbial organ, and it is estimated that our gut harbors about 1014 microorganisms.
gut microbiota
major depressive disorder
physical exercise
1. Introduction
Major depressive disorder (MDD) is a multifactorial psychiatric disorder characterized by at least one depressive episode for a minimum of 2 weeks. The essential feature of a depressive episode is the presence of either a depressed mood or anhedonia accompanied by other neurocognitive and neurovegetative symptoms, such as diminished ability to think or concentrate and sleep disturbances [1]. Approximately 4–5% of the world population is affected by MDD [1]. Individuals with MDD are at a higher risk of suicidality [2], developing physical comorbidities (e.g., cardiovascular, stroke, diabetes, obesity) [3], and negative outcomes in other social aspects of life, such as education, employment and personal relationships [4].
MDD is a complex disorder whose mechanisms are not completely established [1]. Animal models have been an important strategy for investigating the molecular pathways implicated in the pathophysiology of MDD. These pathways include, but are not limited to, genetic and epigenetic alterations, neuroendocrine and immune disturbances, neuroplasticity impairments, mainly with regard to neurogenesis, and alterations in the microbiota–gut–brain axis [5]. An increased understanding of these biological processes may assist in developing new strategies for managing MDD.
The response to stressful stimuli, either psychological or physical, is mediated by the hypothalamic–pituitary–adrenal (HPA) axis [6]. The termination of the stress response is based on a negative feedback mechanism and is mediated by the connection between the hippocampus and the paraventricular nucleus of the hypothalamus [7]. A healthy response to stress requires both a rapid and vigorous response followed by the termination of such response [7]. However, a dysfunction of the HPA axis is present in individuals with MDD [1]. In addition, HPA axis activation may alter the composition of the gut microbiota and increase gastrointestinal permeability [8].
Individuals with MDD present higher serum levels of inflammatory markers [9]. Administration of lipopolysaccharide (LPS) in rodents has been used to mimic the inflammatory response and depressive symptoms observed in individuals with MDD [10]. LPS inflammatory effects extend from the periphery to the brain. Peripherally, pro-inflammatory cytokine production increases, and cytokines can subsequently reach the brain either via macrophage-like cell-mediated signaling or by crossing the blood–brain barrier (BBB), resulting in microglial activation [10]. Microglia are responsible for scavenging damaged brain tissue and pathogenic agents. At rest, they present ramifications that help their function as sentinels. In response to threats, microglia switch to their active phenotype. This results in the activation of the NOD-like receptor pyrin domain-containing-3 (NLRP3) inflammasome and the consequent release of the inflammatory cytokines interleukin (IL)-1β and IL-18, which contributes to neuroinflammation as shown in Figure 1 [11]. Accordingly, image studies have identified increased neuroinflammation in the brain of MDD patients as compared with controls [12][13].
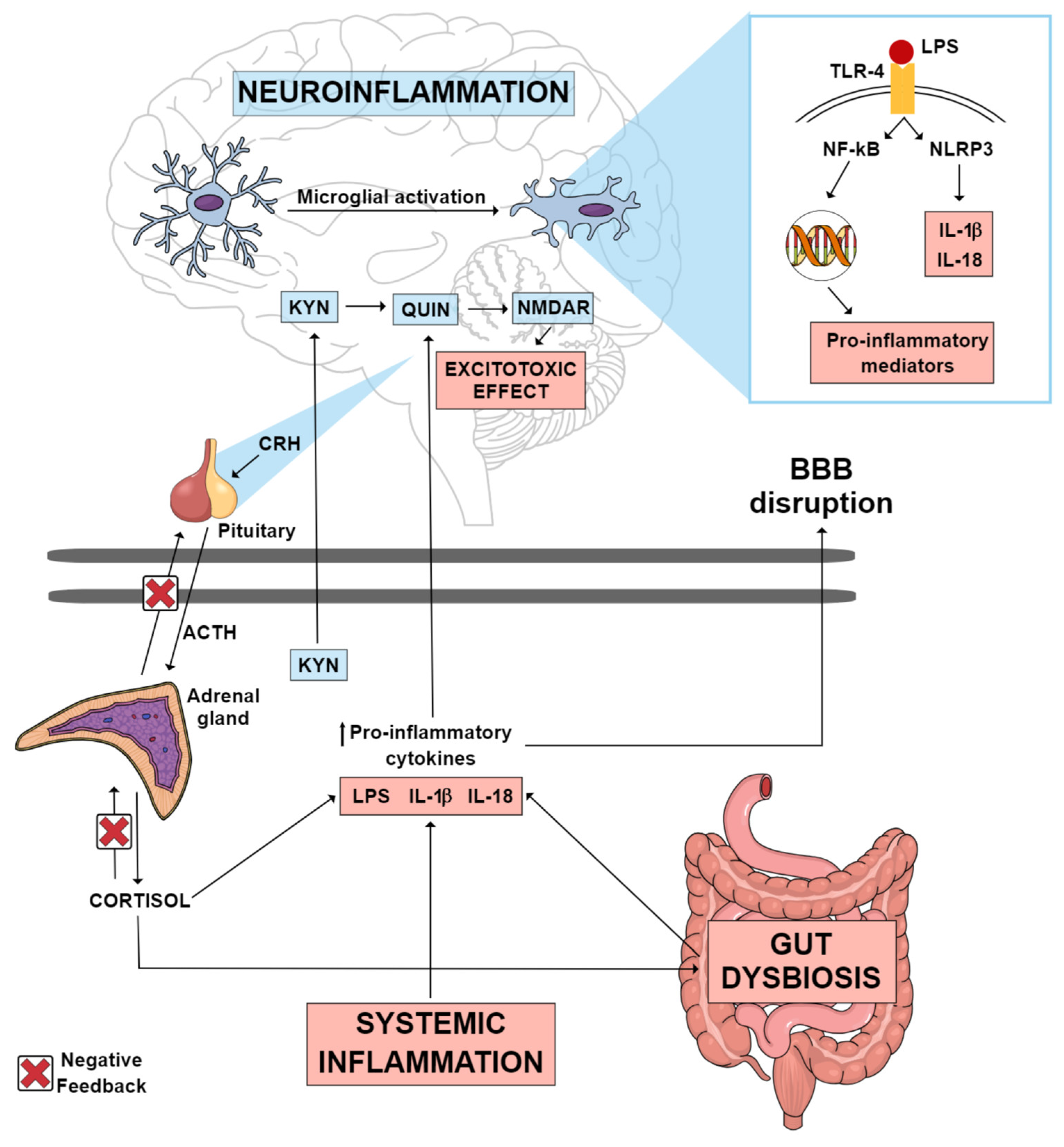
Figure 1. Possible inflammatory mechanisms thought to mediate neuroinflammation in MDD. Systemic inflammation, gut dysbiosis and an imbalance in the HPA axis can lead to an increase in circulating pro-inflammatory cytokines, which consequently may lead to the disruption of the blood–brain barrier. These pro-inflammatory cytokines can reach the CNS, where they can activate various mechanisms that culminate in neuroinflammation. Gut dysbiosis may be one of the factors contributing to the increase in circulating LPS. This endotoxin can reach the brain, where it may activate TLR-4 microglial receptors, promoting the transcription of pro-inflammatory mediators, as well as the activation of the NLRP3 inflammasome, which culminates in the production of IL-1 β and IL-18. A pro-inflammatory state in the CNS causes the KYN pathway to be directed towards the production of QUIN, an excitotoxic NMDAR agonist metabolite, instead of the production of KYNA, a neuroprotective NMDAR antagonist metabolite. Abbreviations: ACTH: adrenocorticotropic hormone; BBB: blood–brain barrier; CNS: central nervous system; CRH: corticotrophin-releasing hormone; HPA: hypothalamic–pituitary–adrenal; IL-18: interleukin 18; IL-1β: interleukin 1 beta; KYN: kynurenine; KYNA: kynurenic acid; LPS: lipopolysaccharide; MDD: major depressive disorder; NF-kB: nuclear factor kappa B; NLRP3: NOD-like receptor pyrin domain-containing-3; NMDAR: N-methyl-D-aspartate receptor; QUIN: quinolinic acid; TLR-4: toll-like receptor 4.
Another key inflammatory mechanism implicated in LPS-induced depressive-like behavior is the tryptophan (Trp)–kynurenine metabolism [14]. Trp metabolism can follow two directions: production of serotonin by tryptophan hydroxylase or production of kynurenine (KYN) by indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO) enzymes [15]. The conversion of Trp into KYN is activated under pro-inflammatory conditions [14]. KYN metabolites can be grouped into two pathways according to their effects: the excitotoxic pathway and the neuroprotective pathway [11]. KYN excitotoxic metabolites are induced in inflammatory states and include 3-hydroxy-kynurenine (3-HK), 3-hydroxy-anthralinic acid, quinolinic acid (QUIN) (a glutamate receptor agonist) and nicotinamide adenine dinucleotide (NAD+). Neuroprotective metabolites include kynurenic acid (KYNA), with opposing effects to QUIN by its antagonistic action on the N-methyl-D-aspartate (NMDA) glutamatergic receptor and reduction in extracellular glutamate release [11]. In MDD, there is an imbalance in the Trp-KYN pathway, favoring the production of neurotoxic over neuroprotective metabolites as illustrated in Figure 1 [16][17].
2. Gut Microbiota and MDD
The gut is the biggest digestive, immune and endocrine organ of the human body and can sometimes be referred to as the second brain [18]. The gut is a microbial organ, and it is estimated that our gut harbors about 1014 microorganisms [19]. The first signature microorganisms are acquired at birth and develop over the course of the first few years of life. Although it is easily modulated by host genetic and environmental factors, such as diet, stress and exposure to other microorganisms or antibiotics, the microbiota adapts to the host, carrying out several important metabolic and biochemical processes. Thus, gut microorganisms can directly influence human health [20].
The bidirectional connections between gut microorganisms and the brain through various biological systems are called the microbiota–gut–brain axis. These connections are fundamental for the maintenance of gastrointestinal, central nervous system (CNS) and microbial homeostasis and occur through direct and indirect communication via the autonomic nervous system, enteric nervous system, neuroendocrine system and immune system and signaling through microbial-derived metabolites and products, chemical transmitters, and neuronal pathways [21].
This close communication between the intestinal microbiota and the CNS is sensitive to several factors, mainly environmental ones, including diet, antibiotic use, stress and infections [22]. Disturbances in the homeostasis of these systems have already been related to the pathophysiology of MDD [23].
Clinical evidence has shown that MDD individuals have an altered microbiota composition when compared to healthy controls, including an increased Bacteroidetes/Firmicutes ratio, which is considered a dysbiosis signature [24]. Of note, a meta-analysis study has demonstrated a decreased abundance of the bacterial families Veillonellaceae, Prevotellaceae and Sutterellaceae, as well as the genera Coprococcus, Faecalibacterium, Ruminococcus, Bifidobacterium and Escherichia, and an increased abundance of the family Actinomycetaceae and the genus Paraprevotella in MDD patients compared to controls [23]. In addition, preclinical studies have shown that probiotics could improve depression-like phenotypes. For example, Tian et al. [25] reported that ingestion of the Bifidobacterium longum subspecies infantis strain CCFM687 improved stress-induced depressive-like behavior, increased BDNF levels and the abundance of butyrate-producing bacteria and modulated the HPA axis in mice. Also, treatment with Akkermansia muciniphila improved chronic-stress-induced depressive-like behavior, modulated corticosterone, dopamine, and BDNF levels and regulated gut microbiota and metabolites in mice [26].
The most direct communication between the gut and brain is through the vagus nerve (cranial nerve X) [27]. This peripheral nervous system (PNS) nerve has an important role in sensory and parasympathetic regulation of gut physiology regulating motility, digestion and tonic secretion of gastric mucus via the neurotransmitter acetylcholine (ACh) [28][29][30]. Vagal neurons synapse with intestinal enteroendocrine cells (EECs), such as neuropod cells that can release neurotransmitters including glutamate and serotonin, glucagon-like peptide-1 (GLP-1) and peptide YY (PYY). These transmit sensory stimuli from the gut to the brain in milliseconds [20][31]. Several studies have suggested that abnormal composition of the gut microbiota is related to depressive and anhedonia-like phenotypes, and this is, at least in part, mediated by vagus nerve communication [32][33]. In fact, intraperitoneal injection of LPS in rats induced a depression-like phenotype that was abolished by subdiaphragmatic vagotomy [34]. Also, depression-like phenotypes, altered microbiota composition, systemic inflammation and downregulation of synaptic proteins in the medial prefrontal cortex were shown to be dependent on the subdiaphragmatic vagus nerve in mice exposed to LPS [35].
The gut microbiota is essential to the digestion of food, absorption of nutrients and production of metabolites like short-chain fatty acids (SCFAs), lipids, vitamins, bile acids, branched-chain amino acids, Trp and indole derivatives [36][37]. SCFAs are one of the most well-characterized metabolites produced by gut microbiota. They are saturated fatty acids with a carbon chain ranging from one to six atoms in length produced through the fermentation of dietary fiber in the colon, with acetate (C2), propionate (C3) and butyrate (C4) being the most common SCFAs [38]. After being absorbed by the colonocytes, SCFAs serve as an energy source, through the production of ATP in the mitochondria, being substrates for the synthesis of cholesterol and fatty acids. Moreover, SCFAs may improve gut and brain health and maintain the integrity of the intestinal and blood–brain barriers by regulating tight junction proteins, affecting mucus production and influencing gastrointestinal motility and appetite through the regulation of neuronal activity and intestinal hormones, such as GLP-1 and PYY. Also, SCFAs can protect from inflammation by modulating pro-inflammatory cytokines and chemokines, inhibiting nuclear factor kappa B (NF-kB) as well as histone deacetylases (HDACs) [39] and regulating microglial homeostasis in the CNS [40][41]. The main mechanism by which SCFAs seem to exert their effects is by activating G-protein-coupled receptors (GPRs) that are expressed in several cell types, including immune cells, adipocytes, skeletal and heart muscle cells, intestinal cells and brain cells [42][43][44]. More specifically, acetate, propionate and butyrate bind to GPR43 and GPR41 (also known as free fatty acid receptor 2 (FFAR2) and FFAR3, respectively), with butyrate also binding to GPR109A (also known as hydroxycarboxylic acid receptor 2 (HCAR2)) [45]. Among other functions, GPR43 and GPR41 are involved in the production and secretion of GLP-1 and PYY and exert anti-inflammatory and anti-tumorigenic activities [46][47].
Butyrate, the only known SCFA agonist for GPR109A, appears to be the principal agonist in the gastrointestinal tract [48], but this receptor can also be activated by niacin (also known as nicotinic acid and vitamin B3) and β-hydroxybutyrate [49][50]. Among other functions, GPR109A is involved in the reduction in chemokine and pro-inflammatory cytokine production, including IL-1β, IL-6 and tumor necrosis factor α (TNF-α) [51][52], as well as the inhibition of the NLRP3 inflammasome [53]. Furthermore, GPR109A can enhance the activity of adenosine monophosphate (AMP)-dependent kinase (AMPK) in microglia, resulting in the activation of sirtuin 1 (SIRT1), which inhibits NF-κB signaling via acetylation [54]. The nuclear factor (erythroid-derived) related factor-2 (Nrf2), which mediates antioxidant and anti-inflammatory signaling, has also been implicated in the pathways associated with the activation of this receptor [55]. Finally, GPR109A signaling has also been shown to be involved in the increase in several neurotrophic factors, including vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) [56][57]. All these pathways have been implicated in the pathophysiology of MDD, suggesting that butyrate production by the gut microbiota and activation of GPR109A may improve depressive symptoms.
SCFAs not only play an important role in CNS homeostasis by maintaining the integrity of the BBB but also have the ability to cross it, where they can regulate brain development, neuroplasticity, neurotransmitter synthesis, epigenetic factors and gene expression, as well as the immune response [21][40]. Indeed, sodium butyrate was shown to prevent microglia activation and depressive-like behaviors in mice [58], and in vitro studies have demonstrated its anti-inflammatory role through the decrease in LPS-induced microglial inflammation [59] in rat primary microglia cultures, hippocampal slices and co-cultures of rat cerebellar granule neurons, astrocytes, and microglial cells [60]. Furthermore, SCFAs have also been shown to reduce depressive-like behaviors by regulating HPA activity. In agreement, oral administration of acetate, propionate and butyrate was able to improve changes in intestinal permeability, HPA hyperactivity and anhedonia in mice submitted to repeated psychosocial stress [61].
As mentioned above, dysfunctions in neuroplasticity and neurogenesis have also been implicated in the pathophysiology of MDD. SCFAs have the capacity to modulate neurotrophins such as BDNF, nerve growth factor (NGF) and glial cell line-derived neurotrophic factor (GDNF) that regulate the growth, survival and differentiation of neurons and synapses, thereby impacting neuroplasticity [62][63]. In fact, subcutaneous administration of sodium butyrate was shown to induce cell proliferation, migration and differentiation in the hippocampal dentate gyrus in a rat model of ischemia [64]. Moreover, a combination of sodium butyrate and pyridoxine promoted cell proliferation and neurogenesis in mice [65]. In addition, systemic administration of sodium butyrate has been reported to induce histone hyperacetylation in the hippocampus and frontal cortex, upregulate BDNF transcript levels and elicit antidepressant-like effects [66].
Another important metabolite of the gut microbiota is lactate, which is produced by the fermentation of dietary fibers by lactic acid bacteria, such as the genera Lactobacillus and Bifidobacterium. Lactate can be further converted into different SCFAs by several bacterial species, including Eubacterium hallii, Anaerostipes spp. and Veillonella spp. [67][68]. Studies have suggested that lactate can be absorbed and cross the BBB and has an important role in CNS, being used as an energy substrate by neurons and contributing to synaptic plasticity [69]. Several lines of evidence have indicated that lactate abnormalities may be associated with MDD. Indeed, an increased urine lactate concentration was observed in severe MDD patients [70]. Moreover, lactate was able to improve stress-induced depressive-like behavior, and this effect was associated with changes in the expression of target genes involved in serotonin receptor trafficking, astrocyte functions, neurogenesis, nitric oxide synthesis and cAMP signaling [71]. Further studies have also supported the antidepressant effect of lactate. Karnib et al. [72] demonstrated that lactate was able to improve depressive-like behaviors and mediate resilience to stress by modulating hippocampal levels and activity of HDACs in mice exposed to social defeat stress. In addition, Carrard et al. [73] showed that L-lactate administration reversed corticosterone-induced depressive-like behavior and promoted the proliferation and survival of new hippocampal neurons in adult mice. Furthermore, pharmacological inhibition of adult hippocampal neurogenesis abolished this effect, demonstrating the role of neurogenesis in the antidepressant-like effect of lactate. In fact, lactate is involved in regulating neuronal plasticity-related genes, including those coding for BDNF, activity-regulated cytoskeleton-associated protein (ARC) and VEGF [74][75].
The gut microbiota can also regulate Trp metabolism and the KYN pathway in MDD, promoting a decrease in KYN and an increase in QUIN levels [76]. In the brain, the enzyme kynurenine aminotransferase (KAT) that converts KYN into KYNA is localized mainly in astrocytes, and microglia and macrophages are responsible mainly for the production of QUIN by kynureninase under inflammatory conditions [77]. Some microbial components present within the gut microbiota, such as LPS and lipoteichoic acids, can activate toll-like receptors (TLRs) and have been identified as key factors in initiating Trp metabolism through the KYN pathway [78]. Furthermore, SCFAs can modulate intestinal barrier integrity and systemic inflammation, which are in turn associated with an altered KYN pathway [79]. In addition, butyrate plays a role in reducing intestinal IDO expression by inhibiting HDAC and IFN-gamma-dependent phosphorylation of signal transducer and activator of transcription 1 (STAT1) and, subsequently, the STAT1-driven transcriptional activity of IDO [80].
The modulation of the KYN pathway by the gut microbiota may be implicated in abnormal KYN levels found in MDD patients. In fact, fecal microbiota transplantation (FMT) from MDD patients to antibiotic-treated rats induced anhedonia and anxiety-like behaviors along with decreased gut microbiota richness and diversity and an elevated KYN/Trp ratio [81]. In addition, a reduction in the Firmicutes phylum and a reduction in SCFA synthesis, which has been associated with increased inflammation and the diversion of KYN metabolism to the neurotoxic pathway with the consequent production of QUIN, have been observed in MDD patients [82][83].
References
- Marx, W.; Penninx, B.W.J.H.; Solmi, M.; Furukawa, T.A.; Firth, J.; Carvalho, A.F.; Berk, M. Major depressive disorder. Nat. Rev. Dis. Primers 2023, 9, 44.
- Cai, H.; Xie, X.M.; Zhang, Q.; Cui, X.; Lin, J.X.; Sim, K.; Ungvari, G.S.; Zhang, L.; Xiang, Y.T. Prevalence of Suicidality in Major Depressive Disorder: A Systematic Review and Meta-Analysis of Comparative Studies. Front. Psychiatry 2021, 12, 690130.
- Penninx, B.W.; Milaneschi, Y.; Lamers, F.; Vogelzangs, N. Understanding the somatic consequences of depression: Biological mechanisms and the role of depression symptom profile. BMC Med. 2013, 11, 129.
- Campbell, D.; Green, M.J.; Davies, N.; Demou, E.; Howe, L.D.; Harrison, S.; Smith, D.J.; Howard, D.M.; McIntosh, A.M.; Munafò, M.; et al. Effects of depression on employment and social outcomes: A Mendelian randomisation study. J. Epidemiol. Community Health 2022, 76, 563–571.
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297.
- Godoy, L.D.; Rossignoli, M.T.; Delfino-Pereira, P.; Garcia-Cairasco, N.; de Lima Umeoka, E.H. A Comprehensive Overview on Stress Neurobiology: Basic Concepts and Clinical Implications. Front. Behav. Neurosci. 2018, 12, 127.
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21.
- de Punder, K.; Pruimboom, L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front. Immunol. 2015, 6, 223.
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5,166 patients and 5,083 controls. Brain Behav. Immun. 2020, 87, 901–909.
- Yin, R.; Zhang, K.; Li, Y.; Tang, Z.; Zheng, R.; Ma, Y.; Chen, Z.; Lei, N.; Xiong, L.; Guo, P.; et al. Lipopolysaccharide-induced depression-like model in mice: Meta-analysis and systematic evaluation. Front. Immunol. 2023, 14, 1181973.
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171.
- Holmes, S.E.; Hinz, R.; Conen, S.; Gregory, C.J.; Matthews, J.C.; Anton-Rodriguez, J.M.; Gerhard, A.; Talbot, P.S. Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biol. Psychiatry 2018, 83, 61–69.
- Richards, E.M.; Zanotti-Fregonara, P.; Fujita, M.; Newman, L.; Farmer, C.; Ballard, E.D.; Machado-Vieira, R.; Yuan, P.; Niciu, M.J.; Lyoo, C.H.; et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 2018, 8, 57.
- O’Connor, J.C.; Lawson, M.A.; André, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522.
- Badawy, A.A. Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects. Int. J. Tryptophan Res. 2017, 10, 1178646917691938.
- Liang, S.; Zhao, L.; Ni, P.; Wang, Q.; Guo, W.; Xu, Y.; Cai, J.; Tao, S.; Li, X.; Deng, W.; et al. Frontostriatal circuitry and the tryptophan kynurenine pathway in major psychiatric disorders. Psychopharmacology 2023.
- Chen, X.; Beltran, D.J.; Tsygankova, V.D.; Woolwine, B.J.; Patel, T.; Baer, W.; Felger, J.C.; Miller, A.H.; Haroon, E. Kynurenines increase MRS metabolites in basal ganglia and decrease resting-state connectivity in frontostriatal reward circuitry in depression. Transl. Psychiatry 2021, 11, 456.
- Liang, S.; Wu, X.; Jin, F. Gut-Brain Psychology: Rethinking Psychology from the Microbiota-Gut-Brain Axis. Front. Integr. Neurosci. 2018, 12, 33.
- Sender, R.; Fuchs, S.; Milo, R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell 2016, 164, 337–340.
- Fülling, C.; Dinan, T.G.; Cryan, J.F. Gut Microbe to Brain Signaling: What Happens in Vagus…. Neuron 2019, 101, 998–1002.
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255.
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73.
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13.
- Yang, J.; Zheng, P.; Li, Y.; Wu, J.; Tan, X.; Zhou, J.; Sun, Z.; Chen, X.; Zhang, G.; Zhang, H.; et al. Landscapes of bacterial and metabolic signatures and their interaction in major depressive disorders. Sci. Adv. 2020, 6, eaba8555.
- Tian, P.; Zou, R.; Song, L.; Zhang, X.; Jiang, B.; Wang, G.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. Ingestion of Bifidobacterium longum subspecies infantis strain CCFM687 regulated emotional behavior and the central BDNF pathway in chronic stress-induced depressive mice through reshaping the gut microbiota. Food Funct. 2019, 10, 7588–7598.
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426.
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230.
- Aubé, A.C.; Blottière, H.M.; Scarpignato, C.; Cherbut, C.; Rozé, C.; Galmiche, J.P. Inhibition of acetylcholine induced intestinal motility by interleukin 1 beta in the rat. Gut 1996, 39, 470–474.
- Godinho-Silva, C.; Cardoso, F.; Veiga-Fernandes, H. Neuro-Immune Cell Units: A New Paradigm in Physiology. Annu. Rev. Immunol. 2019, 37, 19–46.
- Somasundaram, K.; Ganguly, A.K. The effect of subdiaphragmatic vagotomy on the gastric mucus barrier in rats. Clin. Exp. Pharmacol. Physiol. 1987, 14, 735–741.
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136.
- Wang, S.; Ishima, T.; Zhang, J.; Qu, Y.; Chang, L.; Pu, Y.; Fujita, Y.; Tan, Y.; Wang, X.; Hashimoto, K. Ingestion of Lactobacillus intestinalis and Lactobacillus reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve. J. Neuroinflamm. 2020, 17, 241.
- Wang, S.; Ishima, T.; Qu, Y.; Shan, J.; Chang, L.; Wei, Y.; Zhang, J.; Pu, Y.; Fujita, Y.; Tan, Y.; et al. Ingestion of Faecalibaculum rodentium causes depression-like phenotypes in resilient Ephx2 knock-out mice: A role of brain-gut-microbiota axis via the subdiaphragmatic vagus nerve. J. Affect. Disord. 2021, 292, 565–573.
- Konsman, J.P.; Luheshi, G.N.; Bluthé, R.M.; Dantzer, R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur. J. Neurosci. 2000, 12, 4434–4446.
- Zhang, J.; Ma, L.; Chang, L.; Pu, Y.; Qu, Y.; Hashimoto, K. A key role of the subdiaphragmatic vagus nerve in the depression-like phenotype and abnormal composition of gut microbiota in mice after lipopolysaccharide administration. Transl. Psychiatry 2020, 10, 186.
- Agus, A.; Clément, K.; Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 2021, 70, 1174–1182.
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14.
- Miller, T.L.; Wolin, M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Environ. Microbiol. 1996, 62, 1589–1592.
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200.
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478.
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977.
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96.
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319.
- Kimura, I.; Ozawa, K.; Inoue, D.; Imamura, T.; Kimura, K.; Maeda, T.; Terasawa, K.; Kashihara, D.; Hirano, K.; Tani, T.; et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat. Commun. 2013, 4, 1829.
- Liu, H.; Wang, J.; He, T.; Becker, S.; Zhang, G.; Li, D.; Ma, X. Butyrate: A Double-Edged Sword for Health? Adv. Nutr. 2018, 9, 21–29.
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Nøhr, M.K.; Pedersen, M.H.; Gille, A.; Egerod, K.L.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.M.; Grunddal, K.V.; Poulsen, S.S.; Han, S.; et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 2013, 154, 3552–3564.
- Cresci, G.A.; Thangaraju, M.; Mellinger, J.D.; Liu, K.; Ganapathy, V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J. Gastrointest. Surg. 2010, 14, 449–461.
- Soga, T.; Kamohara, M.; Takasaki, J.; Matsumoto, S.; Saito, T.; Ohishi, T.; Hiyama, H.; Matsuo, A.; Matsushime, H.; Furuichi, K. Molecular identification of nicotinic acid receptor. Biochem. Biophys. Res. Commun. 2003, 303, 364–369.
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652.
- Wu, Y.; Gong, Y.; Luan, Y.; Li, Y.; Liu, J.; Yue, Z.; Yuan, B.; Sun, J.; Xie, C.; Li, L.; et al. BHBA treatment improves cognitive function by targeting pleiotropic mechanisms in transgenic mouse model of Alzheimer’s disease. FASEB J. 2020, 34, 1412–1429.
- Viatchenko-Karpinski, V.; Kong, L.; Weng, H.R. Activation of microglial GPR109A alleviates thermal hyperalgesia in female lupus mice by suppressing IL-18 and glutamatergic synaptic activity. Glia 2022, 70, 634–649.
- Qian, J.; Zhu, W.; Lu, M.; Ni, B.; Yang, J. D-β-hydroxybutyrate promotes functional recovery and relieves pain hypersensitivity in mice with spinal cord injury. Br. J. Pharmacol. 2017, 174, 1961–1971.
- Parodi, B.; Rossi, S.; Morando, S.; Cordano, C.; Bragoni, A.; Motta, C.; Usai, C.; Wipke, B.T.; Scannevin, R.H.; Mancardi, G.L.; et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 2015, 130, 279–295.
- Safari, A.; Badeli-Sarkala, H.; Namavar, M.R.; Kargar-Abarghouei, E.; Anssari, N.; Izadi, S.; Borhani-Haghighi, A. Neuroprotective effect of dimethyl fumarate in stroke: The role of nuclear factor erythroid 2-related factor 2. Iran. J. Neurol. 2019, 18, 108–113.
- Shehadah, A.; Chen, J.; Zacharek, A.; Cui, Y.; Ion, M.; Roberts, C.; Kapke, A.; Chopp, M. Niaspan treatment induces neuroprotection after stroke. Neurobiol. Dis. 2010, 40, 277–283.
- Sun, J.; Yuan, B.; Wu, Y.; Gong, Y.; Guo, W.; Fu, S.; Luan, Y.; Wang, W. Sodium Butyrate Protects N2a Cells against A. Mediators Inflamm. 2020, 2020, 7605160.
- Yamawaki, Y.; Yoshioka, N.; Nozaki, K.; Ito, H.; Oda, K.; Harada, K.; Shirawachi, S.; Asano, S.; Aizawa, H.; Yamawaki, S.; et al. Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res. 2018, 1680, 13–38.
- Wang, P.; Zhang, Y.; Gong, Y.; Yang, R.; Chen, Z.; Hu, W.; Wu, Y.; Gao, M.; Xu, X.; Qin, Y.; et al. Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol. Dis. 2018, 111, 12–25.
- Huuskonen, J.; Suuronen, T.; Nuutinen, T.; Kyrylenko, S.; Salminen, A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br. J. Pharmacol. 2004, 141, 874–880.
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944.
- Varela, R.B.; Valvassori, S.S.; Lopes-Borges, J.; Mariot, E.; Dal-Pont, G.C.; Amboni, R.T.; Bianchini, G.; Quevedo, J. Sodium butyrate and mood stabilizers block ouabain-induced hyperlocomotion and increase BDNF, NGF and GDNF levels in brain of Wistar rats. J. Psychiatr. Res. 2015, 61, 114–121.
- Barichello, T.; Generoso, J.S.; Simões, L.R.; Faller, C.J.; Ceretta, R.A.; Petronilho, F.; Lopes-Borges, J.; Valvassori, S.S.; Quevedo, J. Sodium Butyrate Prevents Memory Impairment by Re-establishing BDNF and GDNF Expression in Experimental Pneumococcal Meningitis. Mol. Neurobiol. 2015, 52, 734–740.
- Kim, H.J.; Leeds, P.; Chuang, D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009, 110, 1226–1240.
- Yoo, D.Y.; Kim, W.; Nam, S.M.; Kim, D.W.; Chung, J.Y.; Choi, S.Y.; Yoon, Y.S.; Won, M.H.; Hwang, I.K. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem. Res. 2011, 36, 1850–1857.
- Schroeder, F.A.; Lin, C.L.; Crusio, W.E.; Akbarian, S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol. Psychiatry 2007, 62, 55–64.
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185.
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22.
- Mosienko, V.; Teschemacher, A.G.; Kasparov, S. Is L-lactate a novel signaling molecule in the brain? J. Cereb. Blood Flow. Metab. 2015, 35, 1069–1075.
- Chen, J.J.; Zhou, C.J.; Zheng, P.; Cheng, K.; Wang, H.Y.; Li, J.; Zeng, L.; Xie, P. Differential urinary metabolites related with the severity of major depressive disorder. Behav. Brain Res. 2017, 332, 280–287.
- Carrard, A.; Elsayed, M.; Margineanu, M.; Boury-Jamot, B.; Fragnière, L.; Meylan, E.M.; Petit, J.M.; Fiumelli, H.; Magistretti, P.J.; Martin, J.L. Peripheral administration of lactate produces antidepressant-like effects. Mol. Psychiatry 2018, 23, 392–399.
- Karnib, N.; El-Ghandour, R.; El Hayek, L.; Nasrallah, P.; Khalifeh, M.; Barmo, N.; Jabre, V.; Ibrahim, P.; Bilen, M.; Stephan, J.S.; et al. Lactate is an antidepressant that mediates resilience to stress by modulating the hippocampal levels and activity of histone deacetylases. Neuropsychopharmacology 2019, 44, 1152–1162.
- Carrard, A.; Cassé, F.; Carron, C.; Burlet-Godinot, S.; Toni, N.; Magistretti, P.J.; Martin, J.L. Role of adult hippocampal neurogenesis in the antidepressant actions of lactate. Mol. Psychiatry 2021, 26, 6723–6735.
- Yang, J.; Ruchti, E.; Petit, J.M.; Jourdain, P.; Grenningloh, G.; Allaman, I.; Magistretti, P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. USA 2014, 111, 12228–12233.
- Margineanu, M.B.; Mahmood, H.; Fiumelli, H.; Magistretti, P.J. L-Lactate Regulates the Expression of Synaptic Plasticity and Neuroprotection Genes in Cortical Neurons: A Transcriptome Analysis. Front. Mol. Neurosci. 2018, 11, 375.
- Wang, L.; Feng, Z.; Zheng, T.; Dai, G.; Wang, M.; Zhou, L.; Zheng, Y.; Chen, G. Associations between the kynurenine pathway and the brain in patients with major depressive disorder-A systematic review of neuroimaging studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 121, 110675.
- Guillemin, G.J.; Smith, D.G.; Smythe, G.A.; Armati, P.J.; Brew, B.J. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv. Exp. Med. Biol. 2003, 527, 105–112.
- Orhan, F.; Bhat, M.; Sandberg, K.; Ståhl, S.; Piehl, F.; Svensson, C.; Erhardt, S.; Schwieler, L.; Schizophrenia Project (KaSP) Consortium. Tryptophan Metabolism Along the Kynurenine Pathway Downstream of Toll-like Receptor Stimulation in Peripheral Monocytes. Scand. J. Immunol. 2016, 84, 262–271.
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112 Pt B, 399–412.
- Martin-Gallausiaux, C.; Larraufie, P.; Jarry, A.; Béguet-Crespel, F.; Marinelli, L.; Ledue, F.; Reimann, F.; Blottière, H.M.; Lapaque, N. Butyrate Produced by Commensal Bacteria Down-Regulates. Front. Immunol. 2018, 9, 2838.
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118.
- Huang, Y.; Shi, X.; Li, Z.; Shen, Y.; Wang, L.; Li, G.; Yuan, Y.; Wang, J.; Zhang, Y.; Zhao, L.; et al. Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2018, 14, 3329–3337.
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48.
More
Information
Subjects:
Neurosciences
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
549
Revisions:
2 times
(View History)
Update Date:
12 Dec 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




